Abstract
This paper reports the third-order nonlinear optical features of acid blue 129 dye in aqueous solutions, by Z-scan method working at 635 nm wavelength. The functional groups present in acid blue 129 dye were observed by FT-IR spectra. Acid blue 129 dye exhibits self-defocusing and reverse saturable absorption (RSA) behaviors. The absorption cross section of ground and excited states was calculated, confirming the presence of RSA. Furthermore, the value of nonlinear refractive index obtained from Sheik-Bahae design was compared with thermal lens model. The concentric diffraction ring pattern observed in the transmitted beam profile of the sample was recorded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Searching novel nonlinear optical (NLO) materials with large TONLO properties are the subject of interests among the researchers owing to their possible applications in optical sensors, two-photon microscopy, optical switching and computing, optical limiting, etc. [1, 2]. Materials such as organic dyes [3,4,5], natural pigments [6, 7], nanoparticles [8], and organic compounds [9] are found to exhibit better NLO responses under continuous and pulsed wave regime. Among the various NLO materials of interest, organic dyes are emerging as most widely studied category in nonlinear optics, because they possess a delocalized system of π-electrons which leads to a large value of TONLO susceptibility and quick response time [4]. Anthraquinone dyes are one such group and the most important group of commercial dyes. These dyes contain a highly delocalized system of π-electrons both in its nucleus and the substituent’s [10]. TONLO characteristics of anthraquinone dye molecules have been widely studied [4, 10], and these need to be explored further for appropriate applications in future nonlinear optics. Acid blue 129 is anthraquinone family which is widely applied in optical switches, photovoltaic cells, thermal printing, and organic semiconductors [11]. The wavelength of the chosen laser for the present study is well matches the resonant absorption of the acid blue 129 dye, and this may possibly be a suitable material for studying their TONLO properties.
Z-scan technique [12] is a single beam technique that measures the real and imaginary components of the TONLO susceptibility and sign and magnitude of nonlinear index of refraction and nonlinear coefficient of absorption of the sample can be obtained from simple experimental calculations. During beam propagation, the phase distortion transferred to amplitude distortion is the principle of Z-scan method. TONLO susceptibility (χ(3)) of the sample is examined from real and imaginary factors of the TONLO susceptibility. The real part of TONLO susceptibility is directly proportional to nonlinear index of refraction, while the imaginary part of TONLO susceptibility is directly related to nonlinear coefficient of absorption. Therefore, n2 and β are the essential parameters to calculate the TONLO susceptibility, which is calculated from closed and open aperture Z-scan technique.
Experimental
Materials
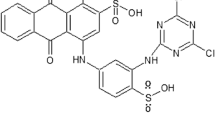
Acid blue 129 dye (Color index: 62,058, M.W: 460.48 g/mol) was obtained from Sigma-Aldrich and has been used directly for the present study. This dye seems to bright blue powder and water soluble. The structure of acid blue 129 dye is shown in Fig. 1. The UV-1601 Shimadzu spectrophotometer was used to record the absorption spectra of acid blue 129 dye, which exhibits two intense absorption peaks, one at 590 nm and another one at 630 nm as shown in Fig. 2. It is clear from Fig. 2 that the absorption peaks appear broad and continuous in the visible region and may possibly be a suitable for photovoltaic applications.
Method
Figure 3 depicts the Z-scan setup used for studying the TONLO properties of acid blue 129 dye. The experiments were performed by using a CW diode laser operating at 635 nm wavelength with 5 mW power. A convex lens with 5 cm of focal length was used to focus the beam. A 1 mm thick cuvette having an aqueous solution of acid blue 129 dye is placed on the micrometer translation stage, and by translating the dye sample between − Z and + Z positions along the direction of propagation of light. An optical power meter was used to measure the transmitted intensity at far-field. The measured Rayleigh length is greater than sample length; consequently, the thin sample estimation is valid in the present study.
Results and discussion
FT-IR study
Figure 4 displays the FT-IR spectra of acid blue 129 dye recorded between 4000 cm−1 and 400 cm−1 at 0.1 cm−1 resolution. A strong band was assigned at 3420 cm−1 is due to N–H group. The weak band at 2922 cm−1 is due to C–H aliphatic stretching frequency of the dye sample. Furthermore, the weak bands at 1599 cm−1, 1577 cm−1 and 1408 cm−1 correspond to aromatic stretching of C=C group. The stretching of C=C gives rise in intermolecular charge transfer between donor and acceptor electrons which in turn increase the NLO responses in acid blue 129 dye. The ICT from donor to acceptor group through single-double band conjugated process can greatly improve both dipole moment and polarizability, since the ICT process contributes to the rise of various C=C stretching modes, which gives the existence of ICT process through π-system. The medium band at 1408 cm−1 is due to bending O–H group of the dye molecule. The band at 1208 cm−1 is owing to amine group. The observed frequencies are confirming the functional groups of acid blue 129 dye.
