Abstract
Three d10 metal complexes, ZnL(OAc)2 (1), CdL(OAc)2 (2) and [CdL2(NO3)2]·CH3CN (3) were synthesized using the ligand (E)-N-(3-methoxy-4-methylphenyl)-1-(quinolin-2-yl)methanimine (L) and characterized by FT-IR spectra, NMR spectra, and CHN elemental analysis. Single-crystal X-ray diffraction analysis revealed that complexes 1 and 2 are isostructural, with the central metal adopting a hexacoordinate octahedral geometry, while complex 3 adopts a triangular dodecahedron geometry. Thermal gravimetric analysis showed that these complexes exhibit good thermal stability. Solid-state fluorescence spectroscopy measurements demonstrated that complexes 1–3 exhibit bright yellow-green fluorescence (λem = 564 nm for 1; 524 nm for 2; 542 nm for 3), suggesting their potential as photoluminescent materials. Furthermore, DFT calculations, including frontier molecular orbitals, energy levels, and surface electrostatic potential, provided insights into the structural and electronic spectral properties of complexes 1–3.
Graphical Abstract
Three d10 metal complexes have been synthesized using bis-dentate N,N-quinoline Schiff Base and their structures, fluorescence properties, as well as theoretical calculations were further characterized and discussed.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff base ligands are obtained by condensation of the aldehydes and amines, which are widely used to prepare non-transition metal complexes and transition metal complexes [1,2,3,4,5]. Due to the advantages of Schiff base ligands, such as simple synthesis, good stability, and complexation ability, organic ligands ranging from rigid to flexible can be constructed by changing the structure of aldehydes and amines. They serve as molecular building blocks (similar to porphyrins) and coordinate with metals to synthesize new materials with outstanding potential [6, 7]. Among them, zinc/cadmium Schiff base complexes have been widely studied, mainly because they can be assembled into supramolecular functional materials with catalytic [8], biological imaging [9], thin film [10] and photophysical properties [11]. Metal complexes, resulting from the coordination of metal ions, balancing anions, and organic ligands, exhibit advantages such as tunable excitation and emission properties, straightforward synthesis methodologies, and a wide range of structural designs. These attributes offer the potential to produce intriguing supramolecular architectures and luminescent characteristics.
It is well-known that quinoline derivatives can construct luminescent metal–organic materials with various metal ions due to their large conjugated system and good coordination ability [12,13,14]. Keasberry et al. presented a study on the synthesis of the [Zn(NNS)2] complex, employing the ligand quinoline-2-formaldehyde-4-methyl-3-thiosemicarbazone (HNNS), and single-crystal X-ray diffraction analysis showed that the central metal zinc exhibited a distorted octahedral configuration. The antibacterial activity of [Zn(NNS)2] was evaluated, and the results indicated that it possessed stronger antibacterial activity compared to the HNNS ligand [15]. Salah et al. synthesized two novel zinc and cadmium complexes using quinoline derivatives, which exhibited solvatochromic behavior with a red shift in π → π* transition as the solvent polarity increased [16]. Demissie and colleagues synthesized a zinc(II) complex using the ligand 2-(2-hydroxyethyl)aminoquinoline-3-carboxaldehyde (H2L) as the starting material in a methanol solution with a metal-to-ligand ratio of 1: 2. Compared to the ligand, the metal complex exhibited intense fluorescence intensity and a blue shift in emission [17]. The fluorescence properties of these d10 metal complexes are extremely important due to their versatile applications in chemical sensors, photochemistry, and electroluminescent displays [18, 19].
Based on the above discussion, in our study, a Schiff base ligand (E)-N-(3-methoxy-4-methylphenyl)-1-(quinolin-2-yl)methanimine (L) with N2 coordination sites was synthesized using quinoline-2-carboxaldehyde and 3-methoxy-4-methylaniline, and the corresponding metal complexes 1–3 were synthesized under reflux conditions. The structures and photophysical properties of complexes 1–3, as well as the relationship between their structure and fluorescence properties, were discussed in detail. The bright fluorescence emission of complexes 1–3 suggests potential applications in photoluminescent materials.
Experimental
Materials and Measurements
All the solvents and reagents (analytical grade) were used as received. All the materials for synthesis were purchased from Haohong Scientific Co., Ltd. (Shanghai, China). Elemental analyses of C, H and N were conducted using a Vario EL elemental analyzer. 1H and 13C NMR spectra of L and complexes 1–3 were obtained using a Bruker Avance–400 MHz spectrometer in DMSO-d6 at 298 K. FT–IR spectra were recorded using a TENSOR II (Bruker) spectrophotometer using a KBr pellet in the range of 4000–400 cm–1. Thermal gravimetry analysis (TGA) experiments were conducted with Versa Therm TGA instrument with a heating rate of 10 °C min−1 from 40 to 800 °C under a nitrogen atmosphere. Sample preparations for the TGA were carried out under air. UV − vis spectra were recorded by using a Shimadzu UH5300 (Japan) spectrophotometer in the range of 200–800 nm at room temperature. Fluorescence spectra were obtained using a Hitachi F-7100 FL spectrophotometer equipped with a 150 W xenon lamp as the excitation and emission source at room temperature, and the slit width of the acetonitrile solution fluorescence spectra was 5.0 nm both the excitation and emission, and that for the solid-state fluorescence spectra was 5.0 nm for excitation and 1.0 nm for emission. The sample concentration for UV − vis and fluorescence spectra testing was 2 × 10–5 mol L–1 in CH3CN.
Synthesis of Complexes 1–3
The Schiff base (E)-N-(3-methoxy-4-methylphenyl)-1-(quinolin-2-yl)methanimine (L) was synthesized following a previously reported method [20]. Complexes 1–3 were synthesized along the reaction route depicted in Scheme 1. A mixture of the ligand L (0.0536 g, 0.2 mmol) and the corresponding metal salts of either (Zn(OAc)2·2H2O (0.0456 g, 0.2 mmol), Cd(OAc)2·2H2O (0.0535 g, 0.2 mmol) or Cd(NO3)2·4H2O (0.0312 g, 0.1 mmol)) in 25 mL CH3CN was refluxed at 80 ℃ for 4–5 h. After several days, yellow crystals of complexes 1–3 were collected by slow evaporation.
