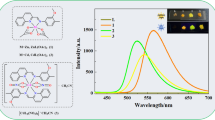
Abstract
Two newly designed dinuclear ZnII and CoII complexes [Co2(L)2]·H2O (1) and [Zn2(L)2]·H2O (2) of a coumarin-based N2O2-donor ligand H2L (5′-(N,N′-diethylamino)-6′-methyl-2,2′-[ethylenedioxybis(nitrilomethylidyne)]phenolcoumarinphenol) were synthesized and characterized by physicochemical and spectroscopic methods. Complexes 1 and 2 are extremely rare asymmetric binuclear structures. The structures of complexes 1 and 2 are isostructural. In both complexes, one metal atom (Co2 or Zn1) adopts a twisted square pyramid configuration, while another metal atom (Co1 or Zn2) adopts a twisted triangular bipyramid configuration with penta-coordination. In addition, complexes 1 and 2 form 3D supramolecular structures through intermolecular hydrogen bonding interactions. Furthermore, the Hirshfeld surfaces analyses and fluorescence properties of complexes 1 and 2 have been discussed in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salen-like complexes have been continuously developed in different fields due to their special three-dimensional structures and unique electronic structures [1,2,3,4,5]. Later, the salamo-like ligands with a N2O2 coordination environment were obtained by modifying the salen-like ligands, which have the advantage of introducing more negatively charged oxygen atoms into the diamine to enhance its stability. Therefore, salamo-like complexes have enriched the types of complexes in structure, have been greatly supplemented in the field of supramolecular chemistry and have played an indispensable role in the field of coordination chemistry [6,7,8,9,10,11]. These metal complexes are used as catalysts [12], magnetic materials [13,14,15,16,17], nonlinear optical materials [18,19,20], luminescence materials [21,22,23,24,25,26,27,28,29,30], molecular identifiers [31,32,33], supramolecular architectures [34,35,36,37], dye and pigments [38], have application in biological fields [3] among other applications, and have great future prospects. In recent years, through the study of the fluorescent properties of metal complexes with different structures, it has been found that for many metal complexes, the interaction between the metal ions and the ligands changes the electronic excited state of the metal center, so that these metal complexes show many interesting fluorescent properties [39,40,41,42].
In this paper, two new dinuclear CoII and ZnII complexes were synthesized by the reactions of H2L with Co(OAc)2·4H2O and Zn(OAc)2·2H2O, respectively; the Hirshfeld surfaces analyses and fluorescence properties of complexes 1 and 2 were investigated.
Experimental
Materials and methods
4-(N,N’-Diethylamino)salicylicaldehyde (99%) and 7-hydroxy-4-methylcoumarin (98%) were purchased from Alfa Aesar (New York, NY, USA) and used without further purification. All other reagents and chemicals are from Tianjin Chemical Reagent Factory. The melting points were measured via an X4 microscopic melting point meter from Beijing Taike Instrument Co., Ltd. Carbon, hydrogen and nitrogen elemental analysis data are obtained from the GmbH VarioEL V3.00 automatic elemental analyzer. Elemental analysis of CoII and ZnII was detected by IRIS ER/S-WP-1 ICP atomic emission spectrometer (Berlin, Germany). UV/Vis spectra were measured by a Shimadzu UV-2550 spectrometer (Hitachi, Tokyo, Japan). The fluorescence spectra were measured on an F-7000 FL spectrophotometer (Hitachi, Tokyo, Japan). The infrared spectra were measured with a VERTEX-70 FT-IR spectrophotometer (Bruker, Germany), and the sample was prepared as KBr (500–4000 cm−1). The single-crystal X-ray structures were determined on a Bruker APEX-II CCD diffractometer.
Preparation of H2L
H2L was prepared according to similar method previously reported [43]. 1,2-Bis(aminooxy)ethane and 8-formyl-7-hydroxy-4-methylcoumarin were prepared according to previous literature [44, 45].
