Abstract
The paper presents the results of research of cordierite ceramics; within its composition, a part of components is introduced using relatively low-melting MABS glass. After firing at a temperature of 1375 °C, densely sintered materials with low values of LCTE (17.7–18.5) × 10–7 deg–1 were obtained, which predetermine their high thermal stability (not lower than 900 °C). The only crystalline phase of experimental ceramics is α-cordierite, which forms its structural matrix. Crystals of α-cordierite of 1–2 μm in size are tightly interconnected via thin layers of the residual glass phase. Cordierite ceramics is characterized by zero values of water absorption and open porosity, as well as high values of mechanical compressive strength. In addition, the dense microstructure allows achieving consistently high dielectric values (ε = 4.9; tan δ = 0.0014) in an ultra-high-frequency electromagnetic field (1010 Hz). The synthesized ceramics can be successively used as radio-transparent materials, including structural ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ceramic dielectric materials are widely used in the development of radio tracking devices in aviation and rocket engineering, in particular, in aircraft antenna fairings and protective windows for shelters of the various types of antenna systems. One of the most important tasks solved in the process of their development lies in creating a reliable structure with the minimum mass, which provides the necessary dielectric and thermo-mechanical characteristics, as well as high erosion resistance. Transparency of these materials for radio waves is ensured by low dielectric losses (relative permittivity ε < 10, dielectric loss angle tan δ = 10–2–10–5) in the ultra-high frequency range 3–30 GHz [1]. There are a large number of high-heat-resistant materials, which are persistent in the oxidizing and reducing environment, with stable dielectric characteristics. Quartz ceramics [2], as well as glass-crystalline materials of aluminosilicate composition [3,4,5], meet these requirements to the greatest extent.
Quartz ceramics is characterized by high thermal stability and the highest level of dielectric characteristics (ε = 3.8, tan δ = (1–4) × 10–4 at the temperature of 25 °C) [2]. At the same time, due to the presence of porosity, it requires moisture protection and sealing, and therefore, it is used in the fairings of aircrafts, the operation of which involves transport and launch containers [1]. In addition, quartz ceramics has relatively low mechanical strength indices, despite all efforts to strengthen it [6,7,8].
More promising are glass-crystalline materials obtained on the basis of various aluminosilicate systems. They are characterized by a set of high physical and technical indicators [4]. However, the effective use of existing lithium aluminosilicate materials (spodumene and eucryptite ones) is limited to the temperature of 900 °C, because of insufficient temperature stability of their physical and technical (dielectric, thermal and mechanical) properties [9, 10].
Apart from spodumene glass–ceramics, which was one of primary materials for antenna fairings of aircrafts in recent decades, celsian and strontium-anortite glass–ceramics types have been created. These materials can operate in the temperature range exceeding 1100 °C. However, such glass–ceramics has a number of disadvantages. It is characterized by high-density index, increased values of the temperature coefficient of linear expansion (LCTE) and dielectric parameters [11,12,13,14].
Therefore, cordierite glass-crystalline materials are the most acceptable ones in terms of achieving high performance. They feature lower density indices compared to other types of alkali-free aluminosilicate glass–ceramics. Low density is an important criterion for reducing the weight of radio-transparent materials. High thermal stability associated with low coefficient of thermal expansion is a characteristic feature of cordierite (2MgO·2Al2O3·5SiO2). Cordierite glass ceramics has low dielectric losses, high mechanical strength and chemical resistance. Glass-crystalline materials developed on the basis of cordierite have deformation temperature of ≥ 1300 °C and thermal stability at the level of 800–1000 °C [15,16,17].
Densely sintered glass-crystalline materials in the MgO–Al2O3–SiO2 (MAS) system are obtained mainly by two technologies: conventional glass and ceramic technologies. Glass technology includes melting of glass, molding of products of molten glass, their annealing and directional crystallization. This method, in addition to high melting temperature of the parent glasses (for glasses of the MAS system, it is mainly 1550–1600 °C), has a number of limitations and disadvantages, namely, stringent requirements for glasses with regard to their melting and molding properties, need for strict compliance with the regime of their heat treatment (during annealing and crystallization), as well as introduction of crystallization catalysts changing the chemical composition of glasses [18, 19]. Besides, the conventional glass technology limits the ability to vary the phase composition of materials and complexity of the products’ shapes.
Currently, the ceramic technology of glass-crystalline materials (powder method) is more actively developing. Powder technology of glass-crystalline materials allows to considerably expanding the range of compositions of the parent glasses. Owing to the possibility of use of different methods of molding, this method allows expanding the range of the manufactured products and complexity of their shapes [15].
Serious disadvantages of the powder method of cordierite glass–ceramics manufacturing are high melting temperatures of the parent MAS glasses (1550–1600 °C), the difficulty of making slips with the required rheological properties, insufficient strength of cast work pieces. In addition, the difference in the grain composition of the dispersed phase of ceramic slips can be the reason for obtaining products (especially large-sized ones) with the areas of different densities. It greatly complicates the provision of stability and reproducibility of their physic-chemical characteristics [16,17,18,19].
The density of glass–ceramics is a critical factor in its application. In order to ensure high resistance to dust and rain erosion, radio-transparent materials should be moisture proof. Otherwise, additional use of protective coatings is required.