(E)-N-(3-methoxy-4-methylphenyl)-1-(quinolin-2-yl)methanimine (L)
Yield: 84.60%, Color: Yellow. Anal. calc. for C18H16N2O: C, 78.24; H, 5.84; N, 10.14. Found: C, 78.29; H, 6.03; N, 10.01. 1H NMR (400 MHz, DMSO-d6, δ): 8.86 (s, 1H, CH = N), 8.50 (d, J = 8.8 Hz, 1H, Quinoline–H3), 8.32 (d, J = 8.4 Hz, 1H, Quinoline–H6), 8.16 (d, J = 8.4 Hz, 1H, Quinoline–H2), 8.07 (d, J = 8.0 Hz, 1H, Quinoline–H9), 7.86 (t, J = 7.2 Hz, 1H, Quinoline–H8), 7.70 (t, J = 7.6 Hz, 1H, Quinoline–H7), 7.22 (d, J = 7.6 Hz, 1H, Phene–H5), 7.06 (s, 1H, Phene–H2), 6.95 (d, J = 8.0 Hz, Phene–H6), 3.90 (s, 3H, –OCH3), 2.20 (s, 3H, –CH3) ppm. 13C NMR (100 MHz, DMSO-d6, δ): 160.21, 158.38, 155.04, 149.76, 147.90, 137.34, 131.19, 130.62, 129.65, 128.89, 128.53, 128.27, 125.29, 118.57, 113.85, 104.10, 55.81, 16.25 ppm. UV–vis (λmax, CH3CN): 252, 298, 348 nm.
ZnL(OAc)2 (1)
Yield: 81.50%, Color: Orange. Anal. calc. for C22H22N2O5Zn: C, 57.47; H, 4.82; N, 6.09. Found: C, 57.55; H, 4.73; N, 6.16. 1H NMR (400 MHz, DMSO-d6, δ): 8.87 (s, 1H, CH = N), 8.54 (d, J = 6.8 Hz, 1H), 8.28 (d, J = 8.8 Hz, 1H), 8.17 (d, J = 7.2 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.85 (t, J = 7.2 Hz, 1H), 7.70 (t, J = 7.6 Hz, 1H), 7.22 (d, J = 7.6 Hz, 1H), 7.08 (s, 1H), 6.98 (d, J = 6.8 Hz, 1H), 3.86 (s, 3H, –OCH3), 2.16 (s, 3H, –CH3), 1.79 (s, 6H, –OOCCH3) ppm. 13C NMR (100 MHz, DMSO-d6, δ): 177.34, 167.76, 160.10, 158.48, 149.29, 131.24, 130.98, 130.96, 129.56, 129.12, 128.63, 125.61, 114.10, 104.31, 55.88, 22.87, 16.32 ppm. UV–vis (λmax, CH3CN): 244, 290, 354 nm.
CdL(OAc)2 (2)
Yield: 85.12%, Color: Lemon-yellow. Anal. calc. for C22H22N2O5Cd: C, 52.14; H, 4.38; N, 5.53. Found: C, 52.20; H, 4.40; N, 5.49. 1H NMR (400 MHz, DMSO-d6): 8.93 (s, 1H, CH = N), 8.59 (d, J = 7.2 Hz, 1H), 8.27 (d, J = 8.4 Hz, 2H), 8.10 (d, J = 8.4 Hz, 1H), 7.87 (t, J = 7.2 Hz, 1H), 7.72 (t, J = 7.2 Hz, 1H), 7.23 (d, J = 7.6 Hz, 1H), 7.15 (s, 1H), 7.04 (d, J = 6.4 Hz, 1H), 3.86 (s, 3H, –OCH3), 2.17 (s, 3H, –CH3), 1.81 (s, 6H, –OOCCH3) ppm. 13C NMR (100 MHz, DMSO-d6, δ): 178.45, 165.67, 160.16, 158.37, 148.39, 143.55, 131.23, 131.08, 130.85, 129.62, 128.63, 126.50, 114.16, 110.43, 106.11, 101.92, 55.90, 22.18, 16.33 ppm. UV–vis (λmax, CH3CN): 252, 300, 354 nm.
[CdL2(NO3)2]·CH3CN (3)
Yield: 50.33%, Color: Orange. Anal. calc. for C38H35N7O8Cd: C, 54.98; H, 4.25; N, 11.81. Found: C, 54.83; H, 4.28; N, 11.75. 1H NMR (400 MHz, DMSO-d6): 8.82 (s, 1H, CH = N), 8.50 (d, J = 8.8 Hz, 1H), 8.28 (d, J = 8.8 Hz, 1H), 8.11 (d, J = 7.6 Hz, 1H), 8.06 (d, J = 8.4 Hz, 1H), 7.83 (t, J = 7.6 Hz, 1H), 7.68 (t, J = 7.2 Hz, 1H), 7.20 (d, J = 7.6 Hz, 1H), 7.02 (s, 1H), 6.94 (d, J = 7.6 Hz, 1H), 3.84 (s, 3H, –OCH3), 2.15 (s, 3H, –CH3), 2.05 (s, 1.5H, CH3CN) ppm. 13C NMR (100 MHz, DMSO-d6, δ): 160.34, 160.08, 158.38, 156.89, 152.35, 147.84, 147.79, 131.24, 130.82, 129.61, 128.63, 128.45, 118.56, 113.98, 110.75, 104.14, 55.87, 16.31, 11.24 ppm. UV–vis (λmax, CH3CN): 248, 230, 374 nm.
