Abstract
Third-order nonlinear optical properties of natural pigment β-carotenoid extracted from medicinal plant phyllanthus niruri are reported herein using a 5-mW-power diode laser with an operating wavelength of 635 nm. The linear optical, vibrational and structural properties of natural pigment were characterized profoundly by UV–Visible absorption, emission, and Fourier transforms infrared (FT-IR) spectroscopy, respectively. The molecular structure of β-carotenoid was confirmed by 1H NMR spectra. The total β-carotenoid concentration in the plant extract was calculated from the absorption spectrum at a specified wavelength. The nonlinear index of refraction (n2) and nonlinear absorption coefficient (β) of the natural pigment were measured to be − 2.00 × 10−7 cm2/W and − 0.63 × 10−4 cm/W. Third-order nonlinear optical susceptibility (χ(3)) of β-carotenoid was calculated to be 6.76 × 10− 7 esu. The natural pigment was satisfied the figure of merit (FOM) conditions for all optical switching device applications. The experimental result shows that the natural pigment β-carotenoid extracted from phyllanthus niruri may be recognized as a novel photonic material for optical switching applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Searching novel nonlinear optical (NLO) materials has been the subject of intensive research in recent decades due to applications in optical communication, optical switching and limiting, information storage, signal processing, computing, etc. [1,2,3,4,5]. NLO behavior of different types of materials has been reported, such as organic dyes [6,7,8], nematic liquid crystal [9], quantum dots [10], organometal halide perovskite [11], etc. Among the variety of available optical materials, delocalized π-conjugated organic molecules are always increasing the interest of the researchers because of excellent optical properties, stability, and large third-order NLO susceptibility [12]. Due to these unique optical properties, a large number of organic materials were synthesized and their NLO characteristics have been reported [13,14,15]. Nevertheless, synthesis of organic materials through different chemical approaches may take longer time and higher costs. With this point of view, significant efforts have been made to synthesize of organic materials in a cost effective and eco-friendly manner.
Natural organic materials such as chlorophylls, carotenoids, xanthophyll, anthocyanin, betanin, etc., obtained from vegetables and leaves are the best alternative to high-cost synthesis process. However, only a few studies on NLO characteristics of natural pigments have been reported [16,17,18,19,20,21,22,23]. Among the different types of available natural pigments, carotenoids are yellow color organic compounds that are abundant in plant leaves, vegetables and fruits. They play an essential role in plant health and defend chlorophyll from photo-damage [24]. Carotenoids act as an antioxidant in the human body and are converted to vitamin A by the human body, which is essential for vision and healthy growth [24]. Phyllanthus niruri is a medicinal plant and mostly cultivated in the tropical regions. This plant is used to relieve problems of liver, kidney, and it is mainly useful for relieving Hepatitis-A. They also help in reducing anemia, hypertension, diabetes, and urinary problems [25].
Third-order NLO parameters of materials can be examined by different experimental methods [26,27,28]. Among them, Z–scan technique [29] is a commonly used experimental method for measuring both real and imaginary parts of the third-order NLO behavior of materials. The simplicity of the technique is the major advantage that led to the researchers for easy handling. This technique allows the simultaneous measurements of nonlinear absorption (NLA) and nonlinear refraction (NLR). The magnitude and sign of n2 and β of the sample can also easily determined by this technique. This paper reports the third-order NLO parameters of natural pigments β-carotenoid extracted from the medicinal plant phyllanthus niruri using low-power CW laser.
2 Experimental details
2.1 Extraction of β-carotenoid from phyllanthus niruri
Column chromatography [30] is a superlative technique used for extracting β-carotenoid from medicinal plant phyllanthus niruri. Figure 1 depicts the β-carotenoid extraction scheme from phyllanthus niruri. The phyllanthus niruri leaves were collected from the garden and washed with running water. A 4 g of phyllanthus niruri leaves was mixed with 5 ml each of acetone and diethyl ether. The mixture was ground, followed by filtration using Whatman filter paper 1. In order to prepare the column chromatography, a 2 g of silica powder dissolved with acetone was loaded into the column. The filtered solution was poured into the column, and some acetone was instantly added drop-wise until the yellow band (β-carotenoid) got separated from the green mixture (chlorophyll). The lower polar solvent β-carotenoid eluted first from the column and was collected in a test tube. Figure 2 shows the molecular structure of β-carotenoid.
