Abstract
Garnet-type Li7 − 3xAlxLa3Zr2O12 (x = 0.2) made of different ratios of powders with mixed pre-treatment conditions is prepared by using a microwave oven. Cubic crystallographic structure is successfully achieved after sintered at 1150 °C for only 30 min, which is much lower than the conventional sintering. The conductivity of the samples, which peaked at 60% of powders treated at higher temperature, is comparable to the ones made at much higher temperature with longer dwelling time in literatures. The comparable conductivity was attributed to the denser microstructure and lower activation energy owing to the effect of powers with mixed pre-treatment conditions. The trend of the conductivity versus the ratio of powders with mixed pre-treatment conditions fit well with the percolation model of dispersed ionic conductors. Therefore, powders with mixed pre-treatment conditions could be used as precursors to prepare lithium ion conductors at lower temperatures with less dwelling time, which is beneficial to retaining lithium stoichiometry and any other problems associated with high-temperature procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lithium ion battery is becoming the main storage device for commercial applications. However, its safety issues still haven’t be resolved due to its organic liquid electrolytes which are volatile and flammable. Inorganic solid lithium electrolytes exhibit several advantages [1] over current liquid organic electrolytes, such as excellent chemical and electrochemical stability, good safety, wide operating temperature range and high resistance to shocks and vibrations. Therefore inorganic solid state lithium ion conductors, including perovskite-type La2/3−xLi3xTiO3 (LLTO) [2], NASICON-type Li1 + xGe2−xAlx(PO4)3 (LAGP) [3] and Li1 + xTi2−xAlx(PO4)3 (LATP) [4], and garnet-type Li7La3Zr2O12 (LLZO), have attracted ever-increasing attention world widely due to its great advantages. Polycrystalline materials consist of multiple grains, hence lithium ion conduction in polycrystalline materials are determined by both grains and grain-boundaries. The migration of lithium ions across the grain boundaries is normally twice slower than in the grains of polycrystalline LLTO, LATP and LAGP, resulting in an intrinsically low overall conductivity. The other drawback of LLTO and LATP is the reduction of Ti4+ into Ti3+ in contact with lithium metal. Of these alternatives, the grain and grain-boundary conductivity in garnet-type Li7La3Zr2O12 are similar and no Ti was involved in this material, which suggests that LLZO could be a suitable candidate for solid electrolytes.
Garnet-type Li7La3Zr2O12 compounds were initially reported by Weppner et al. [5, 6]. Two polymorphs of LLZO have been reported: cubic phase, which is only stable at high temperature, and tetragonal phase, which is stable at low temperature. The ionic conductivity of the cubic phase is almost two orders of magnitude higher than that of the tetragonal phase at room temperature. Supervalent cations (e.g., Al3+, Ga3+, Fe3+, Ga3+, Nb4+, Ta5+, Te6+) substitution at Li, La or Zr position allows formation of cubic LLZO at lower temperature and improves the ionic conductivity [7,8,9,10]. The total conductivity of Al stabilized Li7La3Zr2O12 is in the order of magnitude of 10− 4 S/cm. To obtain stable cubic LLZO, it normally requires high-temperature sintering (e.g., 1230 °C) and long sintering time (e.g., 24 h), which could render problems like the loss of lithium and destabilization of cubic phase. Thus, developing a strategy to prepare LLZO with higher ionic conductivity at even lower temperature would be very useful.
Microwave sintering can uniformly heat the samples throughout the whole volume and may lower the sintering temperature. Therefore, microwave sintering was used to prepare LLZO electrolyte in this study. Starting powders with different pre-treatment conditions were thought to facilitate the lithium migration across the grain boundaries. Hence starting materials were mixed and then thermally treated at two different temperatures. The effect of the ratio of powders with different pre-treatment conditions on the conductivity, crystallographic structure and activation energy was investigated.