Molecular Structure Determination
Crystallographic data were collected on a Rigaku R–AXIS RAPID IP diffractometer with graphite-monochromatized Cu·Kα radiation (λ = 1.54178 Å) at 298 K for complexes 1–3. The structures were solved by the direct methods and refined with full-matrix least-squares on F2 [21]. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were added theoretically. The structural information and results were stored in the Cambridge Crystallographic Data Centre (numbers: 2307441 for 1; 2,330,760 for 2; 2,307,440 for 3).
Computational Details
Gaussian 09 program [22] was employed for DFT calculations. Density functional theory (DFT) calculations were performed using Beck's three-parameter hybrid exchange functional [23] and Lee, Yang and Parr correlation functional [24] B3LYP/6–31G (d). The calculated electronic density plots were prepared using the Gaussview 5.0.8. The Multiwfn [25] and VMD [26] software were used for more detailed analysis.
Results and Discussion
Description of Crystal Structures
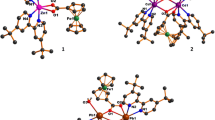
The relevant crystallographic parameters for complexes 1–3 is summarized in Table 1. The complexes ZnL(OAc)2 (1) and CdL(OAc)2 (2) are crystallized in P21/n space group of monoclinic system and \(\text{P}\overline{1 }\) space group of triclinic system, respectively. According to X-ray crystallography analysis, the structures of complexes 1 and 2 are isomorphic, as shown in Fig. 1. The central metal is hexacoordinate, coordinated with quinoline nitrogen (N1), imine nitrogen (N2) and four oxygen atoms (O2, O3, O4, O5) from acetate anions. In the crystal structure of 1, the geometry around the Zn(II) ion is that of an octahedron, the equatorial plane of which is best described by the plane (N1/N2/Zn/O3/O5) and apical sites are taken by two oxygens (O2, O4) of two acetate anions (Fig. S1). In the crystal structure of 2, the octahedral configuration around the Cd(II) ion features an equatorial plane occupied by one nitrogen (N2) and three oxygens (O2, O3 and O5), with the apical positions taken up by one nitrogen (N1) and one oxygen (O4). In both molecular structures, the maximum axial angles of complexes 1 (∠O2–Zn1–O4) and 2 (∠N1–Cd1–O4) are 150.97(15)° and 136.27(10)°, respectively, indicating that the central metal atom is situated in a distorted octahedral environment. Tables S1–S4 present the important bond distances and angles parameters for the two complexes 1 and 2. Notably, the N2–C10 bond distances are 1.264(3) Å for complex 1 and 1.267(4) Å for complex 2, which are consistent with the imine bond in the literature structural data [27, 28]. The M–N bond lengths have significantly differ in the two complexes due to the difference in the radii of zinc and cadmium. Obviously, the bond distances of Zn1–N1 and Zn1–N2 in complex 1 are 2.104(2) Å and 2.142(2) Å, respectively, which are shorter than those of in complex 2, with Cd1–N1 of 2.326(3) Å and Cd1–N2 of 2.351(3) Å. As shown in Fig. S2, the dihedral angles between the quinoline ring (C1-C9/N1) and benzene ring (C11-C16) are 5.494° and 33.981° in complexes 1 and 2, respectively, indicating that the two rings in complex 1 are almost coplanar. Molecules with good coplanarity often possess specific chemical properties and reactivity [29,30,31].
In the crystals of these two complexes, the intermolecular hydrogen bonding interactions (listed in Table 2) further connect the mononuclear molecules to generate a supramolecular architecture. In the supermolecular assembly of 1, no classic hydrogen bonds were found and only weak interactions exhibited intermolecular C–H···O bonds interactions, which played vital role in stabilizing the crystal structure [32, 33]. As shown in Fig. 2, molecules of complex 1 were linked by two hydrogen bonds C3–H3···O4 and C10–H10···O2 to form the different dimers. These dimers were further linked by C7–H7···O3 hydrogen bonding to give rise to 3-D supramolecular structure in space. The molecular packing in the crystals of complex 2 was very distinct from that of complex 1. Two C–H···O hydrogen bonds C10–H10···O3 and C22–H22C···O4 linked adjacent molecules to form a one-dimensional T-shaped chain arrangement along c-axis (Fig. 2).
X-ray crystallography analysis has revealed that complex [CdL2(NO3)2]·CH3CN (3) forms a 2:1 ligand to metal stoichiometry, and crystallizes in the triclinic system with \(\text{P}\overline{1 }\) space group and consists of one Cd(II) ion, two ligand L, and two nitrate anions participating in coordination (Fig. 3). In addition, the asymmetric unit of complex 3 contains a crystalline acetonitrile molecule. Some important bond distances and angles of complex 3 are listed in Tables S5 and S6. Each cadmium ion was associated with two quinoline nitrogen atoms (N1, N3), two imine nitrogen atoms (N2, N4) in the ligand, and four oxygen atoms (O4, O5, O7, O8) from the nitrate ions. The central metal cadmium(II) ion of complex 3 can be best described as a triangular dodecahedron (Fig. 3). Due to the participation of two ligand L molecules in coordination, the structure of complex 3 is relatively distorted, with the dihedral angles between the quinoline ring and the benzene ring being 49.507° (Fig. S3) and 51.396° (Fig. S4), respectively.
In the supermolecular assembly of 3, two C–H···O hydrogen bonds C16–H16···O4 and C30–H30···O3 linked adjacent molecules to form a one-dimensional chain arrangement along b-axis (Fig. 4). In addition, one-dimensional chains were further linked into a two-dimensional layered structure through π–π stacking. For the π–π stacking interaction, the centroid–centroid distance of the two rings (N3/C19-C27) and the two rings (N1/C1-C9) were 3.744 Å and 3.725 Å, respectively. These rings were stacked with a dihedral angle close to 0 ◦.
