Abstract
With the advent of widespread genomic testing for diagnostic indications and disease risk assessment, there is increased need to optimize genetic counseling services to support the scalable delivery of precision medicine. Here, we describe how we operationalized the reciprocal engagement model of genetic counseling practice to develop a framework of counseling components and strategies for the delivery of genomic results. This framework was constructed based upon qualitative research with patients receiving genomic counseling following online receipt of potentially actionable complex disease and pharmacogenomics reports. Consultation with a transdisciplinary group of investigators, including practicing genetic counselors, was sought to ensure broad scope and applicability of these strategies for use with any large-scale genomic testing effort. We preserve the provision of pre-test education and informed consent as established in Mendelian/single-gene disease genetic counseling practice. Following receipt of genomic results, patients are afforded the opportunity to tailor the counseling agenda by selecting the specific test results they wish to discuss, specifying questions for discussion, and indicating their preference for counseling modality. The genetic counselor uses these patient preferences to set the genomic counseling session and to personalize result communication and risk reduction recommendations. Tailored visual aids and result summary reports divide areas of risk (genetic variant, family history, lifestyle) for each disease to facilitate discussion of multiple disease risks. Post-counseling, session summary reports are actively routed to both the patient and their physician team to encourage review and follow-up. Given the breadth of genomic information potentially resulting from genomic testing, this framework is put forth as a starting point to meet the need for scalable genetic counseling services in the delivery of precision medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Genetic counseling is a clinical service to help patients and their families understand and apply genetic information, and to assist in incorporating personalized strategies to help with adjustment to and management of disease(s) and associated risks (National Society of Genetic Counselors’ Definition Task et al. 2006). When provided by genetic counselors, genetic counseling also attends to the emotional ramifications of genetic information in a client-centered manner (Smerecnik et al. 2009). A significant portion of a genetic counseling session includes an informed pre-test consent discussion (if genetic testing is to be undertaken), which includes a discussion of potential testing options, possible results, and discussion of the implications and potential impact of results for the patient and family members. Post-test disclosure typically focuses on application of test results with in-depth discussion and education in the context of personal and family history, psychosocial intervention and support, and case management.
The Reciprocal Engagement Model (REM) of genetic counseling practice best captures the patient-centered genetic counseling process (National Society of Genetic Counselors’ Definition Task et al. 2006; Veach et al. 2007). The REM is built on the tenets of patient-centered education and counseling, understanding and appreciation of the patient’s unique situation, support and guidance to patients to build rapport and trust, and facilitative decision-making. The REM incorporates the patient’s values, prior knowledge, beliefs, and experiences, and allows for the development of a mutual relationship, which is at the core of the genetic counseling process.
To achieve the goals of the REM in the context of genomic service delivery, new “genomic counseling” strategies are needed to address secondary findings identified in broad-scale genomic testing, genomic screening (e.g., carrier testing, population screening), elective genomic testing in healthy individuals, and the combined effects of multiple genetic variants and environmental factors as effectors of disease risk (Hooker et al. 2014; Middleton et al. 2015; Ormond 2013; Wicklund and Trepanier 2014). A more comprehensive application of genomic results, which expands upon traditional genetic counseling approaches for individual indicated conditions, has been termed genomic counseling (Middleton et al. 2015; Mills and Haga 2014; O'Daniel 2010; Ormond 2013; Shelton and Whitcomb 2015). Genomic counseling addresses many different types of medical conditions, and may include a range of different types of risks. Common diseases such as coronary heart disease (CHD), stroke, Alzheimer’s disease, macular degeneration, and non-Mendelian subtypes of cancer that are typically addressed in genomic counseling differ from monogenic Mendelian disease in that there are presumed multiple low/moderate genetic variants, which alongside or in combination with multiple non-genetic influences (e.g., smoking, other behaviors, and environmental influences), confer increased risk (Khera et al. 2016; Skol et al. 2016). Counseling strategies that address the multiple potential conditions of interest that arise as a result of genome testing may help individuals better understand their risk and increase their likelihood of engaging in proactive health behaviors. Furthermore, additional emphasis on health education and disease prevention in genomic counseling may lead to a more motivational style of counseling (Mills and Haga 2014; Ormond 2013).
Further integration of genetic and genomic counseling services within the genomic results delivery process is essential (Collins and Varmus 2015; Kaufman et al. 2012; Lewis et al. 2016). It is also timely given the rise of large, population-wide efforts of genomic sequencing to include the National Institutes of Health All of USSM Research Program (Collins and Varmus 2015), the 100,000 Genomes Project in the UK, and other international initiatives (Manolio et al. 2015) for which the return of individual genomic results is planned. While the focus of clinical care and research has been on diagnostic testing or screening for genomic variants with proven value and clinical utility, there is the expectation that pre-symptomatic and elective genomic screening (e.g., personalized genetic health panels examining medically actionable genes such as the ACMG Secondary Findings v2.0 (Kalia et al. 2017); polygenic risk scores for common disease; pharmacogenomics) and public health screening programs will, at some point, extend to more comprehensive genome sequencing technologies, in the USA and abroad (Carey et al. 2016; Evans et al. 2013; Facio et al. 2013; Linderman et al. 2016; Manolio et al. 2015). The ultimate goal of these initiatives is to provide better predictions of risk for multiple diseases and medical indications, medication safety/efficacy, and other information (e.g., non-genetic risk influences) so that individuals can take a more personalized and preventive approach to health (Collins and Varmus 2015). Their success, and the overall future scaling of genomic technologies into primary care and specialty settings, will require development of novel, creative, and ultimately more effective counseling strategies. In the new era of genomic service delivery, patient-centered practice must continue to promote the tenets and goals of the REM (Redlinger-Grosse et al. 2017), while capitalizing on the strengths of a limited genetic counseling workforce (Force 2016).
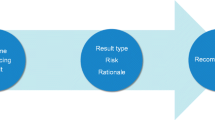
We propose a framework (Fig. 1) of counseling components and strategies that operationalizes the REM for the scalable delivery of genomic results. Our framework is based on theory, literature review, expert panel input, and qualitative research (Sweet et al. 2016), with a specific focus on application of these strategies to large-scale genomic sequencing. Our work seeks to apply existing REM and genetic counseling best practices within the emerging genomic counseling context.
Identification of Genomic Counseling Components and Strategies
Our approach to identifying and developing genomic counseling components and strategies, focused on the disclosure of genomic results and subsequent follow up, was developed using a multi-step iterative process. The process included (1) formative qualitative research with patients (those with chronic disease as well as healthy participants) who had undergone elective genomic testing and subsequently received multiple online potentially actionable results via the OSU-Coriell Personalized Medicine Collaborative to assess their responses to and preferences for counseling (Sweet et al. 2014); (2) extensive literature review of genetic counseling service delivery models and health behavior theory; and (3) reflective analysis (critical discussion) of the data in steps 1 and 2 with an expert panel of counselors with experience in both Mendelian/single-gene genetic counseling and genomic counseling. The formative research study (Sweet et al. 2016), which informed the development of this framework, was approved by Institutional Review Boards at the Ohio State University Wexner Medical Center and the Coriell Institute for Medical Research.
First, a grounded theory approach (Charmaz 2014) was used to build the framework based on themes and patterns that emerged from qualitative research (Sweet et al. 2016). The qualitative research was conducted with participants in the OSU-Coriell Personalized Medicine Collaborative (OSU-CPMC), who each received results for up to 19 complex disease (e.g., age related macular degeneration) and up to 7 drug response reports (e.g., CYP2C19 and Plavix) (Keller et al. 2010). Participants received pre-test education and informed consent, and completed online surveys that collected demographic, medical and family history, lifestyle, and medication information. This information, in concert with genetic results based on genotyping for variants associated with common complex disease and drug response, was incorporated into personalized online risk reports (Gharani et al. 2013; Stack et al. 2011). A study web portal also offered text and multimedia format educational materials to enable study participants to learn more about basic genetic/genomic concepts, pharmacogenomics, family history risk, relative risk and disease etiology, risk factors, and treatment and available preventative or risk-reducing actions for each health condition and drug response reported. OSU-CPMC study participants, who had received either in-person or telephone genomic counseling, were notified of the opportunity to participate in the qualitative interviews to inform development of this framework (Sweet et al. 2016). Although the qualitative research was conducted with individuals who had elected to undergo genomic screening, our sample included patients with chronic disease (hypertension, heart failure) receiving in-person genomic counseling as part of a randomized control trial (Sweet et al. 2016, 2017a, b) in an academic medical setting.
