Abstract
This study emphasis the solvent effect on third-order nonlinear optical (NLO) features of methyl red (MR) dye dissolved in polar solvents including ethanol, methanol, acetone, 1-propanol, DMF and DMSO using low power diode laser. Z–scan technique operating at 405 nm wavelength, is used to estimate the third-order NLO features of MR dye in various solvents. The dye discloses self-defocusing nonlinear index of refraction (n2), which is determined to be the order of 10–7 cm2/W. The nonlinear coefficient of absorption (β) of MR dye displays both negative and positive value owing to saturable absorption (SA) and reverse saturable absorption (RSA), respectively. The real and imaginary components of the third-order NLO susceptibility of MR dye in polar solvents are measured to be the order of 10–6 esu and 10–7 esu, respectively. The dye exhibits a large NLO susceptibility in DMSO, which is estimated to be 1.21 × 10–6 esu. The effect of solvent spectral features on MR dye is determined by applying a multi-parameter scale called Kamlet-Abboud-Taft. The experiment results indicate that MR dye is a promising NLO material that may find applications in photonics and optoelectronics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonlinear optical (NLO) materials are a promising candidate for applications in optical limiting, optical switching, two-photon microscopy, optical computing, optical data storage, optical communication, etc. [1,2,3,4,5]. Z–scan technique [6] is a widely used and useful setup for investigating the third-order optical nonlinearity in materials. In Z–scan technique, the nonlinear coefficient of absorption and nonlinear index of refraction are directly measured from open aperture (OA) and closed aperture (CA) Z–scan methods, respectively [7]. The nonlinear coefficient of absorption of materials arises from either positive or negative absorption owing to RSA and SA. Similarly, the nonlinear index of refraction of materials ascends from positive or negative refraction due to self-focusing or self-defocusing [8,9,10]. The RSA based materials are used in optical limiters for sensor and eye protection while saturable absorber materials are frequently used in mode-locking applications [11].
Variety of materials are employed in NLO applications, and organic materials are considered to be the best choice for photonics and optoelectronics applications [12]. In addition, it has been demonstrated that organic compounds are a good replacement for inorganic materials and are highly desirable due to their relative affordability, flexibility, and ease of device fabrication. These features allow the chemical structure and properties to be customized for specific NLO processes, like low dielectric constants, quick NLO response times, and high laser damage thresholds [13,14,15,16]. Organic dyes exhibit unique optical properties like ease of synthesis and structural tunability, making them a promising material for use in NLO research [17,18,19,20]. The NLO properties of organic dyes have drawn a lot of attention due to their high polarizability and large third-order nonlinear susceptibility [21].
Specific (dielectric enrichment) or non-specific (hydrogen bonding) solute-solvent interactions can give rise to the spectral properties of the solute molecules as a result of the solvent effect. Solvent polarity scale or solvatochromism was used to measure the solvent effect on the solute molecules. Third-order NLO properties of the materials are influenced by the solvent environment, which is a significant factor in the interaction between the solute and solvent [22]. The most important spectral factors influencing the third-order NLO properties of the samples are solvent polarizability, solvent hydrogen bond acceptor, and solvent hydrogen bond donor. Different kinds of solute-solvent interactions are involved and solvent polarity parameters alone cannot be used to describe the influence of solvent effects. Because different solvent parameters are influenced on third-order NLO features of materials and becomes more complex. Consequently, for a precise investigation, Kamlet-Abboud-Taft solvent polarity scale is used to calculate the solute-solvent interaction which comprises of specific and nonspecific interactions [23].
The present work reports the effect polar solvents on third-order NLO features of MR dye in low power regime. The multi-parameter scale is used to study the solvent spectral features on MR dye. The third-order NLO features of MR dye are investigated at a wavelength of 405 nm and a total power of 5 mW.