When using a mechanical mixture of natural materials (kaolinite, talc and crystalline form of alumina), temperature of the beginning of the cordierite formation is in the range of 1160–1270 °C. However, the synthesis of pure cordierite ceramics even at the temperature of 1450 °C during 20–60 h does not allow obtaining a dense material. At the temperatures above 1450 °C, the degradation of the cordierite crystalline structure with subsequent incongruent melting occurs. Cordierite obtained in this way contains a significant amount (up to 20 wt%) of the impurity phases of spinel, mullite, clinoenstatite, which deteriorate the ceramics performance [20, 21].
For obtaining a dense structure of the cordierite ceramics, modifying additives are introduced. They act by different mechanisms, intensifying the sintering process at the lower temperatures.
The papers [22, 23] show an effective mineralizing effect of sodium and potassium compounds, which contribute to the growth of the cordierite phase content and compaction during sintering. At the same time, in case of high-frequency use of such ceramics dielectric losses increase significantly. Adding of lithium-containing materials, in particular, in the form of glass of spodumene composition [24] is more beneficial in this regard. It causes a significant decrease in the cordierite ceramics sintering temperature (up to 1350 °C) without increase in dielectric losses (tan δ = 0.0014). The paper [25] establishes the positive effect of crystalline spodumene in the amount of 10–15 wt% on the compaction, mechanical properties and thermal stability of the cordierite ceramics fired at the temperature of 1380 °C.
Cations of the transition elements Cu, Co, Ni [26,27,28] with the ionic radius close to the ionic radius Mg2+ (0,74 Å) have a favorable effect on the formation of cordierite and sintering of the cordierite ceramics. They form solid solutions upon substitution of Mg2+ ions in the cordierite structure. Isomorphic solid solutions create the defects, which accelerate the diffusion of components and crystallization of α-cordierite until the concentration of the additive exceeds the solubility limit. Increase in the content of additives leads to the formation of new crystalline phases (spinels, silicates) and can significantly change the cordierite ceramics properties.
Authors of [29] found a positive effect of the TiO2-modifying additive in the amount of 10 wt% on the mechanical properties of cordierite ceramics. Sintering temperature is reduced to 1300 °C, but the temperature coefficient of linear expansion (LCTE) is quite high, at 36.1 × 10–7 deg–1.
To promote the process of sintering of the initial mixture of stoichiometric cordierite composition, authors of [30] performed the mechanical activation of this mixture in the vibration mill, and the additional amount of 2.5 wt% of Bi2O3 was introduced. Liquid-phase sintering caused by the presence of bismuth (III) oxide reduces the temperature of formation of the dense structure of cordierite ceramics to 1350–1400 °C, and the time to 2 h.
The action of lead-borosilicate glass was also studied to intensify the processes of α-cordierite formation and compaction of cordierite ceramics. With the addition of such glass, the ceramic material can be sintered at low temperature (1350 °C). This additive slightly deteriorates the dielectric properties of ceramics. It is important that sintering process is not accompanied by the formation of excess amount of the active glass phase, the presence of which adversely affects the properties of cordierite material [31].
Summarizing the above, in the course of creation of radio-transparent into the ultra-high frequency range of glass-crystalline materials with a set of high physical and technical indicators it is advisable to use the MgO–Al2O3–SiO2 system. The current methods for obtaining densely sintered glass-crystalline materials of the cordierite composition are predominantly based on high-temperature heat treatment or do not allow achieving a set of high physical and technical indicators. Therefore, the problem of creating more perfect and energy-efficient technological method of the development of densely sintered glass-crystalline materials of the cordierite composition remains relevant. The essence of the technique is in the directional adjustment of the microstructure and phase composition of the ceramic material by introducing a relatively low-melting glass of the eutectic composition in the pseudo ternary MgO–Al2O3–SiO2 system in the main matrix, the role of which is performed by the cordierite phase.
The purpose of research is to develop the technological aspects of obtaining densely sintered cordierite ceramics at low firing temperatures and to study the physic-chemical patterns of formation of its phase composition and microstructure. It will significantly simplify the technology of glass-crystalline materials at the stage of preparation of ceramic slips and manufacturing of semi-finished products, reduce the energy costs during heat treatment, and control their phase composition and microstructure. In addition, a set of high physical and technical indicators of cordierite ceramics will be provided, which determines its long-term effective operation.
2 Experimental procedures
2.1 Materials
To obtain cordierite ceramics as raw materials components, glass of the eutectic composition in the pseudo ternary MgO–Al2O3–SiO2 system, “cordierite fireclay”, low-temperature fireclay, enriched kaolin zref-1 brand (Ukraine), talc 5SSW brand (India), and technical alumina G-0 brand (Ukraine) were used.
For the melting of glass, raw materials of technical purity: magnesium oxide A brand (MgO ≥ 99 wt%), technical alumina G-0 brand (Al2O3 ≥ 98), silicon dioxide A brand (SiO2 ≥ 99.5 wt%), and boric acid (H3BO3 ≥ 99.8 wt%) were used.
2.2 Glass melting
Composition of the experimental glass was selected in the MgO–Al2O3–SiO2 system at the ternary eutectic point with the temperature of 1365 °C. Additional fusibility of the experimental glass was provided by the introduction of boron oxide into the system (10 weight parts over 100 wt%). Boron oxide promotes reduction of the temperature of formation of the silicate glass melt and crystallization temperature of glass, as well as improvement of its wetting ability in relation to the crystalline phase [32, 33]. Melting of glass in the MgO–Al2O3–B2O3–SiO2 (MABS) system was carried out in the electric furnace with silicon carbide heaters at the temperature of 1350 °C during 1 h. Fireclay crucibles were used for melting.