Second, a transdisciplinary group of investigators from across the country, including genetic counselors with expertise in cancer and cardiovascular genetics/genomics and medical genetics/genomics, as well as investigators with expertise in health communications and health behavior research, worked together to analyze and further refine data from the qualitative research and literature review and group these components into goals and strategies to develop our framework. This was done over the course of several months via conference calls and offline work, and multiple drafts of the genomic counseling framework were circulated, reviewed, and iteratively revised until reaching consensus (May–September 2015). The framework presented here, while based on qualitative research data, literature review, and expert opinion, is largely conceptual—in essence, it is an approach to be refined and built-upon as more empirical evidence is gathered on the effectiveness of these strategies in practice.
Genomic Counseling Framework
The genomic counseling framework incorporates key aspects of the REM and science-based best practices for genetic counseling (National Society of Genetic Counselors’ Definition Task et al. 2006; Veach et al. 2007) and was designed to accompany the receipt of multiple genomic results in varied practice settings (Fig. 1). The counseling approach remains patient-centered, and the counseling agenda is patient-driven and action-oriented, with the end-goal being the activation of patients toward healthy behavior changes to reduce and manage their risk appropriately. Our framework incorporates counseling through four components: (1) pre-test expectation setting and informed consent for genomic testing, (2) automated contracting and modality preference assessment, (3) tailoring of the session based on patient pre-session preferences, and (4) enhancing patient access and empowerment. In the following section, we outline the conceptual and empirical basis for each component (including links to the REM) and provide an overview of activities in each component based on our current practice. While designed for use in a genomic counseling setting where multiple test results are provided, we also provide suggestions for how components of this framework can be modified for other patient care settings.
Component 1: Pre-Test Expectation Setting and Consent
Strategy 1: Assessing and Setting Expectations During Informed Consent
As illustrated in Fig. 1, the counseling process begins prior to genomic testing focusing first on clear and open communication during the informed consent process. Effective genetic counselor-patient communication is a key focus of the REM, as the relationship is integral to genetic counseling (Veach et al. 2007). We propose, as have others (Albada et al. 2012a; Jay et al. 2000), that patients must understand what they can expect from any type of genetic/genomic test results and follow up genomic counseling, prior to beginning the process. Managing the expectations of patients prior to the return of results also has been shown to increase genetic counseling effectiveness and overall patient satisfaction with the counseling process (Albada et al. 2012b; Cacioppo et al. 2016; Jay et al. 2000). Managing expectations (particularly in terms of what genomic testing will not tell patients) is particularly important in genomic results counseling, given the broad scope of results that may be available, representing both rare and common complex disease. Patients may also have unrealistic expectations about the predictive value and benefit of genomic testing given the attention it has received in the media and scientific worlds (Caulfield and Condit 2012). Interpretation of these results also needs to take into account the limitations of the technology used and the available data in curated variant repositories (i.e., ClinVar). Furthermore, the information a patient will receive from genomic testing will depend on the indication (i.e., testing for a diagnostic indication versus pre-symptomatic elective testing in healthy patients); thus, defining and communicating expectations prior to counseling factors prominently into the genomic counseling framework.
The informed consent process should assess expectations regarding the genomic information patients believe they are going to receive, so that misconceptions can be addressed. Furthermore, as suggested by others, less emphasis should be placed on the standard elements of the consent process and technological aspects of the testing (Bernhardt et al. 2015; Wynn 2016) and more on the counseling process itself. This process of setting expectations aligns with the 2013 American College of Medical Genetics and Genomics policy statement on informed consent for genomic sequencing (Directors 2013). Indispensable information communicated to the patient during informed consent should include (1) what the patient will learn (and not learn) from receipt of genomic information, including secondary and incidental findings; (2) how much of their genetic data is included and what is being analyzed; (3) how results might change over time; and (4) the potential range and impact of results on their current and future healthcare (Bernhardt et al. 2015). In our experience, providing familiar examples of genomic results for common disease (e.g., coronary heart disease), while acknowledging that results may not directly correlate with their own personal medical or family history, is essential and helps to stimulate patient questions.
The education component of the pre-test consent process does not necessarily need to be done in-person or by phone, nor by a clinical genetics expert. Past research suggests that educational videos or interactive technologies may be a useful means of communicating and assessing expectations prior to informed consent, and reducing the amount of clinician time necessary to provide this pre-test information while simultaneously shaping patient expectations (Shelton and Whitcomb 2015). Although a direct assessment of interactive tools in pre-test decision-making has not been carried out for genome-wide testing, a review of 15 different studies utilizing interactive e-tools for pre-test decision-making (for more targeted testing use scenarios) found that overall patient satisfaction with consent was equivalent or better than in-person counseling and the knowledge aids appeared to minimize decisional conflict (Birch 2015). In the studies that were used to develop the genomic counseling framework, participants were enrolled in 1-h group sessions (e.g., 5–20 participants per group) by a trained study recruiter who administered a PowerPoint educational presentation (either by face-to-face or by use of a video) that covered background information on DNA, genes, and single nucleotide polymorphisms; the genetic basis of common, complex disease; logistics including access to the online web portal; composition of the online test reports; and the availability of genomic counseling (Sweet et al. 2014). In similar fashion, group pre-consent education and informed consent is already performed in some hereditary disease clinics (Albada et al. 2012a, b; Benusiglio et al. 2017), and this approach could be expanded upon in many areas of genetic counseling practice.
Strategy 2: Obtain Family History and Other Risk Factor Information
Following informed consent and expectation setting, obtaining and assessing medical and family history is an essential next step for comprehensive assessment of disease risk incorporating genomic information. We propose that much of this process could be accomplished outside of the genetic counseling session with the use of online family history tools that assist with routine and systematic collection of family history and standardize the assessment and referral process. A few online family history assessment tools have been developed for this purpose, but much more work needs to be done to explore the effects of online family history collection on genetic counseling outcomes (Orlando et al. 2016; Rubinstein et al. 2011). Prompting patients to use online family history platforms, especially as more robust programs to integrate and assess medical, family history, and lifestyle information become available, may also help busy clinicians (Guttmacher et al. 2004). As progress is made in standardizing electronic health record (EHR) systems, updating of patient medical and family history data via patient EHR portals will continue to become more available, efficient, and reliable. Further development of external patient portals and family history intake by testing laboratories may also facilitate collection of updated medical and family history data from patients. Alternatively, genetic counselor assistants could be utilized to solicit pre-session family history and other lifestyle or medical risk factor information by phone. This approach is already being used in some genetic counseling practice settings (Pirzadeh-Miller et al. 2016).
Although there are limits to the reliability of patient-reported family history information, asking for this information pre-session informs the interpretation of genomic sequence variation and gives the counselor a sense of the patient’s experience allowing for anticipatory guidance. In turn, this could afford more time in the counseling session for the counselor and patient to confirm family history information, reach a shared understanding of the family dynamics and their effects on the patient situation, and to discuss the “social history” aspects of this information (Redlinger-Grosse et al. 2017). In the OSU-CPMC study that inspired the development of this framework, participants were administered online surveys through a secure web portal that collected medical and family history, as well as demographic, lifestyle, and medication information in order to produce personalized genomic risk reports (Keller et al. 2010). The medical and family history information was explored in further detail in the in-person genomic counseling sessions, to provide familial and cultural context into the counseling relationship and for shared decision-making (Sweet et al. 2017a, b), reflecting the tenets of the REM.
Component 2: Assess Patient Preferences Using Prompts
Strategy 1: Assess Patient Preferences for Result Return and/or Discussion
Studies have shown that each individual is likely to have hundreds of risk variants associated with both Mendelian and complex phenotypes (McLaughlin et al. 2014; Tabor et al. 2014), and that most patients and health care professionals prefer broad disclosure and ready access to these results (Middleton et al. 2015; Yu et al. 2014). Woods et al. (2013), in work assessing patient reactions to viewing laboratory test results online, demonstrated high levels of patient satisfaction, predominantly positive experiences, and more patient empowerment. The ability to generate and provide genetic/genomic testing results and summaries online also shows great promise (Buchanan et al. 2015; Schwartz et al. 2014; Tabor et al. 2017). Some individuals may prefer more easily accessible, on-demand (e.g., online or mobile-based), and customizable formats for genomic-based receipt of results and may have a broader notion of what information may be meaningful or actionable than genetic professionals (Gray et al. 2014; Simmons et al. 2014). This work is in line with the recently implemented 2014 US Health and Human Services mandate that allows patients direct access to their lab results (Centers for Medicare & Medicaid Services (CMS) 2014) and supports the tenets of the REM.