Materials and Methods
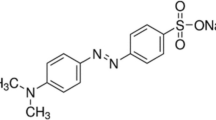
Methyl red dye and solvents ethanol, methanol, acetone, 1-propanol, DMF and DMSO is purchased from Sigma Aldrich. High-grade analytical solvents and dyes are used directly, without any further purification. In polar solvents, the dye dissolves completely at a concentration of 0.01 mM. Table 1 presents the spectral characteristics of the solvents. The molecular structure of MR dye is shown in Fig. 1.
Z–scan technique is used to study the third-order NLO susceptibility of MR dye in polar solvents. Z–scan setup consists of a low power diode laser operating at 405 nm wavelength with 5 mW power. The beam is focused by a convex lens of focal length 5 cm. The MR dye in different solvents is poured into a cuvette of 1 mm thickness. The transmittance of the beam is measured from OA and CA method in order to measure the nonlinear coefficient of absorption and nonlinear index of refraction, respectively. In CA case, the aperture is placed in front of the detector, whereas in OA case the aperture is removed and placed a convex lens with focal length of 20 cm to collect all the beam transmittance. The beam transmittance is measured by a power meter placed at a far field position. All the samples behave like a thin sample because the Rayleigh length is greater than sample length.
Results and Discussion
UV-Visible Absorption Study
The UV-Visible absorption features of MR dye dissolved in ethanol, methanol, acetone, 1-propanol, DMF and DMSO is shown in Fig. 2. The absorption of MR dye is measured from 300 to 800 nm wavelength. The absorption peak is varying with respect to the polarity of the solvent. The absorption peak of MR dye in ethanol, methanol, acetone, 1-propanol, DMF and DMSO is observed at 547 nm, 560 nm, 561 nm, 562 nm and 560 nm, respectively. The linear absorption coefficient of MR dye in different solvents and solvents spectral features are tabulated in Table 1. From Table 1, the linear absorption coefficient of MR in ethanol is higher than that of other polar solvents. This implies that the sample with low polar solvents exhibits large linear absorption coefficient. Also, the linear absorption coefficient of MR dye in DMSO shows minimum and exhibits large value of NLO susceptibility [22].
Third-Order NLO Study
Third-order NLO features of MR dye in ethanol, methanol, acetone, 1-propanol, DMF and DMSO are measured from open aperture (OA) and closed aperture (CA). OA provides the result of nonlinear absorption coefficient which is directly related to imaginary part of the third-order NLO susceptibility. CA method gives the nonlinear index of refraction results which is directly related to real part of the third-order NLO susceptibility. Both real and imaginary part of the third-order NLO susceptibility of MR dye in different solvents gives the third-order NLO susceptibility of MR dye. Figure 3 (a-f) illustrates the OA result of MR dye in ethanol, methanol, acetone, 1-propanol, DMF and DMSO. From Fig. 4 (a-f), MR dye exhibits both positive and negative nonlinear coefficient of absorption owing to SA and RSA absorption of the dye sample. The OA curve of MR dye in ethanol and 1-propanol exhibits SA property, while the dye dissolved in methanol, acetone, DMF and DMSO displays RSA character. Due to the high light intensities at the focus produce SA, the photon absorption increases noticeably before it reaches to the ground state. Conversely, when MR dye is dissolved in methanol, acetone, DMF and DMSO exhibit RSA because of the intense interaction between the sample at the focus and the light intensity. The result of RSA in organic compounds is that the excited state absorption cross-section is greater than the ground state. In OA profile, the nonlinear coefficient of absorption transmittance is given by,
where
where Leff is the sample effective length and Zo is the diffraction length of the sample. The nonlinear coefficient of absorption (β) of MR dye in polar solvents is given by,
CA Z–scan method gives the information about sign and magnitude of the nonlinear index of refraction. The CA index of refraction curve of MR dye in ethanol, methanol, acetone, 1-propanol, DMF and DMSO is shown in Fig. 4 (a-f). The dye sample appears self-defocusing nonlinearity in all the solvents, when the transmittance curve exhibits pre-focal peak followed by post-focal valley. Thermal nonlinearity, which arises from the continuous absorption of the used light source is the cause of self-defocusing. The CW laser irradiation produces a temperature variation inside the sample, which leads to thermal lensing. The sample acts as a defocusing lens when its temperature rises because a negative index of refraction is achieved. The normalized transmittance of MR dye is provided by,
where X = Z/Z0.