2.3 Leaner’s preparation method
To reduce the air shrinkage of ceramic materials, the Leaner’s were introduced into their composition, using “cordierite fireclay” as the main leaner. For preparing the “cordierite fireclay”, kaolin, talc and technical alumina were used in the ratios required to obtain stoichiometric cordierite. The mixture to produce “cordierite fireclay” was prepared by combined wet grinding (W = 35%) in the ball mill until complete passage through the sieve No. 0063 (mesh size 63 μm). Dried material was fired in the electric furnace at the maximum temperature of 1200 °C with 1 h holding. The fired fireclay before use was pre-milled in the ball mill until complete passage through the sieve No. 01 (mesh size 100 μm), and then introduced into the experimental mixtures partially or completely (depending on the composition of the mixture) instead of the appropriate amounts of kaolin, talc and technical alumina. In addition, low-temperature fireclay obtained by preliminary sintering of kaolin zref-1 brand at 800 °C was used.
2.4 Method for ceramic samples’ making
Ceramic slips were prepared of the initial components in the porcelain ball mill by combined wet grinding until complete passage through the sieve No. 0063 (mesh size 63 μm). After the aging process, samples of the slips with the moisture content of 33–35% were cast into gypsum molds in the form of cylinders (d = h = 10 mm), square (5 × 5 × 50 mm) and cylindrical (d = 8 mm, h = 80 mm) rods, and round discs (d = 50 mm, s = 5 mm). After removing the castings from the molds, they were dried to residual moisture content of 1%. Dried samples were fired in the electric furnace in the air environment according to specified temperature and time regime. Maximum sintering temperature was equal to 1250–1375 °C with isothermal holding for 1 h.
2.5 Research methods
Our experimental study was performed according to the standard procedures for determining the properties of glasses and ceramic materials.
Water absorption (W), apparent porosity (P), and imaginary density (ρ) of the samples were measured by saturation and subsequent weighing in the air and water.
The compression strength limit (σ) was determined on cylindrical samples (d = h = 10 mm) using a hydraulic press.
Ceramic samples the size of 5 × 5 × 50 mm were used to measure the relative lengthening of ceramic samples (Δl). The data obtained were used to calculate the average LCTE value in the range of 20‒400 °C at a heating rate of 10 °C/min.
Temperature of softening and crystallization of the LAS matrix glass was measured on the differential scanning calorimeter (DSC, Netzsch 404PC) in the temperature interval of 20–1200 °C at a heating rate of 10 °C/min.
The mineralogical composition of the experimental ceramics was determined by X-ray phase analysis (XPA) at the Philips APD-15 diffractometer in CoKα radiation.
Electron-microscopic studies of ceramic samples at rupture were conducted at the scanning electron microscope SEO-SEM Inspect S50-B.
Heat resistance was determined on the basis of the maximum temperature difference, K, which the samples can withstand before showing the signs of damage.
The measurement of relative permittivity (ε) and the dielectric loss angle (tan δ) were carried out at a measuring unit consisting of the generator G4-83, the spectrum analyzer C4-11, and a biconical resonator. The resonator was connected according to the scheme on the pass. The measurements were carried out at a frequency of 1010 Hz at a temperature of 20 °C [34].
3 Results
3.1 Choosing of glass composition and study of its properties
To obtain densely sintered cordierite ceramics and achieve a set of high physical and technical indicators, the part of components of the MgO–Al2O3–SiO2 system was introduced with the use of glass. In the process of firing of the experimental ceramics, the principle of reaction formation of its microstructure is realized. The final mineralogical composition of ceramic materials is formed due to interaction of a part of components of the experimental glass with crystalline fillers, which is much more intense than the course of reactions in the solid phase. Besides, in the process of sintering there should be the finely dispersed crystallization of the cordierite phase of experimental glass, which would promote increase in the mechanical strength of ceramics.
The glass was chosen in the ternary MgO–Al2O3–SiO2 system. The point corresponding to the composition of cordierite lies in the field of primary crystallization of mullite (mullite crystallizes first) in the high-temperature region (over 1450 °C), stipulating high temperature of the parent glass melting [34]. Given this fact, we chose the point of triple eutectics in the MgO–Al2O3–SiO2 system at the temperature of 1365 °C, located at the junction of the fields of primary crystallization of cordierite (2MgO·2Al2O3·5SiO2), forsterite (2MgO·SiO2) and clinoenstatite (MgO·SiO2). During heat treatment of glass of the composition corresponding to the eutectic point, the physical process of simultaneous crystallization of three compounds with the fields of primary crystallization converging at this point will take place.
Area of the diagram of state of the MgO–Al2O3–SiO2 system where the composition of the experimental glass is chosen is shown in Fig. 1.
To increase the fusibility of the experimental glass, boron oxide (10 wt parts over 100 wt%) was additionally introduced into MAS system. Experimental MABS glass melted at the temperature of 1350 °C in the fireclay crucible is characterized by LCTE equal to 51.5 × 10–7 deg–1.