Despite the growing availability of online report delivery, not all patients will have reliable Internet access and some patients may have privacy concerns and will prefer to receive hard copy results. Nevertheless, genetic counselors can still quickly assess patient preferences prior to testing to include whether they want a phone call or another visit for results disclosure, and when they want this to take place. Regardless of delivery format, allowing patients to access their results prior to genomic counseling gives them the opportunity to evaluate their results, formulate questions, and identify areas of interest or concern for discussion with their genetic counselor to help facilitate more effective genetic counselor-patient communication and to support patient autonomy. For the OSU-CPMC study, participants could choose to view or not view each result, process results in their own time, and access self-directed education and learning tools (Sweet et al. 2014).
Strategy 2: Assess Patient Preferences for Counseling Modality
The genomic counseling framework assesses patient preferences for communication modality [telephone, telegenetic (videoconferencing), or in-person] prior to counseling. Based on our prior work (Sweet et al. 2016), and to help increase efficiency and potential effectiveness of genomic counseling, we developed an online survey (Supplementary Fig. 1) for this purpose. This survey includes an assessment of patient choice of communication mode and allows the counselee to assist with refining the counseling agenda. This approach serves to establish a working contract, helps promote autonomy, and clarifies patient concerns in line with the tenets of the REM. Patients are more satisfied with counseling when given the choice of how they want to discuss the results (Baumanis et al. 2009; Sweet et al. 2016). Alternative service delivery models to the in-person approach may include counseling by phone or video (which can be supplemented with materials routed or accessed by the participant via the Internet or mobile devices) (Cohen et al. 2013; Meropol et al. 2011). Although much of the alternative service delivery work has been done in the cancer setting (Buchanan et al. 2016), this approach is expanding to other diseases (e.g., Alzheimer’s disease) (Christensen et al. 2017). These modes of communication are well-accepted by patients, decrease the amount of provider time (Cohen et al. 2016; Trepanier and Allain 2014), and are effective in educating and supporting patients, facilitating decision-making, and improving quality of life outcomes as compared to in-person genetic counseling (Schwartz et al. 2014). Utilization of technology beyond the standard “in-person” mode of counseling may help facilitate patient access to services that are limited due to geographical or financial barriers, or when in-person counseling is not feasible (Trepanier and Allain 2014). According to a November 2016 Pew Research Center survey, 88% of the general public routinely access the Internet, 77% own a SmartPhone, and 70% utilize social media, suggesting alternative modes of communication are becoming ever more readily accessible (Smith 2016). Trade-offs may include the inability to fully assess non-verbal behaviors/cues, the potential for dropped or interrupted communication, and the need for additional support for targeted patient populations (e.g., minority women) (Peshkin et al. 2016).
Component 3: Tailoring the Genomic Counseling Session
Strategy 1: Establishing a Counseling Agenda
Our previous work suggests that patients desire flexibility and a personally tailored approach to their counseling (Sweet et al. 2016, 2017a, b). Therefore, a key piece of the genomic counseling framework is assessment of participant areas of concern, and points for discussion to help tailor the counseling session. This information is obtained through the use of question prompts (Supplementary Fig. 1) that can be submitted online, emailed/texted, or mailed/captured by phone. Strategies such as tailoring are proposed to influence key determinants of health behavior change and are supported by theories such as the Elaboration Likelihood Model (ELM) (Petty and Cacioppo 1986). The ELM suggests that tailored messages may be more effective because they are more relevant to the individual and stimulate greater cognitive activity or because elaboration on the message increases effectiveness and the likelihood of behavior change (Kreuter and Wray 2003). Leveraging participant preferences and specific questions about results also allows for more focused contracting. In line with the REM (Veach et al. 2007), this process also ensures that counselors know the patient’s concerns. By directly addressing the most significant concerns for a particular test result or disease risk upfront, the educational focus of the counseling session can be weighed toward provision of evidence-based risk information (e.g., genetic and environmental/lifestyle risk influences) associated with the development of the indicated disease in a manner that is more personal and engaging to patients.
Strategy 2: Assess Risk Perceptions
Various health behavior models acknowledge that patient comprehension and subsequent decision-making and action on test results are affected by perceptions of disease risk (e.g., Health Belief Model (Rosenstock 1990), Risk Perception Attitude Framework (Rimal and Real 2003), and Integrative Model of Health Behavioral Prediction (Fishbein 2008)). Risk perception can be influenced by a number of factors, including personal experiences with disease, expectations of risk, as well as one’s emotional state (O'Neill et al. 2010). Our framework focuses on educating through exploring the patient’s perceptions and concepts of risk to help understand the extent to which these align with more objective risk estimates. Disease risk over- or underestimation can be problematic if it is negatively associated with recommended health behaviors and positive health outcomes (e.g., false reassurance from genomic results that inhibits engagement in preventive health behaviors). When significant mismatches between patient perceptions and objective risk estimates occur, counselors should attempt to further explore patient beliefs as well as offer additional insight on known risk factors. Acknowledging the complexity of disease risk estimates simultaneously validates the patient’s existing viewpoint while providing additional data to allow them to re-assess their perceived risk. One approach is to identify and address causal beliefs regarding perceived disease susceptibility, controllability, and etiology (Austin 2015). A second approach is to increase patient comprehension of risk and health actions that might be taken to modify risk. The complexity of disease risk, especially for common disease which often includes multiple low/moderate and sometimes high-risk genetic variants in combination with non-genetic influences, makes the educational aspects of genomic counseling more difficult than for Mendelian single-gene disorders (Austin et al. 2014; Shelton and Whitcomb 2015). Given that most individuals within the general population already have trouble understanding genetic risk information (McKibbin et al. 2014), and particularly struggle with the multiple factors that contribute to risk (DeFrank et al. 2013; Lautenbach et al. 2013), accessible patient education and preventive health strategies are a vital part of the genomic counseling framework.
Strategy 3: Visual Aids
We propose two key strategies to help communicate genomic risk. First, to help patients conceptualize multiple risk factors and magnitudes of risk, we utilize visual aids. Specifically, we used the analogy of pebbles splashing into water (Fig. 2). The size of the pebble (representing a given risk factor) varies depending on the magnitude of risk, and as such, can be used to depict varying levels of risk. It should be acknowledged that there are likely additional ways to present magnitudes of risk and not to assume that a single strategy works for everyone (Lautenbach et al. 2013). Research summarized by Garcia-Retamero et al. has shown that using visual aids that have well-defined elements, and that make use of part-to-whole relationships (e.g., pictographs) may be more effective for vulnerable populations or those with poor numeracy skills (Garcia-Retamero and Cokely 2017). Theories of health communication suggest that the way that information is framed can make the information more salient (noticeable and meaningful) and influence how people evaluate and respond to the information (Entman 1993; Tversky and Kahneman 1981). Counseling on the varied effect of genomic risk variants, the limited predictive contribution of many of these variants, and the polygenic and multifactorial nature of common disease risk may foster a better understanding of complex disease risk. Importantly, the counselor must also attend to the patient’s subjective appraisal of their risk, especially for more common and potentially modifiable conditions, in order to help them understand the steps they may be able to take to identify and engage in risk-reducing health behaviors (Austin 2015).