The nonlinear index of refraction of MR dye is calculated by using the relation
where \(\varDelta {\varnothing }_{0}\)is the phase shift, λ is the wavelength of the light source and I0 be the Intensity of the light beam at the focus. The measured value of nonlinear index of refraction of MR dye in various solvents is tabulated in Table 2. The real and imaginary components of third-order NLO susceptibility of MR dye obtained from n2 and \(\beta\) which are given by,
where c is the velocity of light in vacuum and ε0 is the vacuum permittivity. The third-order NLO susceptibility of MR dye in different solvents is given by,
The third-order NLO susceptibility of MR dye in various solvents are presented in Table 2. It is observed from Table 2 that; the MR dye shows large nonlinear optical susceptibility in DMSO than other polar solvents. The reason behind the large nonlinearity in DMSO is explained below.
Solvent Dependent on Third-Order NLO Study
The solvent environment plays a major role between solute and solvent interaction and influences the third-order NLO characteristics of the materials [24]. Solvent parameters such as solvent hydrogen bond donor, solvent hydrogen bond acceptor and polarizability are the major spectral factors that affecting the third-order NLO properties of the sample. The NLO absorption coefficient of MR dye exhibits both SA and RSA behavior due to polarizability and dipole moment of the solvents. MR dye shows RSA character in high polar solvents such as methanol, acetone, DMF, DMSO and conversely the dye exhibits SA property in low polar solvents like ethanol and 1-propanol.
Different kinds of solute-solvent interactions are involved and solvent polarity parameters alone cannot be used to describe the solvent effects. Because the influence of solvent on third-order NLO characteristics of materials are more complicated. Therefore, for a precise investigation, Kamlet-Abboud-Taft solvent polarity scale is used which comprises of specific and nonspecific interactions [23]. The solvent dependent NLO features of MR dye is derived by using the relation
where A is the solvent dependent parameter, Ao is the regression value of the solute molecules and a,b,s are the regression coefficients.
The obtained results from the above equation are presented in Table 3. From Table 3, a good relationship is observed between multi-parameter scale and third-order NLO features of MR dye. Solvent hydrogen bond donor (α), solvent hydrogen bond acceptor (β) and solvent polarizability (π) with various contributions have played a major role in the third-order NLO properties of MR dye. For a better comparison, the obtained result is transformed into contribution percentage and it is presented in Table 4. From Table 4, the solvent dipolarizability is a dominant contribution on NLO index of refraction and nonlinear coefficient of absorption of MR dye. The solvent hydrogen bond donor and acceptor are also contributed and played a minor role and therefore it is suggested that both specific and nonspecific interactions are involved between solvent and solute molecule.
Figure 5 (a & b) represents the relation between solvent polarizability and dipole moment as a function of third-order NLO susceptibility of MR dye. It appears that, the third-order NLO susceptibility of MR dye is directly related to solvent polarizability and dipole moment. The third-order NLO susceptibility increases with increase in polarizability and dipole moment of the polar solvents [25]. The dye sample in DMSO exhibit large optical nonlinear susceptibility due to large value of dipole moment and polarizability of DMSO.
Conclusion
In conclusion, the third-order NLO features of MR dye in ethanol, methanol, acetone, 1-propanol, DMF and DMSO was examined via low power laser regime. The NLO features including, nonlinear index of refraction and nonlinear coefficient of absorption of MR dye was studied using CA and OA Z–scan technique. The values of n2 and β of MR dye are found to be the order of 10–7 cm2/W and 10–2 cm/W respectively. The MR dye was found to be exhibit large third-order NLO susceptibility when it was dissolved in DMSO and measured to be the order of 10–6 esu. The influence of solvent parameters on MR dye was discussed and multi-parameter scale was used to examine the solute-solvent interaction. From the experimental results, we observed that the dipole moment and polarizability of the solvents influenced the third-order NLO susceptibility of MR dye. The results show that MR dye was a future optoelectronics material for optical switching and optical limiting applications.