2 Experimental
In this study, sample Li7 − 3xAlxLa3Zr2O12 (x = 0.2) was prepared via solid state reaction. The starting materials used were Li2CO3 (> 99.9%), Al2O3 (> 99.9%), La2O3 (> 99.9%) and ZrO2 (> 99.9%), which were all purchased from Tianjin Kemio Chemical Co. Ltd. Firstly, stoichiometric raw powders were weighed, mixed and ground thoroughly in a mortar with a pestle. Then the mixed powders were subjected to two different annealing approaches: the first one was to treat the mixture powders at slightly higher temperature of 1150 °C for 30 min in a microwave oven (INNOV-CS-03M1600, Hunan Innvo-Tech Co. Ltd.), the resultant powder was designated as HTP; the second one was to anneal the mixture powders at slightly lower temperature of 850 °C for 30 min, the resultant powder was designated as LTP. HTP and LTP were mixed in different ratios and then used to make LLZO pellets. The ratios (by weight) of HTP to the mixture powder of HTP and LTP were 0, 20, 40, 60, 80 and 100%, respectively. A high speed blender (Royalstar RZ-218A) was used to mix HTP and LTP to make sure that the mixing was homogenous. Afterwards, the mixtures of HTP and LTP were pressed into a small coin in a 15 mm diameter and subsequently sintered at 1150 °C for 30 min in the microwave oven. The sintered pellets made of mixture powders of 0, 20, 40, 60, 80 and 100% HTP were designated as S00, S20, S40, S60, S80 and S100, respectively.
All the sintered pellets were polished and painted with silver paste on both sides for performing the electrochemical impedance spectra (EIS) analysis. EIS measurements were carried out using a Princeton Versastat3 station by applying a stimulus amplitude of 10 mV over the frequency range of 0.1–1 × 106 Hz at room temperature and elevated temperatures. The conductivities σ were calculated as follows.
where d is the thickness of the pellet, R is the impedance and A is the electrode area of the pellet. The activation energy for lithium ionic conduction was derived from fitting experimental data by using the Arrhenius equation:
where σo is pre-exponential factor, Ea activation energy for ionic conduction, k is the Boltzmann’s constant. X-ray diffraction (XRD, TD-3500X with Cu Kα radiation source) was used for the phase identification of samples. The microstructures were observed by using a field emission scanning electron microscope (JSM-7800F). The density of the samples was determined by the mass and the geometric volume.
3 Results
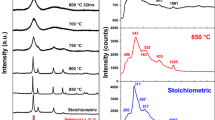
XRD patterns of samples S00, S60 and S100 are shown in Fig. 1. All the main peaks can be assigned to PDF card of 40-0894, which is cubic garnet structure. It indicated that microwave treatment was capable of making cubic garnet-type lithium ion conductors at lower temperatures. A small trace of LaAlO3, La2Zr2O7 and Li2ZrO3 was also found in the samples. Cell volumes of samples S00, S60 and S100 were 2195.57, 2190.57 and 2186.1 Å3, respectively. It can be seen that the cell volumes of samples decreased as the powders pretreated at higher temperature (HTP) increased.
The morphologies of lithium ion conductors made in this study were observed and SEM photos of samples S00, S60 and S100 are shown in Fig. 2. It can be seen that the morphologies of the samples with different amounts of powder HTP appeared to be quite different. Although all the three samples exhibited polycrystalline structure, the appearance of the grains seemed to change with the amount of powder HTP. The big grains in sample S00 were completely covered by a large amount of small micron-size particles, which indicated that the growth of the grains were not quite finished and it may evolve into bigger grains if given time. The average grain size was about 7–8 µm. When the amount of HTP increased to 60%, the small particles found on the surface of grains of sample S00 had disappeared and evolved into flakes covering the big grains. No apparent gaps were found at the grain boundaries and all the grains were well connected. This was attributed to the presence of 40% LTP, which was subjected to a thermal treatment at only 850 °C and therefore acted as (as evidenced in inset in Fig. 2b) bridges between the big grains originated from HTP, which were already thermally treated at 1150 °C. The average grain size was about 15 µm. While for sample S100, the surfaces of the grains were very smooth and no small particles or flakes were found as did for S60 and S100, which proved that the small particles and flakes on sample S00 and S60 originated from powder LTP. The average grain size was about 20 µm. The grain size increased as the amount of powder HTP increased. Since the densities of ionic conductors normally play an important role in the ionic conductivity, the densities of samples were also calculated. The density of samples S00, S60 and S100 was 3.58, 4.09 and 4.04 g/cm3, respectively. The sample S60 achieved the highest density among the samples being studied. This was in well agreement with the observation of morphologies. LTP acted as bridges between big grains in S60 to achieve a denser microstructure. Sample S60 with a denser microstructure was expected to exhibit a higher ionic conductivity.
Complex impedance plots of samples are shown in Fig. 3. It was found that all the complex impedance plots exhibited a semicircle at high frequency range followed by a straight line at low frequency range. The semicircle at higher frequency was ascribed to the grain-boundary relaxation and the straight line at lower frequency was due to the ionic blocking Ag electrode response. The grain response wasn’t able to show at the frequency range swept in this study. All these complex impedance plots showed that the samples made in this study were all ionic conductors.