A risk summary report (Fig. 3) summarizing results can be used to teach patients about the varying contributions of multiple risk factors, and allows for the presentation of risk information in multiple formats. Multiple risk reports from direct-to-consumer labs could be condensed in similar fashion. The risk summary report included an explicit breakdown of the areas of risk (e.g., non-genetic versus genetic) for each disease to facilitate discussion of the management of multiple types of risk information. Although it is generally recommended that absolute risk be communicated (Naik et al. 2012), the OSU-CPMC study chose to report relative risks, which provided context for disease risk, and allowed for the presentation of risk based on multiple influences (genetic, family history, lifestyle) using the same metric taken directly from published literature. Use of absolute risk values in the OSU-CPMC study would have required calculations based on lifetime risk values that were not available for all diseases and reported genotypes (Stack et al. 2011; Keller et al. 2010). For comparison purpose, general population risks also were incorporated into the summary report. Individual relative risk for each disease was described as being increased, decreased, or as “no increase or decrease” in risk (i.e., average risk), with a separate section to relay drug-response information in lay person language. As some people prefer more detailed information, the number of risk alleles and the genotype were also included. The risk summary report can be made available online and/or emailed or mailed to the patient prior to the session for use during the genomic counseling session. This format also allows for direct routing of the risk summary report into the EHR for utilization by the patient’s healthcare team.
Strategy 4: Formulate Action Plan
The inclusion of effective health behavior recommendations, reinforcements, or interventions (Austin 2015), perhaps with use of evidence-based brief intervention strategies for promoting positive health behavior changes (e.g., motivational interviewing), may lead to adoption of health behaviors to reduce risk (Mills and Haga 2014; Shelton and Whitcomb 2015). Focusing genomic counseling on the natural history of a given disease, especially in light of modifiable lifestyle changes, may help develop a personalized action plan (i.e., a set of “next steps” collaboratively developed by the patient and genetic counselor to help the patient manage their risks). In the case of genomic counseling, patient actions may include speaking with a health care provider about disease screening methods (serum lipids; colonoscopy, etc.), making decisions about potential treatments or actions to lower risk, meeting with a health coach to set goals to achieve and maintain risk-reducing health behavior changes, and sharing results with other family members who may be at increased risk. However, formulating successful action plans requires not only determining recommended action steps, but appraising the patient’s attitudes and beliefs that may influence decision-making (Welshimer and Earp 1989), where they are along the continuum from pre-contemplation toward action (Prochaska and Velicer 1997), and perceived or real barriers (e.g., related to access of recommended services) they may face in enacting behavioral change. The Integrative Model of Behavioral Prediction (Fishbein 2008) suggests that factors such as attitudes, perceived social norms for engagement in certain behaviors (the expectations of others for our actions), and efficacy (our confidence to act) are particularly important to consider, as well as broader individual and social factors such as culture and socioeconomic status (SES) that might influence behaviors.
Component 4: Facilitate Patient Activation
Strategy 1: Use of Summary Documents
Summary letters after the genomic counseling process aid patients in their personal understanding of information and also in the dissemination of information to providers, family members, and others who could be affected by their genetic/genomic results. Format, content, and the way in which this is delivered to the patient and the healthcare team are all critical (Sweet et al. 2017a, b). Although patients can often recall important information provided to them in genetic counseling (Michie et al. 1997), summary letters serve as a longer-term reminder of information shared during counseling. We recommend an automated summary letter (Fig. 4) to supplement patient-provider communications (Williams et al. 2016) that is written in patient-friendly language, that outlines test results and reinforces action steps, and that is easily sharable with other providers and family members. This summary letter should include a brief description of the test, separating out results for multiple diseases (with personalized risk factors); specific, personalized bulleted actions steps for prevention and management; and when applicable, supportive language to acknowledge patients already engaged in healthy behaviors. An automated summary letter can be provided to both the patient and provider team via the EHR (e.g., patient portal) or sent to patients by mail.
Strategy 2: Working with Health Care Professionals to Empower Patients
Health care professionals may not raise the topic of genomic test results during consultations with patients, even when they are available in the EHR. This may be due to lack of preparedness to discuss genomic results, or underestimating the importance and/or clinical actionability of certain test results (Guttmacher et al. 2007; Vassy et al. 2013). Each of these possible reasons for inaction can be addressed through active alerting of the physician team regarding genomic consultations about potentially actionable results (Kho et al. 2013; Shoenbill et al. 2014; Sweet et al. 2017a, b) and education and decision support for patients with genomic results. Optimizing the potential for clinical utility of genomic results will also depend on a sustained multidisciplinary approach to education and support for the non-geneticist physician (Talwar et al. 2017; Vassy et al. 2015). Genetic counselors and other health care providers (e.g., nurses, health coaches) familiar with patient activation can also build patient confidence and encourage patients to talk to their physicians about their results.
Strategy 3: Data Sharing
Sharing genomic test results with relatives, especially when a disease(s) has actionable components, allows opportunity to identify other at-risk relatives, facilitate cascade testing as appropriate, and may increase family-centered support and communication. This may be particularly important if test results include “Tier 1” findings (e.g., BRCA 1/2, Lynch syndrome, familial hypercholesterolemia) where cascade screening has shown significant benefits for family members (Khoury et al. 2011). There are increasing opportunities to use social media platforms (e.g., Facebook) or other platforms such as KinTalk to share results with relatives (Lee et al. 2013; Ratzan 2011). A number of clinical testing labs now provide open access and mobile platforms that allow patients to share their genetic/genomic data. These platforms remove many of the barriers to promote open data sharing, encourage research participation, allow patients to become more engaged, and assist them in learning how other people manage similar health concerns through self-advocacy (e.g., FORCE network for BRCA mutation carriers). Easier sharing may also encourage other family members to get tested or to act on results.
Discussion
To help increase the potential effectiveness of genetic counseling practice, as well as its efficiency, we propose a framework of counseling components and strategies that operationalizes the Reciprocal Engagement Model for the scalable delivery of genomic results. The genomic counseling framework incorporates the collection of patient preferences (via online or other tools) before, during, and after genomic testing to increase efficiency of practice; incorporates key strategies to help communicate and enhance patient understanding of complex risk information; and defines a more integrative approach to result delivery in the general medical care settings. We advocate for the expansion of health education on preventative behavior and lifestyle changes in counseling to help further support and accentuate the actual patient-centered psychosocial counseling process. Our goal was to develop a flexible, modifiable counseling approach that is able to be used in emerging genomic counseling settings, as well as other clinical practice settings where a range of genetic test results are returned simultaneously. The genomic counseling framework is also imminently scalable and streamlined via the use of web-based resources for the coming age of large-scale genomic testing and patient access to multiple results.
In this framework, we propose assessing and honoring patient preferences for communication modality (telephone, telegenetic, or in-person) prior to counseling about the results. Utilization of delivery models beyond the standard “in-person” mode of counseling will likely help facilitate patient access to services that are limited due to geographical or financial barriers, or when in-person counseling is not feasible (Trepanier and Allain 2014), and these alternative service delivery models have already been well-accepted by patients in some disease areas (e.g., cancer) (Buchanan et al. 2015). Furthermore, these alternate forms of communication increase patient/client convenience, and expand the scope of practice to include the ability to counsel multiple family members simultaneously who are not all in the same geographic location (Cohen et al. 2016; Trepanier and Allain 2014).
Secondly, we propose that patient areas of concern, or points of discussion for the counseling session, can be assessed with the use of question prompts that could be made available online, elicited by phone, emailed, mailed, or texted to the patient. Having the counselee provide these preferences pre-session could allow for even more pointed contracting and targeted genetic counseling intervention than already typically occurs. A patient not bringing up a particular area of concern is also still helpful and presents opportunities for education and information provision. This approach is especially relevant when an individual is provided results for multiple disease risks, as provided by genomic testing. Tailored visual aids and result summary reports divide areas of risk (genetic variant, family history, lifestyle) for each disease, facilitating viewing of multiple disease risks simultaneously. Post-counseling summary reports can be actively routed to the patient and their physician team electronically to encourage review and follow-up on recommended disease prevention/risk reduction actions. Genetic counselors should continue to explore the utility of social media platforms and emerging mobile health tools (Gallagher et al. 2016). This more participatory approach may be beneficial in helping patients who desire to be more actively engaged in their health care, which, in turn, can improve patient activation and produce positive health outcomes.