TGA, FT-IR and NMR Analysis
The thermal stabilities of complexes 1–3 were examined under an N2 atmosphere from 40 to 800 °C, as depicted in Fig. 5. This investigation aimed to verify the crystalline solvent content and assess the structural stability of these complexes. For complex 1, its TGA curve showed weight loss only when the temperature reached 217 °C, and the product above 600 °C might be ZnO (found: 17.41%; calcd: 17.38%). Similarly, complex 2 began to undergo structural collapse and ligand decomposition at temperatures above 188 °C, and the product above 600 °C should be CdO (found: 25.72%; calcd: 25.63%). For complex 3, the crystallized solvent molecule acetonitrile was released in the temperature range of 78 to 153 °C (found: 4.90%; calcd: 4.95%), and a second weight loss occurred above 238 °C along with structural collapse and ligand decomposition. The final product above 600 °C may be CdO (found: 15.62%; calcd: 15.65%). From the results of the analysis, complex 3 containing crystalline solvent (238 °C) exhibited higher thermal stability compared to complexes 1 (217 °C) and 2 (188 °C).
The FT-IR spectra of the free ligand L and complexes 1–3 (Figs. S5–S8) provide the significant information about the binding of ligand L to the metal atom. Table 3 presents the stretching frequencies for υ(Ar–H), υ(–CH3), υ(C = N), υ(C–O), and δ(–CH3) modes of ligand L with corresponding shifts. In this paper, the Schiff base ligand L can form coordination bonds with Zn(II)/Cd(II) ions via the quinoline nitrogen and imine groups. In complexes 1–3, the stretching bands in the 3046–3079 cm–1 region are assigned to the (C–H) of aromatic ring, bands at 1609–1618 cm–1 signify the (C = N) of imine group, 1228–1234 cm–1 represent the (C–O) corresponding to the methoxy group [34, 35]. Moreover, the stretching vibration and the deformation vibration peaks of –CH3 were found in the FT-IR spectra of complexes 1–3. Due to the formation of complexes, the IR bands of complexes 1–3 were slightly shifted compared with those of ligand L. Notably, at 1628 cm−1, the ligand L has a a characteristic C = N stretching band, which is shifted to lower frequencies by 10–19 cm–1 in complexes 1–3, implying the coordination of the imine nitrogen and Zn(II)/Cd(II) ions [36, 37]. In addition, the new non-ligand stretching bands in the low-frequency regions 519–522 cm–1 are attributed to υ(M–N) [38, 39], which can also be evidence for the formation of M–N coordination bond.
The 1H NMR spectra of the non-magnetic zinc/cadmium complexes provide further evidence for the ligand L bonding mode (Figs. S9–S12). The 1H NMR spectra of L and 1–3 were recorded in DMSO–d6 at room temperature. Compared with the 1H NMR data of ligand L (δ 8.86 ppm), the proton signals of the imine (–CH = N) group appear as singlets at δ 8.87, 8.93, and 8.82 ppm for complexes 1–3, respectively. The chemical shifts of imine proton hydrogen are slightly changed due to the coordination of imine nitrogen with Zn(II)/Cd(II). The proton hydrogens located on the quinoline ring and benzene ring of complexes 1–3 exhibit a precise one-to-one correspondence with their respective chemical structures. Detailed data of NMR spectra, including the chemical shifts, peak patterns, coupling constants of different hydrogen/carbon are listed in Section 2.2. In addition, the proton signals of the methoxy (–OCH3) group appear as singlets at δ 3.86, 3.86, and 3.84 ppm, protons of the methyl (–CH3) group attached to the phene ring as singlet at δ 2.16, 2.17, and 2.15 ppm in complexes 1–3, respectively. In contrast to the ligand L, the presence of the protons of the acetate (–OOCCH3) group signals was observed in complexes 1 and 2, located at 1.79 and 1.81 ppm, respectively. Additionally, the proton peaks of crystallized acetonitrile molecules (δ 2.05 ppm) appeared in complex 3, which is consistent with the structure analyzed by single-crystal X-ray diffraction. The integration values of different proton are consistent with the number of proton atoms in the structure of complexes 1–3, and no organic impurities were observed [40, 41].
In the 13C NMR spectra of the ligand L and complexes 1–3 (Figs. S13–S16), the peaks were consistent in accordance with the total number of carbon atoms in the structure. The peaks corresponds to characteristic imine (–CH = N) carbons, which appeared at 160.21, 177.34, 178.45, 160.34 ppm in L and 1–3, respectively. Peaks are observed in the ranges of 55.81–55.90 ppm and 16.25–22.87 ppm, corresponding to –OCH3 carbon and –CH3 carbon in the structure respectively. To sum up, 1H and 13C chemical shifts in the NMR spectra of complexes 1–3 display the expected shifts of those resonances due to proton and carbon atoms close to N donor atoms involved in bonding to zinc/cadmium.
UV–Vis Spectroscopy
The electronic spectra of the ligand L and its complexes 1–3 (2 × 10–5 mol L–1) were recorded in acetonitrile solution. As shown in Fig. 6, the absorption bands of complexes 1–3 are similar to those of L. The ligand L shows three peaks at 252, 298 (sh), and 348 nm. The high-energy bands at 252 and 298 (sh) nm belong to the π → π* transition of the quinoline ring, and the low energy bands at 348 nm are mainly caused by n → π* transitions of imine group (–CH = N) [42, 43]. the UV–Vis absorption data are summarized in Table 4. The extinction coefficients of these bands fall into the range 104–105 M−1 cm−1. The red shift of peaks in complexes 1–3 is mainly due to ligand-based transitions, displaying that the quinoline nitrogen atom and imine nitrogen atom are coordinated with Zn(II)/Cd(II) ions [44, 45]. Due to the presence of d10 electron configuration in Zn(II)/Cd(II), no low-energy d → d transition was observed in 1–3 [46, 47].