2.2 Z–scan study
Figure 3 displays the schematics of the experimental setup of Z–scan technique. A CW laser working at 635 nm wavelength with total power of 5 mW was used to perform the Z–scan measurements. A convex lens was used to focus the beam with focal length of 50 mm. The natural pigment was placed on the micrometer translational stage and moves between –Z and + Z positions. The transmitted beam through the sample was measured using a power meter in the far-field position. The open and closed aperture Z–scan methods were used to calculate the nonlinear absorption coefficient and nonlinear index of refraction of the natural pigment, respectively. In closed aperture technique, the aperture is placed before the detector, while in open aperture, the aperture is removed, and a convex lens is used to collect the total transmittance of the beam. A thin sample condition was fulfilled in the present study for the validation of Z–scan technique. The UV–Vis absorption and emission spectra of the sample were measured by Perkin Elmer Lambda 35 spectrometer and Perkin Elmer LS 45 spectrophotometer.
3 Results and discussion
3.1 Absorption and Emission study
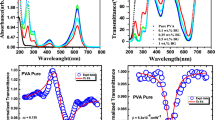
Figures 4 and 5 display the UV–Vis absorption and emission spectra of β-carotenoid extracted from the medicinal plant phyllanthus niruri. The absorption spectrum of β-carotenoid is recorded between 200 and 800 nm wavelength as shown in Fig. 4. In Fig. 4, the peaks observed between 200 and 400 nm are known as Soret band or B band. The Soret band arises in molecules due to the dipole moment that allows π-π* transition [20]. The concentration of β-carotenoid extracted from phyllanthus niruri in 100% acetone was obtained from absorption spectra which is given by [31],
where Ca, Cb, Cx+c and A refer to concentrations of chlorophyll-a, chlorophyll-b, β-carotenoid in μg/ml and absorbance of the sample at corresponding wavelength, respectively. The concentration of β-carotenoid extracted from the medicinal plant phyllanthus niruri was found to be 5.25 μg/ml. The emission spectrum of extracted β-carotenoid was recorded between 400 and 800 nm wavelength, and the intense emission peak was noticed at 656 nm, as shown in Fig. 5.
3.2 FT-IR study
The Perkin Elmer 6X FT-IR spectrometer was used to examine the functional groups present in the natural pigment. Figure 6 depicts the FT-IR spectra of the natural pigment β-carotenoid. The bands observed, respectively, at 3920 cm− 1 and 3461 cm− 1 are owing to the stretching of –OH group. The stretching frequency of the C–H group is assigned for the band at 2979 cm−1 and 2869 cm−1. Further, a strong band observed at 1738 cm−1 is due to the stretching of −C = O group. The weak band at 1605 cm−1 and 1456 cm−1 confirms the stretching of C = C and bending of C-H groups. The strong band noticed at 1373 cm−1 and 1136 cm−1 are due to bending of –OH and stretching of C–O groups. Also, the band at 726 cm−1 and 698 cm−1 are due to the bending of the C = C group.
3.3 1H NMR study
The molecular structure of the natural pigment β-carotenoid has been studied by proton NMR spectroscopy. Figure 7 shows the 1H NMR spectra of β-carotenoid extracted from phyllanthus niruri and the observed characteristic peaks are assigned as follows.
In the spectrum, a triplet at 1.25 ppm is assigned to methyl protons (-CH3) of the compound. A signal appeared at 1.97 ppm is assigned to cyclohexane methylene protons (-CH2). The methine protons (-CH) are assigned to a signal at 2.22 ppm. Furthermore, the signal at 4.47 ppm is assigned to methine protons which are adjacent to methyl groups. This deshielding of ppm is due to steric interaction of bulky groups. The extracted compound formation is confirmed by the presence of methyl, methylene and methine group signals in NMR spectrum.
3.4 Third-order NLO study
The n2 and β of the natural pigment are associated with the real and imaginary components of third-order NLO susceptibility (χ(3)). The scanning process in open aperture Z–scan configuration has been carried out from −Z to + Z, and the transmittance of the beam either increases or decreases with respect to focus (Z = 0). The transmittance is insignificant or negligible at −Z and + Z positions due to decrease in light transmittance. Depending on the increase or decrease in light intensity at the focus, one can easily identify the nature of the nonlinear optical absorption of the sample. Figure 8 depicts the open aperture Z–scan profile of β-carotenoid extracted from the leaves of medicinal plant phyllanthus niruri. From Fig. 8, the transmittance of the light beam through β-carotenoid was increased with an increase in input intensity at the focus, and forms a fine peak, is the result of saturable absorption (SA). The solid line in Fig. 8 is the theoretical fit, which is well-matched with experimental results. SA is the nonlinear optical absorption process that occurs in many NLO materials [32,33,34]. If the ground-state absorption cross-section is large compared to excited-state absorption cross-section, is the characteristic property of SA. The transmittance of natural pigment β-carotenoid is determined from the fitting curve of the open aperture, which is given by
where \( L_{eff} = \left[ {1 - {\text{exp}}\left( { - \alpha_{o} L} \right)} \right]/\alpha_{o}\) is the effective length of the sample, L is the thickness of the sample, and αo is the linear absorption coefficient.