According to results of the differential thermal analysis (Fig. 2), endothermic effect with the minimum at the temperature of 750 °C corresponds to softening of the parent glass, while the intense exo-effect at the temperature of 930 °C is compatible with its crystallization. Insignificant endo-effect at 1180 °C is obviously conditioned by the glass powder sintering.
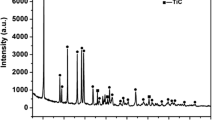
To identify the qualitative crystal-phase composition, the experimental MABS glass was subjected to stepwise heat treatment. Isothermal holding for 1 h was performed first at the temperatures of 750 and 930 °C, which corresponded to softening and crystallization of glass (see Fig. 2), and then at the maximum firing temperature of 1200 °C. Crystal-phase composition of heat-treated glass C (Fig. 3) is mainly represented by α-cordierite (d × 1010 = 8.30; 4.70; 3.98; 3.30; 3.08; 2.96; 1.67 m). Low-temperature form of clinoenstatite and a small amount of forsterite, with diffraction maxima characteristic for these compounds at (d × 1010 = 3.30; 2.96; 2.81; 1.60 m) and (d × 1010 = 5.20; 3.40; 2.73; 2.50 m), respectively, are recorded as well.
Based on the results of X-ray phase analysis, the estimated content of crystalline phases being the products of crystallization of the experimental glass after heat treatment is presented below, wt%: cordierite 53.7; clinoenstatite 31.2; forsterite 5.9.
3.2 Results of research of physical and technical indicators of cordierite ceramics
Crystalline phases of clinoenstatite and forsterite, which are the products of crystallization of the experimental glass, have good dielectric properties (ε = 6–7, tan δ = 0.0015–0.003 at the temperature of 20 °C). At the same time, clinoenstatite and forsterite are characterized by high values of LCTE, 77.0 × 10–7 deg−1 and 90 × 10–7 deg−1 respectively [35]. The above indicators of thermal expansion of these compounds do not allow obtaining ceramics with high thermal stability. Binding of clinoenstatite and forsterite into the phase with low LCTE (cordierite) was implemented using the principle of reaction formation of the structure through addition of the missing components using crystalline compounds.
Binding of clinoenstatite and forsterite into cordierite phase was performed by the reactions below:
In the course of obtaining of cordierite ceramics, MABS glass was introduced in the amount of 10, 20 i 30 wt% (C-1, C-2, C-3 compositions). C-0 composition was also considered for the comparison of results along with compositions containing MABS glass. This composition included kaolin, low-temperature fireclay, talc and technical alumina. Oxide content in the C-0 composition corresponded to their stoichiometric ratio in cordierite (MgO:Al2O3:SiO2 = 2:2:5).
Introduction of clay material (kaolin) into experimental cordierite compositions allows easily controlling the rheological properties of aqueous slips and getting (after disassembly of molds) the work pieces with sufficient strength for the subsequent process stages.
To prevent deformation and cracking in the ceramic samples, kaolin was introduced in the amount not exceeding 35 wt%. The rest was introduced using “cordierite fireclay” and low-temperature fireclay.
Heat treatment is an important process stage in obtaining of glass-crystalline materials. Therefore, it is necessary to establish the most rational parameters of this process, which would allow obtaining specified mineralogical composition and microstructure of the material.
Taking into account the data of differential thermal studies of the parent MABS glass, we performed stepwise firing of the experimental ceramic materials. At the first stage, it was made at the temperatures of nucleation (750 °C) and crystallization (930 °C) of the parent glass with 1 h holding. Furthermore, the maximum firing temperature was reached at 1250–1375 °C with 1 h holding.
The results of measurement of physical and technical properties of experimental ceramics in the form of graphical dependences are presented in Figs. 4 and 5.
Experiments proved that ceramics obtained on the basis of the stoichiometric cordierite without addition of the parent glass (C-0 composition) was characterized by low degree of sintering. Increase in the firing temperature to 1375 °C causes the gradual decrease in water absorption from 31 to 13.9%, and in open porosity from 45 to 25.3% (Fig. 4a, b). As a result, the apparent density increases to 1.84 g/cm3 and compressive strength to 105.5 MPa.
Introduction of a part of components of MAS system into experimental compositions using MABS glass results in significant intensification of their sintering process. The effectiveness is enhanced with the increase in glass content from 10 to 30 wt% and firing temperature from 1250 to 1375 °C.
When MABS glass is introduced in the amount of 10 wt% and firing temperature increases from 1250 to 1375 °C, water absorption and open porosity of the ceramics are reduced to 7.12 and 14.54%, respectively. Besides, the value of ρ increases significantly to 2.10 g/cm3 (Fig. 4c) and σ to 176 MPa (Fig. 5a).
The experimental glass, introduced in the amount of 20–30 wt% (C-2 and C-3 compositions), causes more noticeable changes in the physical and technical properties of the synthesized ceramics. With the increase in firing temperature to 1350 °C water absorption decreases to 1.9–4.5%, open porosity to 3.9–9.1%. The values of ρ, accordingly, grow to 2.01–2.06 g/cm3, and σ to 188.6–214.0 MPa.
The firing temperature of 1375 °C is the most rational in terms of achieving a set of high physical and technical indicators. Cordierite ceramics is characterized by zero values of water absorption and open porosity (Fig. 4a, b); it also has the highest apparent density of 2.42–2.47 g/cm3 (Fig. 4c), which conditions the achievement of maximum values of σ 276.5–307.4 MPa (Fig. 5a).