Fluorescence Properties
As we mentioned before, the Zn(II)/Cd(II) complexes can serve as potential luminescent materials for organic light-emitting diode (OLED) applications [48]. Therefore, we investigated the solid-state luminescent properties of complexes 1–3 at room temperature. As shown in Fig. 7, the ligand L and complexes 1–3 exhibit similar fluorescence emission bands, with maximum emission peaks are located at 518, 564, 524, and 542 nm, respectively. These values exhibit a red shift relative to the position of the maximum emission peak in acetonitrile solution. The fluorescence data are summarized in Table 4. The photoluminescence properties of the Zn(II)/Cd(II) complexes are primarily attributed to intramolecular ligand emissions, which are due to the presence of the d10 electronic configuration [49, 50]. Compared to the free ligand L, the fluorescence emission peaks of complexes 1–3 exhibit a significant red shift and an increase in fluorescence intensity. This can be attributed to the enhanced structural rigidity of complex resulting from coordination [51]. In addition, the differences in fluorescence intensity among complexes 1–3 may be related to the heavy atom effect of cadmium [52]. Under the irradiation of a 365 nm UV lamp, the Zn(II) complex 1 exhibits bright orange-yellow fluorescence (Fig. 7), which may be related to the good coplanarity of its structure (the dihedral angle is 5.494°, close to 0°). The fluorescence of complexes 1–3 in acetonitrile solution is weak (Fig. 7), which may be due to solution quenching [53, 54]. These results show that complexes 1–3 can be used as a potential luminescent materials.
DFT Studies
We calculated the HOMO and LUMO of ligand L and complexes 1–3 using the B3LYP method with the 6–31G(d)/LANL2DZ basis set. The optimized geometries are derived from the single-crystal structures of complexes 1–3. The electron clouds of the highest occupied molecular orbital (HOMO) of L and 1–3 are primarily distributed over the benzene rings and C = N double bonds, whereas the electron clouds of the lowest unoccupied molecular orbital (LUMO) are localized on the quinoline rings. Consequently, the transitions observed in complexes 1–3 can be ascribed to metal-perturbed ligand internal π → π* transitions. As depicted in Fig. 8, the energy gaps between HOMO and LUMO of L and 1–3 are 3.53, 3.02 eV, 3.08 eV and 3.30 eV, respectively. A smaller energy gap implies a longer maximum absorption wavelength [55], which is generally consistent with their experimental spectroscopic results.
ESP Analysis
The electrostatic surface potential (ESP) pertains to the distribution of electrostatic potential across a specific surface enveloping a molecule. It is intimately associated with the electronegativity, electron density, partial charges, dipole moment, and chemical reactivity inherent to the molecule [56, 57]. ESP stands as a pivotal instrument in the elucidation and anticipation of molecular reactions. Divergent colors are employed to visualize the magnitudes of electrostatic potential across distinct surface areas, thereby offering a precise representation of the electrostatic potential distribution on the molecular surface [58]. When the ESP exhibits blue on the molecular surface, it denotes the presence of a negative electrostatic potential, thereby implying a tendency towards the occurrence of electrophilic reactions [59]. As seen from Fig. 9 in ligand L and complexes 1–3, the regions exhibiting negative potential are situated around the benzene rings and nitrogen atoms, suggesting that hydrogen bonds can readily form in this particular region [27].
Conclusions
In this study, we studied the synthesis, characterizations and crystal structures of three Zn(II) and Cd(II) complexes of a Schiff base, (E)-N-(3-methoxy-4-methylphenyl)-1-(quinolin-2-yl)methanimine. In complexes 1–3, intermolecular C-H···O hydrogen bonds and π–π stacking interactions connect molecules to form different supermolecular structures, which further stabilize the crystal structures. Further, fluorescence analysis of complexes 1–3 showed that the coordination interaction between the metal and ligand enhances the fluorescence emission intensity and leads to a red shift in emission. The solid-state fluorescence emissions of 1–3 were observed at 564, 524, and 564 nm, respectively, exhibiting bright yellow-green fluorescence, suggesting their potential as luminescent materials. In addition, the mechanism of fluorescence enhancement in complexes was verified through theoretical calculations.
Data Availability
The data used to support the findings of this study are included within the article.
References
Whiteoak CJ, Salassaa G, Kleij AW (2012) Recent advances with π-conjugated salen systems. Chem Soc Rev 41:622–631. https://doi.org/10.1039/c1cs15170c
Li XJ, Xie SQ, Hu YZ, Xiang J, Wang LM, Li RL, Chen M, Wang FB, Liu Q, Chen XQ (2021) AIEgen modulated per-functionalized flower-like IRMOF-3 frameworks with tunable light emission and excellent sensing properties. Chem Commun 57:2392–2395. https://doi.org/10.1039/d0cc08403d
Laube C, Taut JA, Kretzschmar J, Zahn S, Knolle W, Ullman S, Kahnt A, Kersting B, Abel B (2020) Light controlled oxidation by supramolecular Zn(II) Schiff-base complexes. Inorg Chem Front 7:4333–4346. https://doi.org/10.1039/d0qi00980f