4-(N,N’-diethylamino)-2-[O-(1-ethyloxyamide)]oximephenol: 4-(N, N’-diethylamino) salicylicaldehyde (385.6 mg, 2.00 mmol) was dissolved in a methanol solution (25 mL) and slowly added to a methanol solution (50 mL) of 1,2-bis(aminooxy)ethane (184.3 mg, 2.00 mmol) for 4 h. The above solution was heated at 55 °C for about 5 h. After cooling to room temperature, the red-brown oil obtained by distillation under reduced pressure was separated by column chromatography using ethyl acetate/chloroform (1:20, V/V), and 78.5 mg white solid 4-(N,N’-diethylamino)-2-[O-(1-ethyloxyamide)]oximephenol was gained by distillation under reduced pressure.
H2L: a above-obtained 4-(N,N’-diethylamino)-2-[O-(1-ethyloxyamide)]oximephenol (262.21 mg, 1.00 mmol) in ethanol (25 mL) was added to a solution of 4-methyl-7-hydroxy-8-formylcoumarin (204.17 mg, 1.00 mmol) in ethanol (30 mL). The mixed solution was heated to ca. 80 °C and refluxed for 4–5 h. The solution was cooled to room temperature to obtain a white precipitate H2L, filtered and dried, as given in Scheme 1. Yield 51.7%. m.p.148–149 °C. Anal. Calcd. for C24H27N3O6 (%): C, 63.56; H, 6.00; N, 9.27. Found: C, 63.72; H, 5.89; N, 9.08.
Preparation of complex 1
A solution of Co(OAc)2·4H2O (2.50 mg, 0.01 mmol) in ethanol (5 mL) was added to a solution of H2L (4.53 mg, 0.01 mmol) in acetone (5 mL) at room temperature. The color of the mixture turned to red-brown immediately, and then, the mixture was stirred at room temperature for about 30 min. The mixture was filtered off, and the filtrate was allowed to stand at room temperature for about 1 week. The solvent was partially evaporated, and a transparent red block single crystal suitable for X-ray crystallographic analysis was obtained. Anal. Calcd. for C48H52Co2N6O13 (1038.84) (%): C, 55.50; H, 5.05; N, 8.09; Co, 11.35. Found: C, 51.72; H, 5.01; N, 7.95; Co, 11.12.
Preparation of complex 2
A solution of Zn(OAc)2·2H2O (2.20 mg, 0.01 mmol) in ethanol (5 mL) was added to a solution of H2L (4.53 mg, 0.01 mmol) in dichloromethane (5 mL) at room temperature. The mixture is colorless, and then, the mixture was stirred at room temperature for about 30 min. The mixture was filtered off, and the filtrate was allowed to stand at room temperature for about 1 week. The solvent was partially evaporated, and a transparent colorless needle-like single crystal suitable for X-ray crystallographic analysis was obtained. Anal. Calcd. for C48H52Zn2N6O13 (1051.73) (%): C, 54.82; H, 4.98; N, 7.99; Zn, 12.43. Found: C, 54.98; H, 5.02; N, 7.84; Zn, 12.27.
Crystal structure determinations of complexes 1 and 2
X-ray diffraction data of complexes 1 and 2 were collected at 100.01(10) K, use a BRUKER SMART APEX-II CCD diffractometer with graphite monochromated Cu-Ka and Mo–Ka radiations (λ = 1.54178 Å and λ = 0.71073 Å), respectively. The LP factor and semiempirical absorption corrections were applied to the intensity data. Then, the structures were solved using SHELXS-1997 [46] and by a full matrix least-squares technique based on F2 using SHELXL-2014 and SHELXL-2015 [47]. The anisotropic thermal parameters are assigned to all non-hydrogen atoms. Contributions to scattering due to these highly disordered solvent molecules were removed using the SQUEEZE routine of PLATON; the structures were then refined again using the data generated. Crystal data and experimental parameters relevant to the structure determinations with respect to complexes 1 and 2 are listed in Table 1.
Results and discussion
Salamo-like complexes 1 and 2 have been appropriately prepared and characterized by IR, UV–Vis and fluorescence spectroscopy, X-ray crystallography and Hirshfeld surfaces analyses.