Data Availability
All the data available with the authors.
Change history
23 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10895-024-03673-1
References
Sakthi sabarimoorthi A, Martin Brotto Dhas SA, Jose M (2018) Nonlinear optical properties of Ag@SiO2 core-cell nanoparticles investigated by continuous wave He-Ne laser, Mater. Chem. Phys. 212 224–229. https://doi.org/10.1016/j.matchemphys.2018.03.047
Jeyaram S (2021) Intermolecular charge transfer in donor-acceptor substituted triarylmethane dye for NLO and optical limiting applications. J Mater Sci Mater Elect 32:9368–9376. https://doi.org/10.1007/s10854-021-05600-7
Jeyaram S (2021) Spectral, nonlinear optical and optical switching behavior of carotenoid extracted from Phyllanthus niruri. Ind J Phy 96:1655–1661. https://doi.org/10.1007/s12648-021-02122-0
Jamshidi-Ghaleh K, Salmani S, Majles MH, Ara (2007) Nonlinear response and optical limiting behavior of fast green FCF dye under a low power CW He-Ne laser irradiation. Opt Commun 271:551–554. https://doi.org/10.1016/j.optcom.2006.10.037
Zongo S, Sanusi K, Britton J, Mthunzi P, Nyokong T, Maaza M, Sahraoui B (2015) Nonlinear optical properties of natural laccaic dye studied using Zscan technique. Opt Mater 46:270–275. https://doi.org/10.1016/j.optmat.2015.04.031
Sheik- Bahae M, Said AA, Wei T, Hagan DJ, Van Stryland EW (1990) Sensitive measurement of optical nonlinearities using a single beam. IEEE J Quant Elect QE 26:760–769. https://doi.org/10.1109/3.53394
Jeyaram S, Hemalatha S, Geethakrishnan T (2020) Nonlinear refraction, absorption and optical limiting properties of disperse blue 14 dye. Chem Phys Lett 739:137037. https://doi.org/10.1016/j.cplett.2019.137037
Sudha N, Surendran R, Jeyaram S (2022) Synthesis, spectral, solvent dependent linear and nonlinear optical characteristics of (E)-N-(3-(3-(4 (dimethylamino) phenyl) acryloyl) phenyl) quinolone-2-carboxamide. J Fluoresc 32:1471–1480. https://doi.org/10.1007/s10895-022-02959-6
Jeyaram S, Jeancy Rany D (2023) Extraction of natural pigment from ocimum tenuiflorum using different polar solvents and their nonlinear optical characteristics. J Fluoresc 33:287–295. https://doi.org/10.1007/s10895-022-03061-7
Pramodini S, Poornesh S (2014) Effect of conjugation length on nonlinear optical properties of anthraquinone dyes investigated using He-Ne laser operating in CW mode. Opt Laser Technol 62:12–19. https://doi.org/10.1016/j.optlastec.2014.02.003
Sreenath MC, Hubert Joe I, Rastogi VK (2018) Third-order optical nonlinearities of 1,-diaminoanthraquinone for optical limiting applications. Opt Laser Technol 108:218–234. https://doi.org/10.1016/j.optlastec.2018.06.056
Bredas JL, Adant C, Tackx P, Persoons A (1994) Third-order nonlinear optical response in Organic materials: theoretical and experimental aspects. Chem Rev 94:243–278. https://doi.org/10.1021/cr00025a008
He GS, Xu GS, Prasad PN, Reinhardt BA, Bhatt JC, Dillard A (1995) Two-photon absorption and optical-limiting properties of novel organic compounds. Opt Lett 20:435–437. https://doi.org/10.1364/OL.20.000435
Sudha N, Surendran R, Jeyaram S (2022) Synthesis, characterization, linear and nonlinear optical features of novel organic compound pyridylcarboxamide chalcone for nonlinear optical applications. Opt Mater 131:112668. https://doi.org/10.1016/j.optmat.2022.112668
María A, Díaz-García (2009) Nonlinear optical properties of phthalocyanines and related compounds. J Porphyr Phthalocyanines 13:652–667. https://doi.org/10.1142/S1088424609000784