The trend of impedance against the ratio of HTP wasn’t linear. The lowest impedance was obtained when the ratio of HTP was 60%. According to Eq. (1), the conductivities of samples S00, S60 and S100 were calculated and the values at room temperature were 4.17 × 10− 5, 1.15 × 10− 4 and 3.29 × 10− 5 S/cm, respectively. The sample S60 achieved a total conductivity of 1.15 × 10− 4 S/cm, which is comparable to the value of 2.6 × 10− 4 S/cm reported for Li6.4Al0.2La3Zr2O12 sintered at 1230 °C for 6 h [9].
4 Discussions
The samples made in this study could be thought to bear comparative analogy to dispersed ionic conductors. The grains originated from HTP corresponded to the normal conductor in a dispersed ionic conductor and the grains originated from LTP corresponded to the poor conductor or insulator in a dispersed ionic conductor. According to the percolation model for dispersed ionic conductors [11, 12], the total conductivity of a dispersed ionic conductor could be expressed as:
where σg, σn, p and l corresponds to the conductivity of grains originated from LTP, grains originated from HTP, the concentration of grains originated from LTP and the size of grains originated from HTP. In dispersed ionic conductors, the ionic conductivity strongly correlates with the ratio of the concentration p and the grain size l of the normal conductor. It will increase first and then drops down after passing its maximum. That the conductivity peaked at 60% HTP (i.e., 40% LTP) could be explained by the percolation model for dispersed ionic conductors. From 0% HTP to 60% HTP and then to 100% HTP, the concentration p was decreasing while the grain size l was continuously increasing from 8 to 20 µm. Therefore sample S60 exhibited the maximum conductivity.
Compared to the preparation conditions of 1230 °C for 6 h in Rettenwander’s study, sample S60 which presented a comparable total conductivity was only thermally treated at 1150 °C for 30 min in the microwave oven. The preparation procedure adopted in this study exhibited two advantages over the conventional sintering: one was the high efficiency; the other one was the easier control of lithium content due to the short dwelling time and low sintering temperature employed.
The plots of the temperature dependent ionic conductivities are shown in Fig. 4 and the activation energy for the total lithium conduction was derived from the fitting curves by using Eq. (2). For the sample S00 and S60, the activation energy over the entire temperature range studied was constant. While the activation energy of the sample S100 exhibited two distinct values: 0.30 eV at low temperature range and 0.28 eV at high temperature range. The transition occurred at 130 °C, which indicated that the lithium migration pathway has changed at 130 °C. The activation energy of the sample S00 was 0.29 eV, which was similar to the previously-reported values (0.3–0.37 eV) [13,14,15]. While the sample S60 exhibited much lower activation energy of 0.21 eV, which was thought to be partially responsible for the high ionic conductivity achieved for the sample S60. And the denser microstructure of sample S60 described in the previous section was thought to contribute to the highest ionic conductivity as well.
The transference number of solid electrolyte is used to represent the weight of ionic conduction and the deviation from 1 is thought to originate from the electronic conduction. The transference number of a pure lithium ion conductor should be close to 1. The Li transference number was calculated using Eq. (4) [16].
σDC, resulting from the contribution of the electron conduction, was measured by DC polarization method. σAC, resulting from the combination of grain boundary and intragrain ion hopping conductions, was determined by AC impedance measurement. The transference numbers of samples S00, S60 and S100 were measured and the value was 0.999, 1 and 0.999, respectively. It showed that the samples made in this study had a negligible electronic conduction and all the conductions were attributed to the migration of lithium ions.
5 Conclusions
Garnet-type Li7 − 3xAlxLa3Zr2O12 with different ratios of pre-treated powders was prepared by using a microwave oven. XRD patterns showed that cubic garnet materials were successfully obtained at a lower temperature. The sample S60 which consisted of 60% of powders pretreated at 1150 °C exhibited a total conductivity of 1.15 × 10− 4 S/cm, which was comparable to the literatures. The comparable conductivity of sample S60 obtained at lower preparation temperature was attributed to the denser microstructures and lower activation energy owing to the use of powders with mixed pre-treatment conditions. The samples made in this approach were treated at 1150 °C for only 30 min, which was much advantageous to conventional sintering at 1230 °C for 6 h. Therefore, powders with mixed pre-treatment conditions could be used to prepare lithium ion conductors at lower temperatures with less dwelling time.