Through our previous work, we have found that for individuals undergoing elective genomic testing, the degree of genetic counseling intervention needed varies per patient and per indication (Schmidlen et al. 2014; Sweet et al. 2016). Some individuals require specific genetic counseling for multiple risk reports or disease concerns, while others may more intuitively understand that interpretation of results for one condition is relevant to another. Although our framework was developed to be patient driven, with the primary focus on discussing the results the patient has most interest in and for which there is increased risk, the interpersonal relationship that is central to the genetic counseling process is also important here. For example, in discussing how some variant results can impact medical care (e.g., homozygous status for the AMD variant confers a RR > 6.0) or to support decisions to make personal lifestyle modifications to reduce disease risk, many patients still require active counseling to help alleviate psychological distress and promote a sense of autonomy and control. We also found in our work that patients with chronic disease may have different motivations and represent more varied socioeconomic status (SES) than “healthy” individuals seeking predictive genomic risk information. These groups of patients may also vary in their understanding and response to multiple actionable genomic risk reports, and may treat risk information related to their diagnosis differently than risk information for other diseases. Therefore, even as genetic counseling approaches become more automated and online, there remains the need to explore personal and family dynamics. This can be achieved in a manner similar to current genetic counseling practice: by reviewing aspects of the personal and family history, eliciting responses to concerns about disease risks that may be present in the family, or by simply reflecting upon the reason(s) an individual has elected to undergo testing in the first place.
The genomic counseling framework is based on several key health behavior theories, which provides a framework for integrating concepts of agenda setting and multistep workflow and allows for assessment of behavior constructs such as perceived personal control, risk perception accuracy, and attitudes (Fishbein 2008). This framework also promotes personal health behavior modification on three essential socioecological levels (individual: receipt of potentially actionable genomic results; interpersonal: interaction with a genetic counselor; and organizational: interaction with health care systems) (Division of Cancer Prevention and Control 2015; Golden et al. 2015). The framework is both theoretically and empirically based, taking into account messages and actions to be utilized in genomic counseling to reduce risk and influence health behavior. As such, we suggest that this framework could be further developed and expanded upon to incorporate any type of potentially actionable genomic test result, to include rare Mendelian in addition to common multifactorial disease as many of the essential elements of genetic counseling remain the same. It is notable that genetic counseling approaches for actionable Mendelian disease are in the midst of rapid change given the expansion of (1) clinical molecular testing to encompass multigene panels (Domchek et al. 2013), (2) the growing availability of online test report delivery, and (3) the increasing use of telephone/telegenetic counseling services. Testing labs are also beginning to make available panel-based approaches to medically actionable screening panels (based on the principles of the ACMG 59 gene list) for healthy individuals. These panels are not currently direct-to-consumer and typically incorporate genomic counseling, pre-test education, and informed consent; for some, result delivery is available online. Focused gene panels that capture actionable Mendelian diseases are providing opportunities to meet growing consumer interest in actionable diseases, significantly impacting current genetics practice, and highlighting the need for continued evolution of genomic counseling service delivery and practice.
Given the breadth of genomic information likely to be included in result reports as the use of genomic-based technologies continues to increase, further development of this framework should help provide a scalable approach to the delivery of precision medicine that capitalizes on evidence-based best practices and incorporates patient preferences. To reach this end goal, a number of hurdles must be overcome. Allowing greater access to genomic counseling for multiple types of results, as predicated by this framework, may make it more difficult from a professional time management perspective. Implementation of genomic testing/screening will ultimately be driven by patient and healthcare provider demand, payers’ willingness to reimburse, and by assessing outcomes after receiving genomic information and counseling. Genome-wide testing will produce many findings, some of which are medically actionable, and some which will have uncertain clinical implications (e.g., VUSs, variants that are clinically relevant but lack specific guidelines, variable penetrance, etc.) with the potential to exacerbate some very difficult genetic counseling issues related to uncertainty, patient engagement, and decision-making (Bernhardt et al. 2015). Some of these are addressed by this genomic counseling framework, others might be approached through augmentations to genetic counselor training, as well as more extensive training around the psychology of uncertainty and evidence-based methods of facilitated decision-making (Hooker et al. 2014). There are appreciable startup costs associated with development of web-based interfaces and counseling services. Significant barriers also exist related to under-utilization of health services by lower SES and selected racial/cultural subgroups in the USA and abroad. Lastly, although many genetic/genomic testing services now allow consumers to access their results (and even genetic counselors) online, there remains appreciable gaps by the general public in access to, and use of, available technology and genetic counseling services. Additional research on the expanding roles of technology, genomic counseling service delivery outcomes, and cost-effectiveness is warranted (Madlensky et al. 2017).
Conclusion
The genomic counseling framework was developed to meet the challenges of providing genomic counseling for the coming age of large-scale genomic testing and patient access to multiple results. The components and strategies elaborated in this framework serve the existing tenets of the REM, encompass genetic counseling best practices, and are grounded in empirical and theoretical research. Furthermore, development of this framework was based on formative research with patients receiving multiple disease and pharmacogenomics reports, and consultation with experts in genetic and genomic counseling to provide broad scope and applicability for any type of genomic result. The components and strategies within this framework should undergo further testing and subsequent refinement, with studies currently underway. We believe that this genomic counseling framework presents a first step toward operationalizing the REM for a more scalable delivery of patient-centered genomic counseling with the ultimate goals of (1) reducing risk for both common and rare diseases with genetic and genomic components in a proactive approach and (2) helping patients and families adapt to and cope with genetic health risks.
References
Albada, A., van Dulmen, S., Ausems, M. G., & Bensing, J. M. (2012a). A pre-visit website with question prompt sheet for counselees facilitates communication in the first consultation for breast cancer genetic counseling: findings from a randomized controlled trial. Genetics in Medicine, 14(5), 535–542. https://doi.org/10.1038/gim.2011.42.
Albada, A., van Dulmen, S., Lindhout, D., Bensing, J. M., & Ausems, M. G. (2012b). A pre-visit tailored website enhances counselees' realistic expectations and knowledge and fulfils information needs for breast cancer genetic counselling. Familial Cancer, 11(1), 85–95. https://doi.org/10.1007/s10689-011-9479-1.
Austin, J. (2015). The effect of genetic test-based risk information on behavioral outcomes: a critical examination of failed trials and a call to action. American Journal of Medical Genetics. Part A, 167A(12), 2913–2915. https://doi.org/10.1002/ajmg.a.37289.
Austin, J., Semaka, A., & Hadjipavlou, G. (2014). Conceptualizing genetic counseling as psychotherapy in the era of genomic medicine. Journal of Genetic Counseling, 23(6), 903–909. https://doi.org/10.1007/s10897-014-9728-1.
Baumanis, L., Evans, J. P., Callanan, N., & Susswein, L. R. (2009). Telephoned BRCA1/2 genetic test results: prevalence, practice, and patient satisfaction. Journal of Genetic Counseling, 18(5), 447–463. https://doi.org/10.1007/s10897-009-9238-8.
Benusiglio, P. R., Di Maria, M., Dorling, L., Jouinot, A., Poli, A., Villebasse, S., et al. (2017). Hereditary breast and ovarian cancer: successful systematic implementation of a group approach to genetic counselling. Familial Cancer, 16(1), 51–56. https://doi.org/10.1007/s10689-016-9929-x.
Bernhardt, B. A., Roche, M. I., Perry, D. L., Scollon, S. R., Tomlinson, A. N., & Skinner, D. (2015). Experiences with obtaining informed consent for genomic sequencing. American Journal of Medical Genetics. Part A, 167A(11), 2635–2646. https://doi.org/10.1002/ajmg.a.37256.
Birch, P. H. (2015). Interactive e-counselling for genetics pre-test decisions: where are we now? Clinical Genetics, 87(3), 209–217. https://doi.org/10.1111/cge.12430.
Buchanan, A. H., Datta, S. K., Skinner, C. S., Hollowell, G. P., Beresford, H. F., Freeland, T., et al. (2015). Randomized trial of telegenetics vs. in-person cancer genetic counseling: cost, patient satisfaction and attendance. Journal of Genetic Counseling, 24(6), 961–970. https://doi.org/10.1007/s10897-015-9836-6.
Buchanan, A. H., Rahm, A. K., & Williams, J. L. (2016). Alternate service delivery models in cancer genetic counseling: a mini-review. Frontiers in Oncology, 6, 120. https://doi.org/10.3389/fonc.2016.00120.
Cacioppo, C. N., Chandler, A. E., Towne, M. C., Beggs, A. H., & Holm, I. A. (2016). Expectation versus reality: the impact of utility on emotional outcomes after returning individualized genetic research results in pediatric rare disease research, a qualitative interview study. PLoS One, 11(4), e0153597. https://doi.org/10.1371/journal.pone.0153597.