TONLO study
Figure 5 depicts the open aperture (OA) Z-scan data of acid blue 129 dye with different concentrations of 0.01 mM, 0.02 mM, 0.03 mM and 0.04 mM, respectively. It is observed from Fig. 5 that the transmittance of the sample decreases at focal point with increase in dye concentration. The OA Z-scan traces for all dye concentrations exhibit minimum transmittance at the focus is the characteristics property of reverse saturable absorption (RSA) or positive nonlinear absorption (NLA). It is obvious that various nonlinear absorption (NLA) processes are involved in NLO materials such as two-photon absorption (TPA), excited state absorption (ESA), free carrier absorption (FCA) and reverse saturable absorption (RSA) [11]. The simple five levels model is similar to that of our reported work [11] can be used to explain the NLA behavior of acid blue 129 dye. The five-level model contains various vibrational energy states such as singlet ground state S0, singlet first state S1, excited singlet state S2, lower triplet state T1 and excited triplet state T2. Simultaneous absorption of two photons with same or different energy is transferred from S0 to S1 is known as TPA. Transition from S1 to S2 and/or T1 to T2 is called ESA. The electrons are transferred from the state S1 to T1 through the intersystem crossing (ISC) could greatly enhance the ESA or RSA. The thermal effects from continuous absorption of laser irradiation increase the ESA, and it suggests that ESA-aided RSA is the NLA mechanism observed in the acid blue 129 dye.
Furthermore, the primary mechanism for the presence of RSA in acid blue 129 dye is that the excited state absorption cross section (σexc) is greater than ground state absorption cross section [13] (σgr). The β of the dye sample is related to σgr is given by,
where \(\Delta \sigma ={\sigma }_{exc}-{\sigma }_{gr}\), No is the concentration of the sample and \({I}_{s}=\frac{hc}{\lambda {\sigma }_{gr}\tau }\). \({\sigma }_{gr}={\alpha }_{o}/{N}_{o}C\) is the ground state absorption cross section, where C is the concentration of the dye sample. The order of σgr and σexc was found to be 10−18 cm2 and 10−15 cm2, respectively. The estimated ground and excited state absorption cross section satisfied the criterion for the presence of RSA and therefore, RSA is the most prominent nonlinear absorption mechanism observed in acid blue 129 dye.
The β of acid blue 129 dye in water is given by,
The closed aperture (CA) method is accomplished by placing an aperture before the detector. The data obtained from CA contains both nonlinear refraction and nonlinear absorption. The pure nonlinear refraction was obtained by dividing CA data from OA. Figure 6 shows the pure nonlinear refraction curve of acid blue 129 dye with 0.01 mM, 0.02 mM, 0.03 mM and 0.04 mM concentrations. The peak-valley normalized transmittance of the traces indicates the self-defocusing nonlinearity and consequently the negative nonlinear index of refraction of the sample. The self-defocusing effect in the present study is ascribed to a thermal nonlinearity caused by the continuous absorption of laser irradiation at 635 nm. The heat is generated inside the sample by absorption of light that leads to change in refractive index. It is recognized that the contribution of thermal effects is prominent under CW laser irradiation and cannot be avoided. The peak-valley separation for all concentrations is greater than 1.7 times ZR is the validation of thermal nonlinearity and therefore the obtained result is a cubic nonlinearity [11].
The quantity ΔTp–v as a function of ∣Δφ0∣is given by,
where∣Δφ0 ∣is the on-axis phase shift at the focus\(, S=1-\mathrm{exp}(-2{r}_{0}^{2}/{\upomega }_{0}^{2})\) is the aperture linear transmittance, r0 denotes the aperture radius and ω0is the beam radius at the aperture in the linear regime. The nonlinear refractive index of the sample is given by,
where λ is the laser wavelength, I0 is the input intensity and Leff is the effective length of the sample. According to thermal lens (TL) model, the nonlinear index of refraction is directly related to beam waist of the sample is given by [13],
where α, ωo, k and dn/dT are the absorption coefficient, beam waist at the focus, thermal conductivity and thermo-optic coefficient, respectively. The measured values of nonlinear refractive index obtained from thermal lens model are tabulated in Table 1 and the values are comparable with Sheik-Bahae formulation.
The real and imaginary components of the TONLO susceptibility χ(3) are given by the relation,
where ε0 and c are the permittivity and light velocity in vacuum.
The measured TONLO parameters of acid blue 129 dye are presented in Table 1. A series of anthraquinone dyes and their TONLO susceptibility are tabulated in Table 2. It is meaningful to say that the measured TONLO susceptibility of acid blue 129 dye is larger than that of reported anthraquinone dyes [2, 3, 11, 14,15,16,17,18,19].