FT-IR spectra
Infrared spectra of H2L and its complexes 1 and 2 exhibited different bands in 4000–500 cm−1 region (Table 2). The IR spectral details of H2L and its complexes 1 and 2 are given in Fig. 1. The O–H stretching band of H2L is found at ca. 3417 cm−1 that belongs to the phenolic hydroxyl groups [8]. However, these bands are not observed in complexes 1 and 2, indicating that the phenolic O–H groups of H2L are fully deprotonated. New O–H stretching vibration bands in complexes 1 and 2 are observed at around 3437 and 3442 cm−1 corresponding to crystallizing water molecules [26]. The C=N stretching vibration band of H2L is observed at ca. 1634 cm−1, while those of complexes 1 and 2 are found at ca. 1617 and 1621 cm−1. The typical C=N stretching frequencies are moved to lower frequencies, showing that the CoII and ZnII atoms are coordinated to the oxime nitrogen atoms [9]. The typical Ar–O stretching band of H2L emerges at approximately 1292 cm−1, and after coordination, the stretching bands of complexes 1 and 2 shift to low wave numbers, at approximately 1242 and 1241 cm−1, showing that the Co–O and Zn–O coordinate bonds have been formed [10]. In addition, the stronger absorption band of H2L at 1728 cm−1 is attributed to the stretching vibration of the C=O double bond of coumarin; the C=O stretching vibration of complexes 1 and 2 compared to H2L appears at about 1733 and 1735 cm−1 and is attributed to the formation of M–N/O after coordination between H2L and the metal. New moderate intensity M–O vibrational bands in complexes 1 and 2 appear at 520 and 513 cm−1 [48, 49].
UV–Vis spectra
UV–Vis spectra of the free ligand H2L and its complexes 1 and 2 in 2.5 × 10−5 mol/L−1 ethanol solution are shown in Fig. 2.
The absorption spectra of H2L and its complexes 1 and 2 are given in Fig. 2. In the UV–Vis absorption spectrum of the free ligand H2L, absorption peaks appear at approximately 291, 336 and 368 nm, respectively. The absorption peak at 289 nm is attributed to the π–π* transition of the benzene ring, the second peak at 336 nm could be assigned to the n–π* transition of the C=N bond in the ligand, and the last absorption peak at 368 nm is assigned to the π–π* transition to the carbonyl group in coumarin. Compared with the free ligand H2L, complexes 1 and 2 have obvious absorption peaks at approximately 360 and 366 nm, which are assigned to L → M charge-transfer (LMCT) transitions [50].
Descriptions of the crystal structures
Selected bond lengths and angles are listed separately in Table 3. The corresponding hydrogen bonding interactions of complexes 1 and 2 are summarized in Table 4.
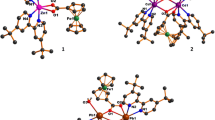
For complexes 1 and 2, all MII atoms are located at the N2O2 site of the deprotonated ligand (L)2− moiety with two atoms (O6 and O12) from the other salamo-type ligand (L)2− moiety, and the two MII atoms are connected in a M–O–M fashion through phenol oxygen atoms, and the crystal structures feature two-fold symmetry. At the same time, the two MII atoms of complexes 1 and 2 are penta-coordinated and adopt slightly twisted trigonal bipyramidal geometries (Co1 and Zn2) and square pyramid configuration (Co2 and Zn1) geometries (Figs. 3b and 5b) [44], which are deduced by calculating the values of τCo1 = 0.57, τCo2 = 0.45, τZn1 = 0.45 and τZn2 = 0.59, respectively [46]. From the calculation results, it can be seen that the τ values of Co1 and Zn2 are both greater than 0.5, forming trigonal bipyramidal geometries, and the τ values of Co2 and Zn1 are less than 0.5, forming square pyramid configurations. X-ray crystal structure measurement shows that complexes 1 and 2 are asymmetric binuclear structures and crystallize in the orthorhombic system, space group Pbca. The structure consists of two MII atoms (M1 and M2), two completely deprotonated (L)2− units, and one lattice water molecule, and forms an extremely rare 2:2 (molar ratio of L2−:MII atom) crystal structure which is different from the structures of 1:1 [19, 22, 51], 2:3 [7, 30, 52], 2:4 [39], 3:3 [19, 22], 4:4 [19], 1:3 [53], 2:6 [54], 1:6 [55] and 1:7 [56] (L:M2+) reported earlier. The molecular structures of complexes 1 and 2 are shown in Figs. 3a and S1a. In the coordination environment of Co2 and Zn1, two phenolic oxygen atoms (O9, O12 or O6, O12) and oxime nitrogen atoms (N4, N5 or N1, N2) are in the cis positions with each other (Figs. 3b and S1b). Due to the long distance between the two MII atoms, there is no interaction between them.