For experimental compositions, the extreme course of dependence of LCTE on firing temperature is recorded. For example, with the increase in firing temperature to 1350 °C, the values of LCTE fall from (21.5–23.2) × 10–7 deg−1 to (12.3–18.0) × 10–7 deg−1. Further sintering at the temperature of 1375 °C causes the insignificant increase in LCTE in the range of 20–400 °C to (14.0–18.5) × 10–7 deg−1. Besides, growing values of LCTE, from (12.3–14.0) × 10–7 deg−1 to (18.0–18.5) × 10–7 deg−1, are observed for the experimental ceramics fired in the temperature range of 1350–1375 °C, with increase in the content of the parent glass to 30 wt%.
For samples of ceramics characterized by the highest physical and technical indicators, dielectric properties were investigated, and thermal stability was determined. It is found that the ceramics of C-2 and C-3 compositions fired at 1375 °C has the low dielectric constant ε = 4.9 and low value of tan δ, which does not change depending on the composition being at the level of 0.0014. The developed cordierite ceramics is also characterized by high thermal stability, which is 900 °C.
3.3 Results of studies of the phase composition and microstructure of cordierite ceramics
Results of X-ray phase analysis (Fig. 6) show that the qualitative mineralogical composition of C-0, which does not contain MABS glass, changes significantly during firing in the temperature range of 1250–1375 °C. After firing at 1250 °C, there is a number of newly formed crystalline structures in the composition which take the form of β-cristobalite (d × 1010 = 4.00; 3.12; 2.47; 1.43 m), clinoenstatite MgO∙SiO2 (d × 1010 = 3.12; 2.94; 2.84; 2.11 m), mullite 3Al2O3∙2SiO2 (d × 1010 = 5.34; 3.40; 3.34; 2.66; 2.18 m) and a small amount of α-cordierite 2MgO∙2Al2O3∙5SiO2 (d × 1010 = 8.21; 3.34; 2.94; 1.67 m). After firing at the temperature of 1375 °C, the formation of cordierite phase of C-0 composition is completed, which is evidenced by the data of X-ray phase analysis, as well as results of dilatometric measurements (LCTE20–400 = 31.2 × 10–7 deg−1)—Fig. 5a. Microstructure of C-0 composition (Fig. 7) is formed by the isotropic mass where it is possible to distinguish the outlines of α-cordierite crystals mainly of 1–2 μm in size, bound by the glassy phase. The content of the glass phase and its activity are not sufficient to fill the volumetric space of the system. As a result, microstructure of the material shows a large number of pores, the longitudinal size of which reaches 7–8 μm.
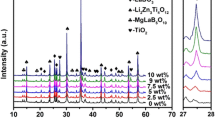
Mineralogical composition of ceramic materials synthesized with the addition of MABS glass, according to data of X-ray phase analysis (Fig. 8), is represented by the cordierite phase. Furthermore, α-cordierite is fully formed at the temperature of 1250 °C, as evidenced by high intensity of the main diffraction maxima corresponding to it (d × 10–10 = 8.16; 4.02; 3.34; 3.10; 2.99; 1.68 m), as well as the absence of other crystalline phases. With the increase in firing temperature and content of MABS glass in the experimental compositions, intensity of the main diffraction maxima of α-cordierite grows slightly. Strengthen of the diffraction pattern for experimental compositions with increasing temperature of synthesis is due to formation of more crystals of the cordierite phase and their clearer structure. Data of X-ray phase analysis show strong correlation with the results of electron microscopic studies (Fig. 9).
Microstructure of ceramics sintered at the temperature of 1375 °C is represented by larger amount of clearly formed small crystals of the α-cordierite phase compared to ceramics sintered at 1250 °C. These crystals are mainly of round shape; they are tightly interconnected by glassy phase and form a dense homogeneous structure (Fig. 9c, d). Increase in the content of MABS glass in the experimental compositions (up to 30 wt%) causes the formation of predominantly small crystals of α-cordierite of max 2 μm in size at the temperature of 1375 °C. This microstructure provides the highest mechanical strength of the experimental ceramics.
4 Discussion
Experimental studies confirmed the fact that high-temperature solid-phase synthesis of cordierite ceramics of stoichiometric composition of the mixture of natural and technical raw materials does not provide the dense sintering of the material. Despite the complete binding of the initial components of the raw material mixture in the cordierite phase after firing at the temperature of 1375 °C, the ceramic material is characterized by high open porosity of 25% (Fig. 4b).
In the work, to obtain densely sintered cordierite ceramics and achieve a set of high physical and technical indicators, a part of components of the MAS system was introduced using pre-designed MABS glass.
Experiments prove that physical and technical indicators of the experimental ceramics, its quantitative mineralogical composition and microstructure are determined by the content of MABS glass and firing temperature. Increase in the concentration of the parent glass from 10 to 20–30 wt% and firing temperature from 1250 to 1375 °C enhances the ceramics sintering processes considerably. The developed ceramics has the single-crystal phase composition and is represented by α-cordierite, and this crystalline phase forms the structural matrix of experimental ceramics. Cordierite is formed due to intense interaction of the main components of the experimental MABS glass with crystalline fillers (Al2O3·2SiO2 i MgO) as a result of chemical reactions (1) and (2) in the course of sintering of ceramics. These findings are clearly confirmed by the results of X-ray phase analysis shown in Figs. 6 and 8. Besides, α-cordierite is a product of finely dispersed crystallization of MABS glass (see Fig. 3).