Li T, Wang YF, Yin Z, Li J, Peng X, Zeng MH (2022) The sequential structural transformation of a heptanuclear zinc cluster towards hierarchical porous carbon for supercapacitor applications. Chem Sci 13:10786–10791. https://doi.org/10.1039/d2sc03987g
Li L, Wang L, Peng X, Tao S, Zeng MH (2022) Nickel–salen as a model for bifunctional OER/UOR electrocatalysts: pyrolysis temperature–electrochemical activity interconnection. Inorg Chem Front 9:1973–1983. https://doi.org/10.1039/d2qi00226d
Mondal I, Basak T, Banerjee S, Chattopadhyay S (2020) A theoretical insight on the rigid hydrogenbonded network in the solid state structure of two zinc(II) complexes and their strong fluorescence behaviors. CrystEngComm 22:3005–3019. https://doi.org/10.1039/d0ce00125b
Nirumand L, Farhadi S (2021) Cobalt(II) Schiff base chemically grafted onto magnetic amino-functionalized reduced graphene oxide nanosheets for highly rapid and selective removal of methyl orange. New J Chem 45:11946–11959. https://doi.org/10.1039/d1nj02118d
Mazet C, Jacobsen EN (2008) Dinuclear (salen)Al complexes display expanded scope in the conjugate cyanation of α, β-unsaturated imides. Angew Chem Int Ed 47:1762–1765. https://doi.org/10.1002/chin.200827090
Chakraborty T, Mukherjee S, Parveen R, Chandra A, Samantac D, Das D (2021) A combined experimental and theoretical rationalization of an unusual zinc(II)-mediated conversion of 18-membered Schiff-base macrocycles to 18-membered imine–amine macrocycles with imidazolidine side rings: an investigation of their bio-relevant catalytic activities. New J Chem 45:2550–2562. https://doi.org/10.1039/d0nj05635a
Koo BH, Lim KS, Ryu DW, Lee WR, Kohb EK, Hong CS (2013) Synthesis, structures and magnetic characterizations of isostructural tetranuclear Ln4 clusters (Ln = Dy, Ho, and Eu). Dalton Trans 42:7204–7209. https://doi.org/10.1039/c3dt00056g
Qiao D, Wang JY, Zhang LY, Dai FR, Chen ZN (2019) Aggregation-induced emission enhancement and reversible mechanochromic luminescence of quinoline-based zinc(II)–Schiff base complexes. Dalton Trans 48:11045–11051. https://doi.org/10.1039/c9dt02177a
Yuan GZ, Zhang Q, Wang ZP, Song K, Yuan XM, Wang YZ, Zhang LY (2017) Assembly of four 8-quinolinate-based multinuclear complexes: the effect of substituents on core structures and photoluminescent properties. Inorg Chem Fron 4:764–772. https://doi.org/10.1039/c7qi00082k
Bella SD (2021) Lewis acidic zinc(II) salen-type Schiff-base complexes: sensing properties and responsive nanostructures. Dalton Trans 50:6050–6063. https://doi.org/10.1039/d1dt00949d
Yan XJ, Song XX, Mu XY, Wang Y (2019) Mechanochromic luminescence based on a phthalonitrile-bridging salophen zinc(II) complex. New J Chem 43:15886–15891. https://doi.org/10.1039/c9nj03704g
Nurin Sakinatul Hayati Haji Damit, Malai Haniti Sheikh Abdul Hamid, Nur Sabrina Rahayu Haji Abdul Rahman, Siti Nor Hashimah Haji Ilias, Natasha Ann Keasberry (2021) Synthesis, structural characterisation and antibacterial activities of lead(II) and some transition metal complexes derived from quinoline-2-carboxaldehyde 4-methyl-3-thiosemicarbazone, Inorg Chim Acta 527:120557. https://doi.org/10.1016/j.ica.2021.120557
Ibrahim MA, Emara AAA, Taha A, Adly OMI, Nabeel AI, Aziz MA, Salah N (2023) Synthesis, characterization, TD-DFT, molecular docking, biological applications, and solvatochromic studies of some new metal complexes derived from semicarbazone of pyrano[3,2-c]quinoline-3-carboxaldehyde. Appl Organomet Chem 37:e7169. https://doi.org/10.1002/aoc.7169
Damena T, Alem MB, Zeleke D, Desalegn T, Eswaramoorthy R, Demissie TB (2022) Novel zinc(II) and copper(II) complexes of 2-((2-hydroxyethyl)amino)quinoline-3-carbaldehyde for antibacterial and antioxidant activities: a combined experimental, DFT, and docking studies. ACS Omega 30:26336–26352. https://doi.org/10.1021/acsomega.2c02205
Ilmi R, Kansız S, Al Rasbi NK, Husband J, Dege N, Khan MS (2023) Synthesis, X-ray crystal structure and determination of non-covalent interactions through Hirshfeld surface analysis of a pure red emitting asymmetrical octacoordinated Sm(III) complex. Polyhedron 246:116673. https://doi.org/10.1016/j.poly.2023.116673
Liu X, Hamon JR (2019) Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord Chem Rev 389:94–118. https://doi.org/10.1016/j.ccr.2019.03.010
Dong YW, Wang Y, Song Y, Yu Z, Yu Z, Zhao Z, Wang LY (2024) A simple and rapid quinoline Schiff base as a fluorescent probe for Zn2+ and its application in test strips. ChemistrySelect 9:e202400389. https://doi.org/10.1002/slct.202400389
Sheldrick GM (1999) SHELXTL NT Crystal Structure Analysis Package, Version 5.10, Bruker AXS, Analytical X-ray System, Madison, WI
Huang H, Wang YX, Wang B, Zhuang SQ, Pan B, Yang X, Wang L, Yan CL (2013) Controllably tunable phenanthroimidazole–carbazole hybrid bipolar host materials for efficient green electrophosphorescent devices. J Mater Chem C 1:5899–5908. https://doi.org/10.1039/c3tc30832d
Chen S, Wu YK, Zhao Y, Fang DN (2015) Deep blue organic light-emitting devices enabled by bipolar phenanthro[9,10-d]imidazole derivatives. RSC Adv 5:72009–72018. https://doi.org/10.1039/c5ra13814k
Yuan Y, Chen JX, Lu F, Tong QX, Yang QD, Mo HW, Ng TW, Wong FL, Guo ZQ, Ye J, Chen Z, Zhang XH, Lee CS (2013) Bipolar phenanthroimidazole derivatives containing bulky polyaromatic hydrocarbons for nondoped blue electroluminescence devices with high efficiency and low efficiency roll-off. Chem Mater 25:4957–4965. https://doi.org/10.1021/cm4030414