References
P. Knauth, Inorganic solid Li ion conductors: an overview. Solid State Ion. 180, 911–916 (2009)
B.A. Lineva, S.D. Kobylyanskaya, L.L. Kovalenko, O.I. V’yunov, A.G. Belous, Effect of impurities on the electrical properties of the defect perovskite Li0.33La0.57TiO3. Inorg. Mater. 53, 326–332 (2017)
Y. Cui, M.M. Mahmoud, M. Rohde, C. Ziebert, H.J. Seifert, Thermal and ionic conductivity studies of lithium aluminum germanium phosphate solid-state electrolyte. Solid State Ion. 289, 125–132 (2016)
T. Hupfer, E.C. Bucharsky, K.G. Schell, A. Senyshyn, M. Monchak, M.J. Hoffmann, H. Ehrenberg, Evolution of microstructure and its relation to ionic conductivity in Li1 + xAlxTi2 – x(PO4)3. Solid State Ion. 288, 235–239 (2016)
M. Ramaswamy, T. Venkataraman, W. Weppner, Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007)
C.A. Geiger, E. Alekseev, B. Lazic, M. Fisch, T. Armbruster, R. Langner, M. Fechtelkord, N. Kim, T. Pettke, W. Weppner, Crystal chemistry and stability of “Li7La3Zr2O12” garnet: a fast lithium-ion conductor. Inorg. Chem. 50, 1089–1097 (2011)
X. Tong, V. Thangadurai, E.D. Wachsman, Highly conductive Li garnets by a multielement doping strategy. Inorg. Chem. 54, 3600–3607 (2015)
D. Rettenwander, C.A. Geiger, G. Amthauer, Synthesis and crystal chemistry of the fast Li-ion conductor Li7La3Zr2O12 doped with Fe. Inorg. Chem. 52, 8005–8009 (2013)
D. Rettenwander, G. Redhammer, F. Preishuber-Pflügl, L. Cheng, L. Miara, R. Wagner, A. Welzl, E. Suard, M.M. Doeff, M. Wilkening, J. Fleig, G. Amthauer, Structural and electrochemical consequences of Al and Ga cosubstitution in Li7La3Zr2O12 solid electrolytes. Chem. Mater. 28, 2384–2392 (2016)
W. Luo, Y. Gong, Y. Zhu, K.K. Fu, J. Dai, S.D. Lacey, C. Wang, B. Liu, X. Han, Y. Mo, E.D. Wachsman, L. Hu, Transition from super lithiophobicity to super lithiophilicity of garnet solid-state electrolyte. J. Am. Chem. Soc. 138, 12258–12262 (2016)
H.E. Roman, A continuum percolation model for dispersed ionic conductors. J. Phys. Condens. Matter 2, 3909–3917 (1990)
G. Albinet, J.M. Debierre, P. Knauth, C. Lambert, L. Raymond, Enhanced conductivity in ionic conductor–insulator composites: numerical models in two and three dimensions. Eur. Phys. J. B 22, 421–427 (2001)
C. Bernuy-Lopez, W. Manalastas, J.M. Lopez del Amo, A. Aguadero, F. Aguesse, J.A. Kilner, Atmosphere controlled processing of Ga-substituted garnets for high Li-ion conductivity ceramics. Chem. Mater. 26, 3610–3617 (2014)
H. El Shinawi, J. Janek, Stabilization of cubic lithium-stuffed garnets of the type “Li7La3Zr2O12” by addition of gallium. J. Power Sources 225, 13–19 (2013)
S. Afyon, F. Krumeich, J.L.M. Rupp, A shortcut to garnet-type fast Li-ion conductors for all-solid state batteries. J. Mater. Chem. A 3, 18636–18648 (2013)
X. Lu, D. Yang, Effect of sintering conditions on perovskite lithium-based ion conductor. Emerg. Mater. Res. 6, 1–5 (2017)
Acknowledgements
This research was financially supported by Natural Science Foundation of Hebei Province (No. E2018502014), the Fundamental Research Funds for the Central Universities (No. 2017MS138) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lu, X., Yang, D. Preparation of Garnet-Type Li7 − 3xAlxLa3Zr2O12 at Lower Temperature by Using Powders of Mixed Pre-treatment Conditions. J Inorg Organomet Polym 28, 2023–2027 (2018). https://doi.org/10.1007/s10904-018-0859-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0859-y