It is important to note that thermo-physical properties (LCTE) of ceramic materials are mainly affected by their firing temperature. With the increase in firing temperature to 1350 °C, we observe falling of LCTE values from (21.5–23.2) × 10–7 deg−1 to (12.3–18.0) × 10–7 deg−1 (Fig. 5b). This change in LCTE is caused by higher amount of crystals of the cordierite phase and their clearer structure. Further sintering at the temperature of 1375 °C causes a slight growth of LCTE in the range of 20–400 °C to (14.0–18.5) × 10–7 deg−1.
It is explained by the fact that phase composition of the cordierite ceramics C-1, C-2 i C-3, along with the crystalline phase of α-cordierite, is represented by boron-containing glass phase. Its amount is higher, and viscosity at the ceramics’ firing temperatures is obviously lower, compared to the amount and viscosity of the glass phase of magnesium aluminosilicate composition, which is formed in the C-0 composition. Determination of the viscosity of the glass phase of the experimental cordierite ceramics does not seem possible because of change in its composition with the firing temperature increase. At the same time, it is known [36] that boron oxide improves the wetting ability of the silicate glass melt in relation to many crystalline phases, including cordierite. Therefore, it can be assumed that with an increase in the temperature of firing of the cordierite ceramics to 1375 °C, the activity of the glass melt with regard to the crystalline phase of α-cordierite increases. It results in the increase in the degree of dissolution of cordierite crystals in the boron-containing glass melt. As a consequence, a minor increase in the LCTE of the cordierite ceramics is recorded.
The noticeable change in the LCTE of the cordierite ceramics with the increase in the content of experimental glass from 10 to 20–30 wt% is observed in the temperature range of 1350–1375 °C. It is explained by the increase in the total amount of B2O3 in the material composition. Boron oxide is known [36] to cause an increase in the LCTE of silicate glasses. The result is the growth of the LCTE of the cordierite ceramics itself from (12.3–14.0) × 10–7 deg−1 to (16.0–18.5) × 10–7 deg−1 (Fig. 5b).
The increasing the content and activity of the glass melt has a positive effect on the physical and mechanical properties of the experimental ceramics.
When the glass is introduced in the amount of 10 wt% even at the maximum firing temperature of 1375 °C, a porous structure of the material (P = 14.5%) is formed, as a result of insufficient glass phase content in the system.
Increase in the concentration of the parent glass to 20–30 wt% contributes to the formation of a homogeneous dense microstructure. It is formed by strong binding of small crystals of α-cordierite (1–2 μm) with the use of thin layers of glassy phase (Fig. 9b, d). The smaller the size of the crystals, the more often in the path of sliding dislocations there are obstacles at the grain boundary and, accordingly, higher stresses are required for the material deformation at the initial stages. In addition, dense arrangement of crystals of the main phase of α-cordierite in the structure of the material brings the density of ceramics closer to the theoretical one. As a consequence, ceramics is characterized by zero values of water absorption and open porosity (Fig. 4a, b) and maximum values of mechanical compressive strength, at 277–307 MPa (Fig. 5a).
The uniform distribution of finely dispersed cordierite phase and high density of the structural matrix of C-2 and C-3 compositions fired at 1375 °C allows achieving consistently high dielectric values (ε = 4.9; tan δ = 0.0014) in the ultrahigh frequency electromagnetic field of 1010 Hz. Thermal stability of ceramics is also high (900 °C). Therefore, the developed cordierite materials can be successfully used as radio-transparent materials, including structural ones.
5 Conclusions
Consequently, the technique of introducing a part of components into the composition of cordierite ceramics with the use of relatively low-melting MABS glass, which is used in this work, allows to significantly intensifying the process of formation of α-cordierite phase and sintering of the obtained materials. Therefore, it is possible to cut the energy costs relating to the manufacturing process of densely sintered cordierite materials by significantly reducing the melting temperature of the parent glass from 1550–1600 to 1350 °C. In addition, this approach opens the possibility of effective control of the microstructure and phase composition of cordierite ceramics. As a result, at temperature of 1375 °C, the synthesized ceramics has a set of high physical and technical indicators (zero water absorption, high mechanical compressive strength up to 307 MPa). Cordierite ceramics is characterized by low LCTE, (17.7–18.5) × 10–7 deg–7, which conditions its high thermal stability (not lower than 900 °C). The dense microstructure and specified phase composition of the developed ceramics give an opportunity to achieve high dielectric indicators (ε = 4.9; tan δ = 0.0014) and to use it as high-temperature radio-transparent material, including structural one.
The developed compositions of cordierite ceramics allow receiving the articles of various complexity of shapes with use of all basic molding methods traditionally employed in the ceramic technology. Wet method of ceramic mass making provides high degree of its homogenization and, as a consequence, good reproducibility of the properties of fired ceramics. These materials can be used for the manufacturing of antenna fairings for aircrafts and protective windows for shelters of various types of antenna systems. At the same time, to obtain cordierite ceramics with a set of high physical and technical indicators, first of all, it is necessary to strictly adhere to the temperature and time regime of firing and the optimal content of MABS glass.