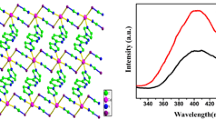
In addition, the supramolecular structures of complexes 1 and 2 are similar. In the crystal structure of complex 1, there are two groups of intramolecular hydrogen bonds and six groups of intermolecular hydrogen bonds; these intermolecular hydrogen bonding interactions form a 3D supramolecular structure, as shown in Table 4 and Fig. 4 [57, 58]. For the crystal structure of complex 2, there are two sets of intramolecular hydrogen bonds and five sets of intermolecular hydrogen bonds as shown in Fig. S2 [59,60,61]. The 3D supramolecular structure of complex 2 is similar to complex 1.
Hirshfeld surfaces analyses
The Hirshfeld surfaces [62,63,64] of complex 1 are mapped over (a) dnorm, (b) the shape index and (c) di in Fig. 5. As shown in Fig. 6, the 2D fingerprint chart shows different intermolecular interactions. In Fig. 5, Hirshfeld surface analysis was performed on complex 1 [65], which shows the surfaces mapped on dnorm, the shape index and di. The bright red area on the depression of Hirshfeld surface indicates the presence of O···H interactions in complex 1, and light red spots indicate C–H···O interactions, while other raised blue areas indicate the presence of C···H, H···H and C···C interactions in complex 1. The Hirshfeld surfaces mapped with dnorm, shape index and di of complex 2 are similar to those of complex 1 because the complexes 1 and 2 are isomorphic. Figure 6 shows the generated 2D fingerprints [66], corresponding to O···H, C···H and H···H interactions on the Hirshfeld surface of complexes 1 (a) and 2 (b), respectively. To provide context, the outline of the full fingerprint is shown in gray, and the blue area shows individual contact. For complexes 1 and 2, the proportions of O∙∙∙H/H∙∙∙O, C∙∙∙H/H∙∙∙C and H∙∙∙H interactions in each molecule are similar to the total surface, while the differences in fingerprint plots of complexes 1 and 2 are caused by different geometrical parameters of disordered Et-groups in these two structures. Because of the existence of these hydrogen bonds, complexes 1 and 2 can be stabilized.
Fluorescence properties
The fluorescence spectra of H2L and its complexes 1 and 2 were measured in ethanol (2.5 × 10−5 M) at room temperature, as shown in Fig. 7. At an excitation wavelength of 350 nm, the ligand H2L has a broad absorption peak at approximate 431 nm, which is attributed to π–π* intraligand transition [67]. Compared with the ligand H2L, the absorption peak intensities of complexes 1 and 2 at 431 nm are significantly different. Compared with the ligand H2L, the fluorescence intensity of complex 1 is significantly reduced, indicating that CoII ions have fluorescence quenching properties. On the other hand, the fluorescence intensity of complex 2 was significantly enhanced due to the coordination of the ligand (L)2− units with ZnII ions and enhanced the coplanarity of the conjugated system.
Conclusion
In this paper, two new dinuclear complexes were synthesized via H2L and Co(OAc)2·4H2O and Zn(OAc)2·H2O, respectively. A series of structural characterizations were performed for complexes 1 and 2. The X-ray single-crystal structure determinations indicate that both CoII and ZnII atoms in complexes 1 and 2 are penta-coordinated. The coordination configurations of the two metal atoms in complexes 1 and 2 are different: one is a twisted trigonal bipyramid configuration, and the other is a twisted square pyramid configuration. The Hirshfeld surfaces and 3D fingerprints can explain the atom-to-pair contact of crystals, which can quantify the intermolecular interactions. In addition, the coordination of CoII and ZnII ions with the ligand (L)2− units results in fluorescence quenching and enhancement, respectively.
Supplementary material
Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication, CCDC Nos. 1975065 and 1975064 for complexes 1 and 2, respectively. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Telephone: +44-01223-762910; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk). These data can be also obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html.