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Yan YB, Yang RW, Zhang HW, Zhang Y, Dong WK (2024) Crystal structure and luminescent mechanochromism of a quinoline-appended acylhydrazone ligand and its Zn(II) complex. J Mol Struct 1299:137148. https://doi.org/10.1016/j.molstruc.2023.137148
Chai LQ, An HL, Chen TT, Cai YY (2024) Structural, spectroscopic, theoretical calculation and Hirshfeld surface analyses of 3-D supramolecular dinuclear zinc(II) and copper(II) complexes. J Mol Struct 1299:137151. https://doi.org/10.1016/j.molstruc.2023.137151
Leong DW, Shao YW, Ni Z, Bhuvanesh N, Ozerov OV (2024) A bis(PCN) palladium pincer complex with a remarkably planar 2,5-diarylpyrazine core. Dalton Trans 53:6520–6523. https://doi.org/10.1039/d3dt04248k
Kumar D, Muniappan K, Chinnusamy S, Shanmugam E (2024) Substituent position dependent photophysical properties of indole-barbituric and thiobarbituric acid based molecular rotors. ChemistrySelect 9:e202301776. https://doi.org/10.1002/slct.202301776
Ji XZ, Cheng HW, Schuster NJ, LeCroy GS, Zhang S, Wu YL, Michalek L, Nguyen BNT, Chiong JA, Schrock M, Tomo Y, Rech J, Salleo A, Gam S, Lee GH, Tok JBH, Bao ZA (2023) Tuning the mobility of indacenodithiophene-based conjugated polymers via coplanar backbone engineering. Chem Mater 6:256–265. https://doi.org/10.1021/acs.chemmater.3c02006
Guerrero M, Pou R, Bay´es-García L, Font-Bardia M, Sort J, Pons J, Ayll´on JA (2018) Syntheses, supramolecular architectures and photoluminescence properties of Zn (II) complexes based on 3,5-dihydroxybenzoic and pyridine/pyrazole derived ligands. Inorg Chem Commun 96:34–38.https://doi.org/10.1016/j.inoche.2018.07.047
Xu ZX, Yu TZ, Zhao YL, Zhang H, Zhao GY, Li JF, Chai LQ (2016) A new starshaped carbazole derivative with polyhedral oligomeric silsesquioxane core: crystal structure and unique photoluminescence property. J Fluoresc 26:149–154. https://doi.org/10.1007/s10895-015-1694-y
Mukherjee D, Sarkar K, Reja S, Bakibillah M, Guha S, Mandal NK, Naskar JP, Das RK (2023) Mononuclear Zn(II) complex based on N2O ligand compartment: first case to detect nitro explosives. J Fluoresc. https://doi.org/10.1007/s10895-023-03431-9
Li J, Ren HJ, Li JT (2023) Application values of two Cu(II) schiff base coordination complexes on blue fluorescent materials. J Fluoresc. https://doi.org/10.1007/s10895-023-03423-9
El-Shalakany H, Ramadan R, Sayed M (2024) New bivalent metal chelates based on an NO-donor Schiff base ligand: synthesis, structural characterization, DFT simulation, biological evaluation, and molecular docking analysis. Inorg Chem Commun 159:111826. https://doi.org/10.1016/j.inoche.2023.111826
Marchetti F, Pettinari R, Verdicchio F, Tombesi A, Scuri S, Xhafa S, Olivieri L, Pettinari C, Choquesillo-Lazarte D, García-García A, Rodríguez-Diéguez A, Galindo A (2022) Role of hydrazone substituents in determining the nuclearity and antibacterial activity of Zn(II) complexes with pyrazolone-based hydrazones. Dalton Trans 51:14165–14181. https://doi.org/10.1039/d2dt02430f
Paul S, Barman P (2024) Exploring diaminomaleonitrile-derived Schiff base ligand and its complexes: synthesis, characterization, computational insights, biological assessment, and molecular docking. J Mol Struct 1296:136941. https://doi.org/10.1016/j.molstruc.2023.136941
Elantabli F, Mohamed R, El-Medani S, Haukka M, Ramadan R, Afifi M (2024) Structural investigations of new tridentate-phenylacetohydrazide Schiff base metal chelates: X-ray diffraction, Hirshfeld surface analyses, DFT, antibacterial and molecular docking studies. J Mol Struct 1299:137230. https://doi.org/10.1016/j.molstruc.2023.137230
Bal M, Köse A (2024) Schiff bases containing 1,2,3-triazole group and phenanthroline: synthesis, characterization, and investigation of DNA binding properties. J Photochem Photobiol A Chem 448:115320. https://doi.org/10.1016/j.jphotochem.2023.115320
Kose A, Erkan S, Tümer M (2023) A series of phenanthroline-imine compounds: Computational, OLED properties and fluorimetric sensing of nitroaromatic compounds. Spectrochim, Acta Part A Mol Biomol Spectrosc 286:122006. https://doi.org/10.1016/j.saa.2022.122006
Divyashree NR, Hosakere DR, Yathirajan HS, Bhavya NR, Mahendra M, Muzaffar I, Chandan S, Raghavendra G, Shiva P (2023) Highly selective and sensitive fluorescent “TURN-ON” furan-based Schiff base for zinc(II) ion probing: chemical synthesis, DFT studies, and X-ray crystal structure. New J Chem 44:17420–17433. https://doi.org/10.1039/d3nj02466k
Naskar B, Dhara A, Maiti D, Kukułka M, Mitoraj M, Srebro-Hooper M, Prodhan C, Chaudhuri K, Goswami S (2019) Aggregation induced emission based sensing platform for selective detection of Zn2+: experimental and theoretical investigations. ChemPhysChem 20:1630–1639. https://doi.org/10.1002/cphc.201900113
Himadri PG, Anmol S, Pranjit B, Diganta C (2022) A new potential ONO Schiff-Base ligand and its Cu(II), Zn(II) and Cd(II) Complexes: synthesis, structural elucidation, theoretical and bioactivity studies. Inorg Chem Commun 146:110153. https://doi.org/10.1016/j.inoche.2022.110153
Kargar H, Fallah-Mehrjardi M, Behjatmanesh-Ardakani R, Munawar KS, Ashfaq M, Tahir MN (2022) Diverse coordination of isoniazid hydrazone Schiff base ligand towards iron(III): synthesis, characterization, SC-XRD, HSA, QTAIM, MEP, NCI, NBO and DFT study. J Mol Struct 1250:131691. https://doi.org/10.1016/j.molstruc.2021.131691