This approach, which proves itself well in the synthesis of densely sintered cordierite ceramics, can be further implemented in the technology of other types of heat-resistant aluminosilicate ceramics.
References
P.D. Sarkisov, D.V. Grashchenkov, L.A. Orlova et al., Modern achievements in the field of creating high-temperature radiotransparent materials (in Russian). Tech. Technol. Silic. 1, 2–10 (2009)
Y.E. Pivinskii, The half of a century period of the domestic ceramics technology development (in Russian) Part 1. Novye Ogneup. (New Refract.) 3, 105–112 (2017). https://doi.org/10.17073/1683-4518-2017-3-105-112
M.T. Sebastian, R. Ubic, H. Jantunen, Low-loss dielectric ceramic materials and their properties. Int. Mater. Rev. 60, 392–412 (2015). https://doi.org/10.1179/1743280415Y.0000000007
E.D. Zanotto, A bright future for glass-ceramics. Am. Ceram. Soc. Bull. 89, 19–27 (2010)
D. Hotza, A.P.N. De Oliveira, New silicate glass-ceramic materials and composites. Adv. Sci. Technol. 68, 1–12 (2010). https://doi.org/10.4028/www.scientific.net/AST.68.1
A.V. Zaychuk, A.A. Amelina, Search for the ways to improve the physical and technical parameters of quartz ceramics. Voprosy Khimii i Khimicheskoi Tekhnologii 6, 63–67 (2017)
E.S. Khomenko, A.V. Zaichuk, E.V. Karasik, A.A. Kunitsa, Quartz ceramics modified by nanodispersed silica additive. Funct. Mater. 25, 613–618 (2018). https://doi.org/10.15407/fm25.03.613
E.S. Khomenko, A.V. Zaichuk, E.V. Karasik, V.D. Ivchenko, N.M. Sribniak, B.M. Datsenko, Improvement of strength characteristics of quartz ceramics. Funct. Mater. 27, 264–269 (2020). https://doi.org/10.15407/fm27.02.264
V.O. Soares, G.R. Paula, O. Peitl et al., Effect of ion exchange on the sinter-crystallisation of low expansion Li2O–Al2O3–SiO2 glass-ceramics. Glass Technol. Eur. J. Glass Sci. Technol. Part A 52, 50–54 (2011)
A.V. Zaichuk, A.A. Amelina, Y.S. Khomenko, A.S. Baskevich, Y.R. Kalishenko, Heat-resistant ceramics of β-eucryptite composition: peculiarities of production, microstructure and properties. Voprosy Khimii i Khimicheskoi Tekhnologii 2, 52–59 (2020). https://doi.org/10.32434/0321-4095-2020-129-2-52-59
Y.M. Sung, W.C. Kwak, Influence of various heating procedures on the sintered density of Sr-celsian glass-ceramic. J. Mater. Sci. Lett. 21, 841–843 (2002). https://doi.org/10.1023/A:1015710309425
O. Zaichuk, A. Amelina, Yu. Hordieiev, Y. Kalishenko, N. Sribniak, S. Halushka, D. Borodai, A. Borodai, Patterns in the synthesis processes, the microstructure and properties of strontium-anorthite ceramics modified by glass of spodumene composition. East. Eur. J. Enterp. Technol. 6, 15–26 (2020). https://doi.org/10.15587/1729-4061.2020.216754
C.M. López-Badillo, J. López-Cuevas, C.A. Gutiérrez-Chavarría, J.L. Rodríguez-Galicia, M.I. Pech-Canul, Synthesis and characterization of BaAl2Si2O8 using mechanically activated precursor mixtures containing coal fly ash. J. Eur. Ceram. Soc. 33, 3287–3300 (2013). https://doi.org/10.1016/j.jeurceramsoc.2013.05.014
A.V. Zaichuk, A.A. Amelina, Yu.S. Hordieiev, Y.R. Kalishenko, N.N. Sribniak, Synthesis and characteristic of celsian ceramics with the use of glass in the system Li2O–Al2O3–B2O3–SiO2. Funct. Mater. 27, 827–835 (2020). https://doi.org/10.15407/fm27.04.827
A.S. Chainikova, M.V. Voropaeva, L.A. Alekseeva et al., Current state of development in the field of radio-transparent cordierite sitalls (review) (in Russian). Aviat. Mater. Technol. S6, 45–51 (2014)
Z. Shamsudin, A. Hodzic, C. Soutis et al., Characterisation of thermo-mechanical properties of MgO–Al2O3–SiO2 glass ceramic with different heat treatment temperatures. J. Mater. Sci. 46, 5822–5829 (2011). https://doi.org/10.1007/s10853-011-5538-0
H. Ohsato, J. Varghese, T. Vahera et al., Micro/millimeter-wave dielectric indialite/cordierite glass-ceramics applied as LTCC and direct casting substrates: current status and prospects. J. Korean Ceram. Soc. 56, 526–533 (2019). https://doi.org/10.4191/kcers.2019.56.6.01
L. Stoch, J. Lelatko, Mechanisms of crystal structure organization in magnesium aluminosilicate glass: HREM and analytical study. Glass Technol. Eur. J. Glass Sci. Technol. Part A 48, 183–188 (2008)
M. Guignard, L. Cormier, V. Montouillout, Environment of titanium and aluminum in a magnesium alumino-silicate glass. J. Phys. Condens. Matter. 21, 1–10 (2009). https://doi.org/10.1088/0953-8984/21/37/375107