References
Chai LQ, Huang JJ, Zhang HS (2014) Spectrochim Acta A 131:526–530
Sun YX, Pan YQ, Xu X, Zhang Y (2019) Crystals 9:607
Song XQ, Peng YQ, Chen GQ, Wang XR, Liu PP (2015) Inorg Chim Acta 427:13–21
Li J, Zhang HJ, Chang J, Sun YX, Huang YQ (2018) Crystals 8:176
Liu PP, Sheng L, Song XQ, Xu WY, Liu YA (2015) Inorg Chim Acta 434:252–257
Jia HR, Chang J, Zhang HJ, Li J, Sun YX (2018) Crystals 8:272
Yu M, Mu HR, Liu LZ, Li N, Bai Y, Dong XY (2019) Chin J Inorg Chem 35:1109–1120
Chang J, Zhang HJ, Jia HR, Sun YX (2018) Chin J Inorg Chem 34:2097–2107
Dong XY, Zhao Q, Kang QP, Li XY, Dong WK (2018) Crystals 8:230
An XX, Zhao Q, Mu HR, Dong WK (2019) Crystals 9:101
Zhang LW, Zhang Y, Cui YF, Yu M, Dong WK (2020) Inorg Chim Acta 506:119534
Li XY, Kang QP, Liu C, Zhang Y, Dong WK (2019) New J Chem 43:4605–4619
Wang F, Liu LZ, Gao L, Dong WK (2018) Spectrochim Acta A 203:56–64
Zhang LW, Li XY, Kang QP, Liu LZ, Ma JC, Dong WK (2018) Crystals 8:173
Song XQ, Liu PP, Liu YA, Zhou JJ, Wang XL (2016) Dalton Trans 45:8154–8163
Zhang HJ, Chang J, Jia HR, Sun YX (2018) Chin J Inorg Chem 34:2261–2270
Petrick M, Florian B, Katja L, Guntram R, Réne P (2012) Molecules 17:7121–7150
Bella SD, Fragala I (2000) Synth Met 115:191–196
Kang QP, Li XY, Wang L, Zhang Y, Dong WK (2019) Appl Organomet Chem 33:e5013
Azam M, Al-Resayes SI, Trzesowska-Kruszynska A, Kruszynski R, Kuma P (2017) Polyhedron 124:177–183
Liu LZ, Wang L, Yu M, Zhao Q, Zhang Y, Sun YX, Dong WK (2019) Spectrochim Acta A 222:117209
Kang QP, Li XY, Wei ZL, Zhang Y, Dong WK (2019) Polyhedron 165:38–50
Gao L, Liu C, Wang F, Dong WK (2018) Crystals 8:77
Yamamura M, Takizawa H, Sakamoto N, Nabeshima T (2013) Tetrahedron Lett 54:7049–7052
Manna AK, Mondal J, Chandra R, Rout K, Patra GK (2018) Sens Actuators B 10:2317–2326
Pan YQ, Xu X, Zhang Y, Zhang Y, Dong WK (2020) Spectrochim Acta A 229:117917
Pushkarev AP, Balashova TV, Kukinov AA, Arsenyev MV, Yablonskiy AN, Kryzhkov DI, Andreev BA, Rumyantcev RV, Fukin GK, Bochkarev MN (2017) Dalton Trans 46:10408–10417
Zhao Q, An XX, Liu LZ, Dong WK (2019) Inorg Chim Acta 490:6–15
Liu LZ, Yu M, Li XY, Kang QP, Dong WK (2019) Chin J Inorg Chem 35:1283–1294
Zhang Y, Liu LZ, Peng YD, Li N, Dong WK (2019) Transit Met Chem 44:627–639
Wei ZL, Wang L, Wang JF, Guo WT, Zhang Y, Dong WK (2020) Spectrochim Acta A 228:117775
Hao J, Li XY, Zhang Y, Dong WK (2018) Materials 11:523
Pan YQ, Zhang Y, Yu M, Zhang Y, Wang L (2020) Appl Organomet Chem 34:e5441
Zhang LW, Liu LZ, Wang F, Dong WK (2018) Molecules 23:1141
Dong XY, Zhao Q, Wei ZL, Mu HR, Zhang H, Dong WK (2018) Molecules 23:1006
Ren ZL, Hao J, Hao P, Dong XY, Bai Y, Dong WK (2018) Z Naturforsch B 73:203–210
Liu C, An XX, Cui YF, Xie KF, Dong WK (2020) Appl Organomet Chem 34:e5272