Damena T, Alem MB, Zeleke D, Desalegn T, Eswaramoorthy R, Demissie TB (2022) Synthesis, characterization, and biological activities of zinc(II), copper(II) and nickel(II) complexes of an aminoquinoline derivative. Front Chem 10:1–21. https://doi.org/10.3389/fchem.2022.1053532
Majumdara D, Dasc D, Sreejithd S, Nage S, Deyb S, Mondalb S, Bankuraa K, Mishra D (2019) Synthesis, characterizations and single crystal structure of di-nuclear azidobridged Cd(II) coordination polymer with Schiff base precursor (H2LpentOMe): DFT, fluorescence, solvatochromism and in vitro antimicrobial assay. Inorg Chim Acta 496:119069. https://doi.org/10.1016/j.ica.2019.119069
Barwiolek M, Jankowska D, Chorobinski M, Kaczmarek-Kędziera A, Łakomska I, Wojtulewskic S, Muzioła T (2021) New dinuclear zinc(II) complexes with Schiff bases obtained from o-phenylenediamine and their application as fluorescent materials in spin coating deposition. RSC Adv 11:24515–24525. https://doi.org/10.1039/d1ra03096e
Ullmann S, Schnorr R, Laube C, Abel B, Kersting B (2018) Photoluminescence properties of tetrahedral zinc(II) complexes supported by calix[4]arene-based salicylaldiminato ligands. Dalton Trans 47:5801–5811. https://doi.org/10.1039/c8dt00757h
Wong CC, Tsai MJ, Wu JY (2022) Zinc– and copper–salicyaldimine complexes: Simultaneous observation of both metal–ligand coordination and weak CH···N contact about a single N-donor and the transmetallation reactions. J Solid State Chem 316:123622. https://doi.org/10.1016/j.jssc.2022.123622
Tsai MJ, Wong CC, Wu JY (2023) Structures, photophysical properties, and optical sensing of zinc– and copper–salicyaldimine complexes. J Photochem Photobiol A Chem 443:114808. https://doi.org/10.1016/j.jphotochem.2023.114808
Williams NJ, Gan W, Reibenspies JH, Hancock RD, Steric P (2009) Control of the relative strength of chelation enhanced fluorescence for zinc(II) compared to cadmium(II): metal ion complexing properties of tris(2-quinolylmethyl)amine, a crystallographic, UV-visible, and fluorometric study. Inorg Chem 48:1407–1415. https://doi.org/10.1021/ic801403s
Lin JY, Liu B, Yu MN, Lin ZQ, Zhang XW, Sun C, Cabanillas-Gonzalez J, Xie LH, Liu F, Ou CJ, Bai LB, Han YM, Xu M, Zhu WS, Trevor AS, Paul NS, Donal DCB (2019) Ultrastable supramolecular self-encapsulated wide-bandgap conjugated polymers for large-area and flexible electroluminescent devices. Adv Mater 31:1804811. https://doi.org/10.1002/adma.201804811
Solanki JD, Siddiqui I, Gautam P, Gupta VK, Jou JH, Surati KR (2022) Blue fluorescent Zinc (II) complexes bearing schiff base ligand for solution-processed organic light-emitting diodes with CIEy≤ 0.09. Opt Mater 134:113222. https://doi.org/10.1016/j.optmat.2022.113222
Zhang SN, Huang MM, Lu H, Ma ZY, Wang ZJ, Yang JP (2022) Three-arm star-shaped aniline derivatives: Tunable photoluminescence, aggregation-induced emission and reversible acid-base vapor fluorescence response. J Photochem Photobiol A 432:114098. https://doi.org/10.1016/j.jphotochem.2022.114098
Enbaraj E, Jeyashri KR, Logeshwari G, Manikandan H, Sivakumar K (2023) Synthesis, crystal structure, Hirshfeld surface analysis and theoretical studies of bis(substituted benzylidene)ethan-1,2-diamines. J Mol Struct 1275:134605. https://doi.org/10.1016/j.molstruc.2022.134605
Elangovan N, Alomar SY, Sowrirajan S, Rajeswari B, Nawaz A, Kalanthoden AN (2023) Photoluminescence property and solvation studies on (E)-N-(pyrimidin-2yl)-4-((3,4,5-trimethoxy benzylidede) amino) benzene sulfonamide; synthesis, structural, topological analysis, antimicrobial activity and molecular docking studies. Inorg Chem Commun 155:111019. https://doi.org/10.1016/j.inoche.2023.111019
Vennila M, Rathikha R, Muthu S, Jeelani A, Irfan A (2022) Theoretical structural analysis (FT-IR, FT-R), solvent effect on electronic parameters NLO, FMO, NBO, MEP, UV (IEFPCM model), Fukui function evaluation with pharmacological analysis on methyl nicotinate. Comput Theor Chem 1217:113890. https://doi.org/10.1016/j.comptc.2022.113890
Zhan HB, Zhang HW, Jiang JJ, Wang Y, Fei X, Tian J (2022) Influence of intramolecular hydrogen bond formation sites on fluorescence mechanism. Chin Phys B 31:038201. https://doi.org/10.1088/1674-1056/ac29ad
Funding
This work was supported by National Natural Science Foundation of China (22102107 and 22302131), the Young and Middle-aged Science and Technology Innovation Talents Program of Shenyang (RC220317).
Author information
Authors and Affiliations
Contributions
Zhiyu Jia: writing–original draft, methodology, investigation, data curation. Jiahui Cao: methodology, investigation, data curation. Wei Chen: Crystal structure analysis. Zhou Yu: investigation, resources. Yangyang Song: DFT studies, resources. Yuwei Dong: writing–original draft, methodology, investigation.
Corresponding authors
Ethics declarations
Ethical Approval
As this work does not deal with any human or animal object so no need of ethical approval is needed.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, Z., Cao, J., Chen, W. et al. Synthesis, Crystal Structure, Fluorescence and Theoretical Calculations of Three Zn(II)/Cd(II) Complexes with Bis-dentate N,N-Quinoline Schiff Base. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03786-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03786-7