A.A. Poteshkina, J.A. Uvarenkova, V.I. Ivanova, B.M. Ivanov, Ceramic dielectric with low permeability for high frequency technology (in Russian). St. Petersbg. State Univ. Bull. S.4 2, 285–293 (2015)
A. Aşkin, I. Tatar, Ş Kilinç, Ö. Tezel, The utilization of waste magnesite in the production of the cordierite ceramic. Energy Procedia 107, 137–143 (2017). https://doi.org/10.1016/j.egypro.2016.12.151
D.M. Ivanov, N.A. Lukyanova, V.I. Ivanova, V.V. Petukhova, Synthesis of cordierite for high-frequency application (in Russian). St. Petersbg. State Univ. Bull. S.4 4, 77–82 (2009)
J. Banjuraizah, H. Mohamad, Z.A. Ahmad, Effect of impurities content from minerals on phase transformation, densification and crystallization of α-cordierite glass-ceramic. J. Alloys Compd. 509, 7645–7651 (2011). https://doi.org/10.1016/j.egypro.2016.12.151
A.V. Zaichuk, A.A. Amelina, Y.V. Karasik, Y.S. Khomenko, V.A. Lementareva, D.Y. Saltykov, Radio-transparent ceramic materials of spodumene-cordierite composition. Funct. Mater. 26, 174–181 (2019). https://doi.org/10.15407/fm26.01.174
J. Wu, C. Hu, X. Xu et al., Preparation and thermal shock resistance of cordierite-spodumene composite ceramics for solar heat transmission pipeline. Ceram. Int. 42, 13547–13554 (2016). https://doi.org/10.1016/j.ceramint.2016.05.147
A.E. Reda, F. Abd-El-Raoof, S.E. Ahmed, Sintering and dielectric behavior for doped cordierite by xCuO within MgO(1–x)–Al2O3–SiO2 ceramics. Mater. Chem. Phys. 243, 122616 (2020). https://doi.org/10.1016/j.matchemphys.2019.122616
F.J. Torres, U.R. Rodríguez-Mendoza, V. Lavín, E. Ruiz de Sola, J. Alarcón, Evolution of the structural and optical properties from cobalt cordierite glass to glass-ceramic based on spinel crystalline phase materials. J. NonCryst. Solids 353, 4093–4101 (2007). https://doi.org/10.1016/j.jnoncrysol.2007.06.014
M.A. Zalapa-Garibaya, D. Torres-Torres, A.M. Arizmendi-Morquecho, S.Y. Reyes-López, Effect of NiO and MoO3 addition on the crystallinity and mechanical properties of α-cordierite and β-cordierite in the MgO–Al2O3–SiO2 system. Results Phys. 13, 4093–4101 (2019). https://doi.org/10.1016/j.rinp.2019.102227
M. Senthil Kumar, A. Elaya Perumal, T.R. Vijayaram, Synthesis, characterization and sintering behavior influencing mechanical, thermal and physical properties of pure cordierite and cordierite-ceria. J. Adv. Ceram. 4, 22–30 (2015). https://doi.org/10.1016/j.jmrt.2013.03.016
N. Obradović, N. Dordević, S. Filipović et al., Influence of mechanochemical activation on the sintering of cordierite ceramics in the presence of Bi2O3 as a functional additive. Powder Technol. 218, 157–161 (2012). https://doi.org/10.1016/j.powtec.2011.12.012
S.-L. Fu, L.-S. Chen, J.-H. Chou, Sintering of cordierite glass-ceramic with lead borosilicate glass. Ceram. Int. 20, 67–72 (1994). https://doi.org/10.1016/0272-8842(94)90010-8
H. Gui, C. Li, C. Lin et al., Glass forming, crystallization, and physical properties of MgO–Al2O3–SiO2–B2O3 glass-ceramics modified by ZnO replacing MgO. J. Eur. Ceram. Soc. 39, 1397–1410 (2019). https://doi.org/10.1016/j.jeurceramsoc.2018.10.002
N.A. Minakova, A.V. Zaichuk, Y.I. Belyi, The structure of borate glass. Glass Ceram. 65, 70–73 (2008). https://doi.org/10.1007/s10717-008-9017-2
M.V. Andreev, O.O. Drobakhin, Y.N. Privalov, D.Y. Saltykov, Measurement of dielectric material properties using coupled biconical resonators. Telecommun. Radio Eng. 73, 1017–1032 (2014). https://doi.org/10.1615/TelecomRadEng.v73.i11.70/
T. Gasparik, System MgO–Al2O3–SiO2, in Phase Diagrams for Geoscientists (Springer, New York, 2014). https://doi.org/10.1007/978-1-4614-5776-3_3
A.A. Appen, Himiya stekla [Chemistry of glass] (Chemistry, Leningrad, 1974) (in Russian)
Funding
This study was funded by Ministry of Education and Science of Ukraine (Grant no. 0120U101969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaichuk, A., Amelina, A., Kalishenko, Y. et al. Aspects of development and properties of densely sintered of ultra-high-frequency radio-transparent ceramics of cordierite composition . J. Korean Ceram. Soc. 58, 483–494 (2021). https://doi.org/10.1007/s43207-021-00125-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-021-00125-5