Carey, D. J., Fetterolf, S. N., Davis, F. D., Faucett, W. A., Kirchner, H. L., Mirshahi, U., et al. (2016). The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genetics in Medicine, 18(9), 906–913. https://doi.org/10.1038/gim.2015.187.
Caulfield, T., & Condit, C. (2012). Science and the sources of hype. Public Health Genomics, 15(3–4), 209–217. https://doi.org/10.1159/000336533.
Centers for, M, Medicaid Services, H. H. S, Centers for Disease, C, Prevention, H. H. S, & Office for Civil Rights, H. H. S. (2014). CLIA program and HIPAA privacy rule; patients' access to test reports. Final rule. Federal Register, 79(25), 7289–7316.
Charmaz, K. (2014). Constructing grounded theory (2nd edition): SAGE Publications Ltd.
Christensen, K. D., Uhlmann, W. R., Roberts, J. S., Linnenbringer, E., Whitehouse, P. J., Royal, C. D. M., et al. (2017). A randomized controlled trial of disclosing genetic risk information for Alzheimer disease via telephone. Genetics in Medicine. https://doi.org/10.1038/gim.2017.103.
Cohen, S. A., Marvin, M. L., Riley, B. D., Vig, H. S., Rousseau, J. A., & Gustafson, S. L. (2013). Identification of genetic counseling service delivery models in practice: a report from the NSGC Service delivery model task Force. Journal of Genetic Counseling, 22(4), 411–421. https://doi.org/10.1007/s10897-013-9588-0.
Cohen, S. A., Huziak, R. C., Gustafson, S., & Grubs, R. E. (2016). Analysis of advantages, limitations, and barriers of genetic counseling service delivery models. Journal of Genetic Counseling, 25(5), 1010–1018. https://doi.org/10.1007/s10897-016-9932-2.
Collins, F. S., & Varmus, H. (2015). A new initiative on precision medicine. The New England Journal of Medicine, 372(9), 793–795. https://doi.org/10.1056/NEJMp1500523.
DeFrank, J. T., Salz, T., Reeder-Hayes, K., & Brewer, N. T. (2013). Who gets genomic testing for breast cancer recurrence risk? Public Health Genomics, 16(5), 215–222. https://doi.org/10.1159/000353518.
Directors, A. B. o. (2013). Points to consider for informed consent for genome/exome sequencing. Genetics in Medicine, 15(9), 748–749. https://doi.org/10.1038/gim.2013.94.
Division of Cancer Prevention and Control, C. f. D. C. a. P (2015). Social Ecological Model. Retrieved from http://www.cdc.gov/cancer/crccp/sem.htm.
Domchek, S. M., Bradbury, A., Garber, J. E., Offit, K., & Robson, M. E. (2013). Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? Journal of Clinical Oncology, 31(10), 1267–1270. https://doi.org/10.1200/JCO.2012.46.9403.
Entman, R. M. (1993). Framing: toward clarification of a fractured paradigm. Journal of Communication, 43(4), 51–58. https://doi.org/10.1111/j.1460-2466.1993.tb01304.x.
Evans, J. P., Berg, J. S., Olshan, A. F., Magnuson, T., & Rimer, B. K. (2013). We screen newborns, don't we?: realizing the promise of public health genomics. Genetics in Medicine, 15(5), 332–334. https://doi.org/10.1038/gim.2013.11.
Facio, F. M., Eidem, H., Fisher, T., Brooks, S., Linn, A., Kaphingst, K. A., et al. (2013). Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study. European Journal of Human Genetics, 21(3), 261–265. https://doi.org/10.1038/ejhg.2012.179.
Fishbein, M. (2008). A reasoned action approach to health promotion. Medical Decision Making, 28(6), 834–844. https://doi.org/10.1177/0272989X08326092.
Force, N. S. o. G. C. D. T (2016). 2016 Professional Status Survey Report. Retrieved from http://www.nsgc.org/page/whoaregeneticcounselors.
Gallagher, L., McCuaig, J., Benoit, L., & Davies, C. (2016). It's time for the genetic counseling profession to embrace social media. Journal of Genetic Counseling, 25(6), 1338–1341. https://doi.org/10.1007/s10897-016-9950-0.
Garcia-Retamero, R., & Cokely, E. T. (2017). Designing visual aids that promote risk literacy: a systematic review of health research and evidence-based design heuristics. Human Factors, 59(4), 582–627. https://doi.org/10.1177/0018720817690634.
Gharani, N., Keller, M. A., Stack, C. B., Hodges, L. M., Schmidlen, T. J., Lynch, D. E., et al. (2013). The Coriell personalized medicine collaborative pharmacogenomics appraisal, evidence scoring and interpretation system. Genome Medicine, 5(10), 93. https://doi.org/10.1186/gm499.
Golden, S. D., McLeroy, K. R., Green, L. W., Earp, J. A., & Lieberman, L. D. (2015). Upending the social ecological model to guide health promotion efforts toward policy and environmental change. Health Education & Behavior, 42(1 Suppl), 8S–14S. https://doi.org/10.1177/1090198115575098.
Gray, S. W., Martins, Y., Feuerman, L. Z., Bernhardt, B. A., Biesecker, B. B., Christensen, K. D., et al. (2014). Social and behavioral research in genomic sequencing: approaches from the clinical sequencing exploratory research consortium outcomes and measures working group. Genetics in Medicine, 16(10), 727–735. https://doi.org/10.1038/gim.2014.26.
Guttmacher, A. E., Collins, F. S., & Carmona, R. H. (2004). The family history—more important than ever. The New England Journal of Medicine, 351(22), 2333–2336. https://doi.org/10.1056/NEJMsb042979.
Guttmacher, A. E., Porteous, M. E., & McInerney, J. D. (2007). Educating health-care professionals about genetics and genomics. Nature Reviews. Genetics, 8(2), 151–157. https://doi.org/10.1038/nrg2007.
Hooker, G. W., Ormond, K. E., Sweet, K., & Biesecker, B. B. (2014). Teaching genomic counseling: preparing the genetic counseling workforce for the genomic era. Journal of Genetic Counseling, 23(4), 445–451. https://doi.org/10.1007/s10897-014-9689-4.
Jay, L. R., Afifi, W. A., & Samter, W. (2000). The role of expectations in effective genetic counseling. Journal of Genetic Counseling, 9(2), 95–116. https://doi.org/10.1023/A:1009493424814.
Kalia, S. S., Adelman, K., Bale, S. J., Chung, W. K., Eng, C., Evans, J. P., et al. (2017). Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genetics in Medicine, 19(2), 249–255. https://doi.org/10.1038/gim.2016.190.
Kaufman, D. J., Bollinger, J. M., Dvoskin, R. L., & Scott, J. A. (2012). Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. Journal of Genetic Counseling, 21(3), 413–422. https://doi.org/10.1007/s10897-012-9483-0.
Keller, M. A., Gordon, E. S., Stack, C. B., Gharani, N., Sill, C. J., Schmidlen, T. J., et al. (2010). Coriell personalized medicine collaborative (R): a prospective study of the utility of personalized medicine. Personalized Medecine, 7(3), 301–317. https://doi.org/10.2217/Pme.10.13.
Khera, A. V., Emdin, C. A., Drake, I., Natarajan, P., Bick, A. G., Cook, N. R., et al. (2016). Genetic risk, adherence to a healthy lifestyle, and coronary disease. The New England Journal of Medicine, 375(24), 2349–2358. https://doi.org/10.1056/NEJMoa1605086.
Kho, A. N., Rasmussen, L. V., Connolly, J. J., Peissig, P. L., Starren, J., Hakonarson, H., & Hayes, M. G. (2013). Practical challenges in integrating genomic data into the electronic health record. Genetics in Medicine, 15(10), 772–778. https://doi.org/10.1038/gim.2013.131.
Khoury, M. J., Bowen, M. S., Burke, W., Coates, R. J., Dowling, N. F., Evans, J. P., et al. (2011). Current priorities for public health practice in addressing the role of human genomics in improving population health. American Journal of Preventive Medicine, 40(4), 486–493. https://doi.org/10.1016/j.amepre.2010.12.009.
Kreuter, M. W., & Wray, R. J. (2003). Tailored and targeted health communication: strategies for enhancing information relevance. American Journal of Health Behavior, 27(1), S227–S232.