Muhammad Khalid M, Khan K, Mahmood M, Arshad M, Imran AAC, Braga R Hussain (2022) Theoretical designing of non-fullerene derived organic heterocyclic compounds with enhanced nonlinear optical amplitude: a DFT based prediction. 12:20220. https://doi.org/10.1038/s41598-022-21894-x
Vinitha G, Ramalingam A (2008) Single beam Z–scan measurement of the third-order optical nonlinearities of triarylmethane dyes, Laser Phys. 18: 1176–1182. https://doi.org/10.1134/S1054660X08100113
Jeyaram S, Geethakrishnan T (2017) Third-order nonlinear optical properties of acid green 25 dye by Z–scan method. Opt Laser Technol 89:179–185. https://doi.org/10.1016/j.optlastec.2016.10.006
Alsous MB, Zidan MD, Ajji Z, Allahham A (2014) Z–scan measurements of optical nonlinearity in acid blue 29 dye. Optik 125:5160–5163. https://doi.org/10.1016/j.ijleo.2014.06.012
Jeyaram S (2022) Nonlinear optical responses in organic dye by Z–scan method. J Opt 51:666–671. https://doi.org/10.1007/s12596-022-00834-y
Ali QM, Palanisamy PK (2006) Z-scan determination of the third-order optical nonlinearity of organic dye nile blue chloride. Mod Phys Lett B 11:623–632. https://doi.org/10.1142/S0217984906010779
Zkerhamidi MS, Nasrollahzadeh Z, Seyed Ahmadian SM (2018) Solvent specific and nonspecific interactions effects on nonlinear optical responses of crystal violet dye. J Mol Liq 268:529–535. https://doi.org/10.1016/j.molliq.2018.07.084
Sadigh MK, Zakerhamidi MS (2018) Media polarity and concentration roles on the third order nonlinear behaviors of thiazine dyes. Opt Laser Technol 100:216–224. https://doi.org/10.1016/j.optlastec.2017.10.007
Jeyaram S (2021) Study of third-order nonlinear optical properties of basic violet 3 dye in polar protic and aprotic solvents. J Fluoresc 31:1637–1644. https://doi.org/10.1007/s10895-021-02796-z
Jeyaram S, Naseer J, Punitha S (2021) Effect of solvent on third-order nonlinear optical behavior of reactive blue 19 dye. J Fluoresc 31:1895–1906. https://doi.org/10.1007/s10895-021-02808-y
Acknowledgements
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R398) King Saud University, Riyadh, Saudi Arabia.
Funding
No funding agencies.
Author information
Authors and Affiliations
Contributions
Conceptualization‒ K. Sivaranjani & Sandhanasamy Devanesan, Methodology‒Mohamad S. AlSalhi & M. Vimalan, Validation-Rajabhuvaneswari Ariyamuthu & S. Jeyaram, Writing-review and editing and Supervision‒S. Jeyaram.
Corresponding author
Ethics declarations
Ethical Approval
The submitted work should be original and should not have been published elsewhere in any form or language.
Consent to Participate
Yes.
Consent for Publication
Yes granted.
Competing Interests
The authors have declared that no competing interests exist.
Informed Consent
Not applicable.
Research Involving Human Participants and/or Animals
Research involving human participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original version of this article a missed to include the Acknowledgement section. The following is the statement. “The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R398) King Saud University, Riyadh, Saudi Arabia.”
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivaranjani, K., Ariyamuthu, R., Devanesan, S. et al. Influence of Polar Solvents on Third-Order Nonlinear Optical Features of Methyl Red Dye. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03658-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03658-0