Bartocci S, Sabaté F, Bosque R, Keymeulen F, Bartik K, Rodríguez L, Cort AD (2016) Dyes Pig 135:94–101
Shi YS, Li YH, Cui GH, Dong GY (2020) CrystEngComm 22:905–915
Yang YJ, Li YH, Liu D, Cui GH (2020) CrystEngComm 22:1166–1175
Xiao QQ, Dong GY, Li YH, Cui GH (2019) Inorg Chem 58:15696–15699
Wei XJ, Liu D, Li YH, Cui GH (2019) J Solid State Chem 272:138–147
Su Q, Zhao Q, An XX, Wang YB, Li XY, Dong WK (2019) Chin J Inorg Chem 35:524–536
Wu HL, Pan GL, Bai YC, Zhang YH, Wang H, Shi FR, Wang XL, Kong J (2014) J Photochem Photobiol B 135:33–43
Zhang Y, Yu M, Pan YQ, Zhang Y, Xu L, Dong XY (2020) Appl Organomet Chem 34:e5442
Sheldrick GM (2008) Acta Cryst A64:112–122
Sheldrick GM (2015) Acta Cryst C71:3–8
An XX, Liu C, Chen ZZ, Xie KF, Zhang Y (2019) Crystals 9:602
Dong XY, Kang QP, Li XY, Ma JC, Dong WK (2018) Crystals 8:139
Peng YD, Wang F, Gao L, Dong WK (2018) J Chin Chem Soc 65:893–899
Akine S, Nabeshima T (2009) J Chem Soc Dalton Trans 46:10377–10628
Akine S, Taniguchi T, Nabeshima T (2004) Inorg Chem 43:6142–6144
Akine S, Kagiyama S, Nabeshima T (2010) Inorg Chem 49:2141–2152
Akine S, Sunaga TS, Nabeshima T (2011) Chem Eur J 17:6853–6861
Akine S, Sunaga TS, Taniguchi T, Miyazaki H, Nabeshima T (2007) Inorg Chem 46:2959–2961
Yamamura M, Miyazaki H, Iida M, Akine S, Nabeshima T (2011) Inorg Chem 50:5315–5317
Kang QP, Li XY, Zhao Q, Ma JC, Dong WK (2018) Appl Organomet Chem 32:e4379
Dong WK, Zhu LC, Dong YJ, Ma JC, Zhang Y (2016) Polyhedron 117:148–154
Sun YX, Wang L, Dong XY, Ren ZL, Meng WS (2013) Synth React Inorg Met-Org Nano-Met Chem 43:599–603
Chai LQ, Liu G, Zhang YL, Huang JJ, Tong JF (2013) J Coord Chem 66:926–3938
Wu HL, Bai Y, Yuan JK, Wang H, Pan GL, Fan XY, Kong J (2012) J Coord Chem 65:2839–2851
Wu HL, Pan GL, Bai YC, Wang H, Kong J (2015) Res Chem Intermed 41:3375–3388
Spackman MA, McKinnon JJ, Jayatilaka D (2008) CrystEngComm 10:377–388
Spackman MA, Jayatilaka D (2009) CrystEngComm 11:19–32
McKinnon JJ, Spackman MA, Mitchell AS (2004) Acta Crystallogr B 60:627–668
Fabbiani FPA, Byrne LT, Mckinnon JJ, Spackman MA (2007) CrystEngComm 9:728–731
Parkin A, Barr G, Dong W, Gilmore CJ, Jayatilaka D, Mckinnon JJ, Spackman MA, Wilson CC (2007) CrystEngComm 9:648–652
Acknowledgements
Fundings from the National Science Foundation (21761018) and the Program for Excellent Team of Scientific Research in Lanzhou Jiaotong University (201706) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
She, WJ., Cui, YF., Liu, C. et al. Structurally characterized dinuclear zinc(II) and copper(II) coumarin-based N2O2-donor complexes: syntheses, Hirshfeld analyses and fluorescent properties. Transit Met Chem 45, 363–372 (2020). https://doi.org/10.1007/s11243-020-00388-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00388-7