Lautenbach, D. M., Christensen, K. D., Sparks, J. A., & Green, R. C. (2013). Communicating genetic risk information for common disorders in the era of genomic medicine. Annual Review of Genomics and Human Genetics, 14, 491–513. https://doi.org/10.1146/annurev-genom-092010-110722.
Lee, S. S., Vernez, S. L., Ormond, K. E., & Granovetter, M. (2013). Attitudes towards social networking and sharing behaviors among consumers of direct-to-consumer personal genomics. J Pers Med, 3(4), 275–287. https://doi.org/10.3390/jpm3040275.
Lewis, K. L., Hooker, G. W., Connors, P. D., Hyams, T. C., Wright, M. F., Caldwell, S., et al. (2016). Participant use and communication of findings from exome sequencing: a mixed-methods study. Genetics in Medicine, 18(6), 577–583. https://doi.org/10.1038/gim.2015.133.
Linderman, M. D., Nielsen, D. E., Green, R. C. (2016). Personal genome sequencing in ostensibly healthy individuals and the PeopleSeq consortium. Journal of Perinatal Medecine, 6(2). https://doi.org/10.3390/jpm6020014.
Madlensky, L., Trepanier, A. M., Cragun, D., Lerner, B., Shannon, K. M., & Zierhut, H. (2017). A rapid systematic review of outcomes studies in genetic counseling. Journal of Genetic Counseling, 26(3), 361–378. https://doi.org/10.1007/s10897-017-0067-x.
Manolio, T. A., Abramowicz, M., Al-Mulla, F., Anderson, W., Balling, R., Berger, A. C., et al. (2015). Global implementation of genomic medicine: we are not alone. Science Translational Medicine, 7(290), 290ps213. https://doi.org/10.1126/scitranslmed.aab0194.
McKibbin, M., Ahmed, M., Allsop, M. J., Downey, L., Gale, R., Grant, H. L., et al. (2014). Current understanding of genetics and genetic testing and information needs and preferences of adults with inherited retinal disease. European Journal of Human Genetics, 22(9), 1058–1062. https://doi.org/10.1038/ejhg.2013.296.
McLaughlin, H. M., Ceyhan-Birsoy, O., Christensen, K. D., Kohane, I. S., Krier, J., Lane, W. J., et al. (2014). A systematic approach to the reporting of medically relevant findings from whole genome sequencing. BMC Medical Genetics, 15, 134. https://doi.org/10.1186/s12881-014-0134-1.
Meropol, N. J., Daly, M. B., Vig, H. S., Manion, F. J., Manne, S. L., Mazar, C., et al. (2011). Delivery of internet-based cancer genetic counselling services to patients' homes: a feasibility study. Journal of Telemedicine and Telecare, 17(1), 36–40. https://doi.org/10.1258/jtt.2010.100116.
Michie, S., McDonald, V., & Marteau, T. M. (1997). Genetic counselling: information given, recall and satisfaction. Patient Education and Counseling, 32(1–2), 101–106. https://doi.org/10.1016/S0738-3991(97)00050-5.
Middleton, A., Hall, G., & Patch, C. (2015). Genetic counselors and genomic counseling in the United Kingdom. Molecular Genetics & Genomic Medicine, 3(2), 79–83. https://doi.org/10.1002/mgg3.123.
Mills, R., & Haga, S. B. (2014). Genomic counseling: next generation counseling. Journal of Genetic Counseling, 23(4), 689–692. https://doi.org/10.1007/s10897-013-9641-z.
Naik, G., Ahmed, H., & Edwards, A. G. (2012). Communicating risk to patients and the public. The British Journal of General Practice, 62(597), 213–216. https://doi.org/10.3399/bjgp12X636236.
National Society of Genetic Counselors' Definition Task, F, Resta, R., Biesecker, B. B., Bennett, R. L., Blum, S., Hahn, S. E., et al. (2006). A new definition of genetic counseling: National Society of genetic Counselors' task Force report. Journal of Genetic Counseling, 15(2), 77–83. https://doi.org/10.1007/s10897-005-9014-3.
O'Daniel, J. M. (2010). The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. Journal of Genetic Counseling, 19(4), 315–327. https://doi.org/10.1007/s10897-010-9302-4.
O'Neill, S. C., McBride, C. M., Alford, S. H., & Kaphingst, K. A. (2010). Preferences for genetic and behavioral health information: the impact of risk factors and disease attributions. Annals of Behavioral Medicine, 40(2), 127–137. https://doi.org/10.1007/s12160-010-9197-1.
Orlando, L. A., Wu, R. R., Myers, R. A., Buchanan, A. H., Henrich, V. C., Hauser, E. R., & Ginsburg, G. S. (2016). Clinical utility of a web-enabled risk-assessment and clinical decision support program. Genetics in Medicine, 18(10), 1020–1028. https://doi.org/10.1038/gim.2015.210.
Ormond, K. E. (2013). From genetic counseling to “genomic counseling”. Molecular Genetics & Genomic Medicine, 1(4), 189–193. https://doi.org/10.1002/mgg3.45.
Peshkin, B. N., Kelly, S., Nusbaum, R. H., Similuk, M., DeMarco, T. A., Hooker, G. W., et al. (2016). Patient perceptions of telephone vs. in-person BRCA1/BRCA2 genetic counseling. Journal of Genetic Counseling, 25(3), 472–482. https://doi.org/10.1007/s10897-015-9897-6.
Petty, R. E., & Cacioppo, J. T. (1986). The elaboration likelihood model of persuasion. In Berkowitz (Ed.), Advances in Experimental Social Psychology (Vol. 19). San Diego: Academic Press.
Pirzadeh-Miller, S., Robinson, L. S., Read, P., & Ross, T. S. (2016). Genetic counseling assistants: an integral piece of the evolving genetic counseling service delivery model. Journal of Genetic Counseling. https://doi.org/10.1007/s10897-016-0039-6.
Prochaska, J. O., & Velicer, W. F. (1997). The transtheoretical model of health behavior change. American Journal of Health Promotion, 12(1), 38–48.
Ratzan, S. C. (2011). Our new "social" communication age in health. Journal of Health Communication, 16(8), 803–804. https://doi.org/10.1080/10810730.2011.610220.
Redlinger-Grosse, K., Veach, P. M., LeRoy, B. S., & Zierhut, H. (2017). Elaboration of the reciprocal-engagement model of genetic counseling practice: a qualitative investigation of goals and strategies. Journal of Genetic Counseling, 26(6), 1372–1387. https://doi.org/10.1007/s10897-017-0114-7.
Rimal, R. N., & Real, K. (2003). Perceived risk and efficacy beliefs as motivators of change: use of the risk perception attitude (RPA) framework to understand health behaviors. Human Communication Research, 29(3), 370–399. https://doi.org/10.1111/j.1468-2958.2003.tb00844.x.
Rosenstock, I. M. (1990). The health belief model: explaining health behavior through expectancies. In K. F. Glanz, M. Lewis, & B. Rimer (Eds.), Health behavior and health education (Vol. 2, pp. 328–335). San Francisco: Jossey-Bass Publishers.
Rubinstein, W. S., Acheson, L. S., O'Neill, S. M., Ruffin, M. T. T., Wang, C., Beaumont, J. L., et al. (2011). Clinical utility of family history for cancer screening and referral in primary care: a report from the family healthware impact trial. Genetics in Medicine, 13(11), 956–965. https://doi.org/10.1097/GIM.0b013e3182241d88.
Schmidlen, T. J., Wawak, L., Kasper, R., Garcia-Espana, J. F., Christman, M. F., & Gordon, E. S. (2014). Personalized genomic results: analysis of informational needs. Journal of Genetic Counseling, 23(4), 578–587. https://doi.org/10.1007/s10897-014-9693-8.
Schwartz, M. D., Valdimarsdottir, H. B., Peshkin, B. N., Mandelblatt, J., Nusbaum, R., Huang, A. T., et al. (2014). Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. Journal of Clinical Oncology, 32(7), 618–626. https://doi.org/10.1200/JCO.2013.51.3226.
Shelton, C. A., & Whitcomb, D. C. (2015). Evolving roles for physicians and genetic counselors in managing complex genetic disorders. Clin Transl Gastroenterol, 6, e124. https://doi.org/10.1038/ctg.2015.46.