The closed aperture Z–scan measurements were performed to measure the magnitude and sign of n2 of the sample. In closed aperture method, the detector is sensitive to both the nonlinear index of refraction and nonlinear absorption coefficient. Therefore, it is essential to separate the nonlinear index of refraction from nonlinear absorption coefficient. In order to obtain a pure nonlinear index of refraction of β-carotenoid, a simple division method was used, i.e., the closed aperture data are divided by corresponding open aperture data. The natural pigment β-carotenoid moves from −Z position to focus (Z = 0), and the light transmittance increases at the focus and obtained a maximum value. Further, the natural pigment moves from focus to + Z position; the transmittance is decreased and reached minimum value. As a result of scanning, the normalized transmittance curve shows either peak followed by a valley or valley followed by a peak. The former one indicates the characteristic signature of self-defocusing or negative nonlinearity, and the latter one is self-focusing or positive nonlinearity. Figure 9 shows the pure nonlinear refraction curve of β-carotenoid extracted from phyllanthus niruri. The peak–valley normalized transmittance curve obtained from Fig. 9 indicates that the natural pigment β-carotenoid exhibits self-defocusing behavior and, consequently, the negative nonlinear index of refraction. The observed self-defocusing effect in the natural pigment β-carotenoid is the result of thermal nonlinearity. The normalized peak-valley difference ΔTp–v as a function of on-axis phase shift ∣Δφ0∣is given by
where \(S = 1 - \exp \left( { - 2r_{0}^{2} /\omega_{0}^{2} } \right) \) is the linear aperture transmittance, ω0 is the beam radius, and r0 denotes the aperture radius. The transmittance of the natural pigment is given by
where X = Z/Zo. The nonlinear index of refraction n2 of β-carotenoid is given by
where λ is the diode laser wavelength, and Leff is the effective length of the sample. The real and imaginary components of third-order NLO susceptibility (χ(3)) of β-carotenoid are given by
where ε0 and c are the permittivity of the vacuum and the velocity of light. The calculated third-order NLO parameters of β-carotenoid extracted from phyllanthus niruri are presented in Table 1. The value of third-order NLO susceptibility of β-carotenoid extracted from phyllanthus niruri is higher than that of some recently reported materials [35,36,37,38].
3.5 Optical switching
Two figures of merit (FOM) criteria were used to evaluate the NLO characteristics of various materials for all optical switching devices applications. The conditions T = 2βλ/n2 and its inverse W = n2/2βλ are called FOM conditions. In order to design an efficient optical switching device, the FOM > 10 is desirable and the better FOM can also bring down the switching power in optical switching devices [39]. In the present study, the appreciable FOM of ~ 24.99 was achieved and hence the natural pigment β-carotenoid extracted from phyllanthus niruri can be used for optical switching device application.
4 Conclusions
In conclusion, the third-order NLO parameters of natural pigment β-carotenoid extracted from the medicinal plant phyllanthus niruri were studied by Z–scan technique using 5 mW power CW laser. The column chromatography method was used to extract and isolate the natural pigments from phyllanthus niruri. The functional group and molecular structure of β-carotenoid were recognized by FT-IR and 1H NMR spectra, respectively. The nonlinear optical index of refraction and nonlinear optical absorption of natural pigments were ascribed to self-defocusing and saturable absorption, respectively. The real and imaginary components of the third-order NLO susceptibility were found to the order of 10−8 esu. The natural pigment meets the FOM requirements, i.e., W > 1 and T < 1, and therefore it is strongly recommended that the extracted sample is highly suitable for all optical switching device applications. The obtained results show that the natural pigment β-carotenoid extracted from the leaves of phyllanthus niruri is a promising material for future photonics applications.
References
M A Kramer, W R Tompkin and R W Boyd Phys. Rev. A. 34 2026 (1986)
L W Tutt and A Kost Nature 356 225 (1992)
F Z Hernandez, A O Marcano, V Alvarado, A Biondi and H Maillotte Opt. Commun. 152 77 (1998)
S Zongo, M S Dhlamini, P H Neethling, A Yao, M Maaza and B Sahraoui Opt. Mater. 50 138 (2015)
M Maaza, D Hamidi, A Simo, T Kerdja, A K Chaudhary and J B K Kana Opt. Commun. 285 1190 (2012)
S Jeyaram Optoelectron. Adv. Mat. 15 63 (2021)
S. Jeyaram J. Mater. Sci. Mater. Elect. (2021) doi: https://doi.org/10.1007/s10854-021-05600-7
S Jeyaram and T Geethakrishnan Opt. Laser Technol. 89 179 (2017)
Z Dehghani, N Dalir, M Nadafan, M H M Ara and E S Iranizad J. Mol. Liq. 225 502 (2017)
S Valligatla, K Kanta Haldar, A Patra and N R Desai Opt. Laser Technol. 84 87 (2016)
S Mirershadi, S Ahmadi-Kandjani, A Zawadzka, H Rouhbakhsh and B Sahraoui Chem. Phys. Lett. 647 7 (2016)
I Ledoux and J Zyss J. Nonlinear Opt. Phys. 3 287 (1994)
S Li, C Gao, F Liu and W Wei React. Funct. Polym. 73 828 (2013)
W Yu, J Jia, J Gao, L Han and Y Li Chem. Phys. Lett. 624 47 (2015)
M S Wong, Z H Li, M F Shek, M Somac, A Somac and B L Davies Chem. Mater. 14 2999 (2002)
B Kouissa, K Bouchouit, S Abed and Z Essaidi Opt. Commun. 293 75 (2003)
K Bouchouit, B Derkowska, A Milgalska-Zalas, S Abed, N Benali-cherif and B Sahraoui Dyes Pigm. 86 161 (2010)
S Jeyaram and T Geethakrishnan Result Opt. 1 100010 (2020)
S Zongo, K Sanusi, J Britton, P Mthunzi, T Nyokong, M Maaza and B Sahraoui Opt. Mater. 46 270 (2015)
S Jeyaram and T Geethakrishnan Opt. Mater. 107 110148 (2020)
A Diallo, S Zongo, P Mthunzi, S Rehman, S Y Alqaradawi, W Soboyejo and M Maaza Appl. Phys. B 117 861 (2014)
S Zongo, K Sanusi, J Britton, M Maaza and B Sahraoui Opt. Mater. 46 270 (2015)
S Zongo, M S Dhlamini, A P Kerasidou, B Sahraoui and M Maaza Appl. Phys. B 120 389 (2015)
S A Paiva and R M Russell J. Am. Coll. Nutr. 18 426 (1999)
J Patel, P Tripathi, V Sharma, N S Chauhan and V K Dixit J. Ethnopharmacol. 138 286 (2011)
M J Moran, C Y She and R L Carman IEEE J. Quant. Elect. 11 259 (1975)
A Owyoung IEEE J. Quant. Elect. 9 1064 (1973)
W E Williams, M J Soileau and E M Van Stryland Opt. Commun. 50 256 (1984)
M Sheik-Bahae, A A Said, T Wei, D J Hagan and E W Van Stryland IEEE J. Quant. Elect. 26 760 (1990)
S Jeyaram and T Geethakrishnan Opt. Laser Technol. 116 31 (2019)
H K Lichtenthaler Methods Enzymol. 148 350 (1987)
J G Breitzer, D D Dlott, L K Iwaki, S M Kirkpatrick and T B Raunchfuss J. Phys. Chem. A 103 6930 (1999)
M Sukumar, R Ramesh Babu and K Ramesh Babu Appl. Phys. B 2121 369 (2015)
Y S Tamgadge, G G Muley, K U Deshmukh and V G Pahurkar Opt. Mater. 86 185 (2018)
S Zongo, A P Kerasidou, B Sone, A Diallo, P Mthunzi, K Illiopoulos, M Nkosi, M Maaza and B Sahraoui Appl. Surf. Sci. 340 72 (2015)
K Illiopoulos, R Czaplicki, H El Quazzani, J Y Balandier, M Chas, S Goeb, M Salle, D Gindre and B Sahraoui Appl. Phys. Lett. 97 101104 (2010)
B Kulyk, D Guichaoua, A Ayadi, A El-Ghayoury and B Sahraoui Dyes Pigm. 145 256 (2017)
B Kulyuk, K Waszkowska, A Busseau, C Villegas, P Hudhomme, S Dabos-Seignon, A Zawadzka, S Legoupy and B Sahraoui Appl. Surf. Sci. 533 147468 (2020)
L Chen, F Chen, S Dai, G Tao, L Yan, X Shen, H Ma, X Zhang and Y Xu Mater. Res. Bull. 70 204 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeyaram, S. Spectral, third-order nonlinear optical and optical switching behavior of β-carotenoid extracted from phyllanthus niruri. Indian J Phys 96, 1655–1661 (2022). https://doi.org/10.1007/s12648-021-02122-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-021-02122-0