Shoenbill, K., Fost, N., Tachinardi, U., & Mendonca, E. A. (2014). Genetic data and electronic health records: a discussion of ethical, logistical and technological considerations. Journal of the American Medical Informatics Association, 21(1), 171–180. https://doi.org/10.1136/amiajnl-2013-001694.
Simmons, L. A., Wolever, R. Q., Bechard, E. M., & Snyderman, R. (2014). Patient engagement as a risk factor in personalized health care: a systematic review of the literature on chronic disease. Genome Medicine, 6(2), 16. https://doi.org/10.1186/gm533.
Skol, A. D., Sasaki, M. M., & Onel, K. (2016). The genetics of breast cancer risk in the post-genome era: thoughts on study design to move past BRCA and towards clinical relevance. Breast Cancer Research, 18(1), 99. https://doi.org/10.1186/s13058-016-0759-4.
Smerecnik, C. M., Mesters, I., Verweij, E., de Vries, N. K., & de Vries, H. (2009). A systematic review of the impact of genetic counseling on risk perception accuracy. Journal of Genetic Counseling, 18(3), 217–228. https://doi.org/10.1007/s10897-008-9210-z.
Smith, A. (2016). Record shares of Americans now own smartphones, have home broadband. Retrieved from http://pewrsr.ch/2jbjymk.
Stack, C. B., Gharani, N., Gordon, E. S., Schmidlen, T., Christman, M. F., & Keller, M. A. (2011). Genetic risk estimation in the Coriell personalized medicine collaborative. Genetics in Medicine, 13(2), 131–139. https://doi.org/10.1097/GIM.0b013e318201164c.
Sweet, K., Gordon, E. S., Sturm, A. C., Schmidlen, T. J., Manickam, K., Toland, A. E., et al. (2014). Design and implementation of a randomized controlled trial of genomic counseling for patients with chronic disease. J Pers Med, 4(1), 1–19. https://doi.org/10.3390/jpm4010001.
Sweet, K., Hovick, S., Sturm, A. C., Schmidlen, T., Gordon, E., Bernhardt, B., et al. (2016). Counselees' perspectives of genomic counseling following online receipt of multiple actionable complex disease and Pharmacogenomic results: a qualitative research study. Journal of Genetic Counseling. https://doi.org/10.1007/s10897-016-0044-9.
Sweet, K., Sturm, A. C., Schmidlen, T., Hovick, S., Peng, J., Manickam, K., et al. (2017a). EMR documentation of physician-patient communication following genomic counseling for actionable complex disease and pharmacogenomic results. Clinical Genetics, 91(4), 545–556. https://doi.org/10.1111/cge.12820.
Sweet, K., Sturm, A. C., Schmidlen, T., McElroy, J., Scheinfeldt, L., Manickam, K., et al. (2017b). Outcomes of a randomized controlled trial of genomic counseling for patients receiving personalized and actionable complex disease reports. Journal of Genetic Counseling. https://doi.org/10.1007/s10897-017-0073-z.
Tabor, H. K., Auer, P. L., Jamal, S. M., Chong, J. X., Yu, J. H., Gordon, A. S., et al. (2014). Pathogenic variants for Mendelian and complex traits in exomes of 6,517 European and African Americans: Implications for the return of incidental results. American Journal of Human Genetics, 95(2), 183–193. https://doi.org/10.1016/j.ajhg.2014.07.006.
Tabor, H. K., Jamal, S. M., Yu, J. H., Crouch, J. M., Shankar, A. G., Dent, K. M., et al. (2017). My46: a web-based tool for self-guided management of genomic test results in research and clinical settings. Genetics in Medicine, 19(4), 467–475. https://doi.org/10.1038/gim.2016.133.
Talwar, D., Tseng, T. S., Foster, M., Xu, L., & Chen, L. S. (2017). Genetics/genomics education for nongenetic health professionals: a systematic literature review. Genetics in Medicine, 19(7), 725–732. https://doi.org/10.1038/gim.2016.156.
Trepanier, A. M., & Allain, D. C. (2014). Models of service delivery for cancer genetic risk assessment and counseling. Journal of Genetic Counseling, 23(2), 239–253. https://doi.org/10.1007/s10897-013-9655-6.
Tversky, A., & Kahneman, D. (1981). The framing of decisions and the psychology of choice. Science, 211(4481), 453–458.
Vassy, J. L., Green, R. C., & Lehmann, L. S. (2013). Genomic medicine in primary care: barriers and assets. Postgraduate Medical Journal, 89(1057), 615–616. https://doi.org/10.1136/postgradmedj-2013-132093.
Vassy, J. L., Korf, B. R., & Green, R. C. (2015). How to know when physicians are ready for genomic medicine. Science Translational Medicine, 7(287), 287fs219. https://doi.org/10.1126/scitranslmed.aaa2401.
Veach, P. M., Bartels, D. M., & Leroy, B. S. (2007). Coming full circle: a reciprocal-engagement model of genetic counseling practice. Journal of Genetic Counseling, 16(6), 713–728. https://doi.org/10.1007/s10897-007-9113-4.
Welshimer, K. J., & Earp, J. A. L. (1989). Genetic counseling within the context of existing attitudes and beliefs. Patient Education and Counseling, 13(3), 237–255. https://doi.org/10.1016/0738-3991(89)90019-0.
Wicklund, C., & Trepanier, A. (2014). Adapting genetic counseling training to the genomic era: more an evolution than a revolution. Journal of Genetic Counseling, 23(4), 452–454. https://doi.org/10.1007/s10897-014-9690-y.
Williams, J. L., Rahm, A. K., Stuckey, H., Green, J., Feldman, L., Zallen, D. T., et al. (2016). Enhancing genomic laboratory reports: A qualitative analysis of provider review. American Journal of Medical Genetics. Part A, 170A(5), 1134–1141. https://doi.org/10.1002/ajmg.a.37573.
Woods, S. S., Schwartz, E., Tuepker, A., Press, N. A., Nazi, K. M., Turvey, C. L., & Nichol, W. P. (2013). Patient experiences with full electronic access to health records and clinical notes through the my HealtheVet personal health record pilot: Qualitative study. Journal of Medical Internet Research, 15(3), e65. https://doi.org/10.2196/jmir.2356.
Wynn, J. (2016). Genomic testing: a genetic counselor's personal reflection on three years of consenting and testing. Journal of Genetic Counseling, 25(4), 691–697. https://doi.org/10.1007/s10897-015-9868-y.
Yu, J. H., Harrell, T. M., Jamal, S. M., Tabor, H. K., & Bamshad, M. J. (2014). Attitudes of genetics professionals toward the return of incidental results from exome and whole-genome sequencing. American Journal of Human Genetics, 95(1), 77–84. https://doi.org/10.1016/j.ajhg.2014.06.004.
Acknowledgements
We thank Stephanie J. Schulte for her kind assistance with the extensive literature review and summary. We are grateful to the anonymous reviewers for their detailed comments and thoughtful insight.
Funding
Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number R21HG006575. This work was also supported in part by the Ohio State University Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Coriell Personalized Medicine Collaborative was funded by the William G. Rohrer Foundation, the RNR Foundation, and a grant from the endowment of the Coriell Institute for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
ESG is currently a paid employee of Genome Medical. She worked for the Coriell Institute for Medical Research at the time that this study was developed and the majority of the data collection period.
Tara Schmidlen, Amy C. Sturm, Shelly Hovick, Laura Scheinfeldt, J. Scott Roberts, Lindsey Morr, Joseph McElroy, Amanda E. Toland, Michael Christman, Julianne M. O’Daniel, Barbara A. Bernhardt, Kelly E. Ormond, and Kevin Sweet declare that they have no conflict of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the local medical ethical boards of the Ohio State University Wexner Medical Center and the Coriell Institute for Medical Research and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Studies
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Supplementary Fig. 1
(PDF 225 kb)
Rights and permissions
About this article
Cite this article
Schmidlen, T., Sturm, A.C., Hovick, S. et al. Operationalizing the Reciprocal Engagement Model of Genetic Counseling Practice: a Framework for the Scalable Delivery of Genomic Counseling and Testing. J Genet Counsel 27, 1111–1129 (2018). https://doi.org/10.1007/s10897-018-0230-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-018-0230-z