The influence of dye concentration on n2 and β of acid blue 129 dye was studied. From Figs. 7 and 8, it is very clear that the values of n2 and β are increases with increase in concentrations of the sample. This is because as the dye molecules increases with concentration, more number of particles get thermally agitated, resulting in an enhanced optical nonlinearity. It is obvious that the nonlinearity of the dye is related to chemical structure of the compound and this can be explained based on the electron donating/accepting ability of the groups present in the dye molecules. The molecule in which donor and acceptor groups connected at the terminal position of π-bridge to create highly polarized molecules and this could exhibit large NLO responses. Acid blue 129 dye possesses methyl group in 2-, 4-, 6- positions and amino groups in 1- and 4- positions act as electron donors. The quinone moieties, oxygen of carbonyl group and sulfonate in the 2- position act as electron acceptors. As the sample concentration increases, i.e., with increase in dye molecules, the presence of donor and/or acceptor groups in the sample increases and consequently the electron density gets enhanced [11]. As a result, the charge transfer between donor and acceptor electrons may take place, resulting in a longer conjugation length. With increase in conjugation length, the dipole moment and the related optical nonlinearity of the dye sample increase.
Figure 9 depicts the image of self-diffraction ring pattern of acid blue 129 dye with 0.04 mM concentration. When the Gaussian laser beam passes through the sample, the sample absorbs the radiation, resulting in an increase in temperature. Due to increase in temperature, there may be an induced local spatial variation of refractive index in the sample which produces self-diffraction ring pattern in the far-field position [20].
Conclusion
TONLO characteristics of acid blue 129 dye are examined by Z–scan instrument operating at 635 nm wavelength. The values of the measured nonlinear index of refraction (n2) and nonlinear coefficient of absorption (β) of acid blue 129 dye were determined to be the order of 10–7 cm2/W and 10–3 cm/W, respectively. The order of magnitude of the measured TONLO susceptibility (χ3) of the dye sample was found to be 10–5 esu. TONLO parameter of the sample was found to increase linearly as the dye concentrations increase. The far-field self-diffraction ring pattern observed in the transmitted beam profile was also recorded. The results are suggested that the studied acid blue129 dye probably be a good candidate for applications in photonics and optoelectronics.
References
M.A. Kramer, W.R. Tompkin, R.W. Boyd, Nonlinear optical interactions in fluorescein-doped boric acid glass. Phys. Rev. A 34, 2026 (1986)
M.C. Sreenath, I. Hubert Joe, V.K. Rastogi, Third-order optical nonlinearities of 1,5 diaminoanthraquinone for optical limiting applications. Opt. Laser Technol. 108, 218 (2018)
S. Jeyaram, S. Hemalatha, T. Geethakrishnan, Nonlinear refraction, absorption and optical limiting properties of disperse blue 14 dye. Chem. Phys. Lett. 739, 137037 (2020)
S. Jeyaram, Intermolecular charge transfer in donor-acceptor substituted triarylmethane dye for NLO and optical limiting applications. J. Mater. Sci. Mater. Elect. 32, 9368 (2021)
K.J. Ghaleh, S. Salmai, M.H.M. Ara, Nonlinear responses and optical limiting behavior of fast green FCF dye under a low power CW He-Ne laser irradiation. Opt. Commun. 271, 551 (2007)
S. Jeyaram, T. Geethakrishnan, Spectral and third-order nonlinear optical characteristics of natural pigment extracted from coriandrum sativum. Opt. Mater. 107, 110148 (2020)
S. Zongo, K. Sanusi, J. Britton, P. Mthunzi, T. Nyokong, M. Maaza, B. Sahraoui, Nonlinear optical properties of natural laccaic dye studied using Z-scan technique. Opt. Mater. 46, 270 (2015)
A.I. Ryasnyansky, B. Palpant, S. Debrus, R.I. Khalibullin, A.L. Stepanov, Nonlinear optical properties of copper nanoparticles synthesized in indium tin oxide matrix by ion implantation. J. Opt. Soc. Am. B 23, 1348 (2006)
G.S. He, G.S. Xu, P.N. Prasad, B.A. Reinhardt, J.C. Bhatt, A.G. Dillard, Two-photon absorption and optical limiting properties of novel organic compounds. Opt. Lett. 20, 435 (1995)
S. Jeyaram, T. Geethakrishnan, Third-order nonlinear optical properties of acid green 25 dye using Z-scan method. Opt. Laser Technol. 89, 179 (2017)
P. Dahiya, M. Kumbhakar, T. Mukherjee, H. Pal, Effect of the amino and hydroxy substituents on the photophysical properties of 1,4-distributed-9,10-anthraquinone dyes. J. Mol. Struct. 40, 798 (2006)
M. Sheik-Bahae, A.A. Said, T. Wei, D.J. Hagan, E.M. Van Stryland, Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quant. Elect. 26, 760 (1990)
F.L.S. Cuppo, A.M.F. Neto, S.L. Gomez, P.P. Muhoray, Thermal-lens model compared with the Sheik-Bahae formalism in interpreting Z-scan experiments on lyotropic liquid crystals. J. Opt. Soc. Am. B 19, 1342 (2002)
S. Pramodini, P. Poornesh, K.K. Nagaraja, Thermally induced nonlinear optical response and optical limiting of acid blue 40. Curr. Appl. Phys. 13, 1175 (2013)
S. Pramodini, P. Poornesh, Third-order nonlinear optical studies of anthraquinone dyes using CW He-Ne laser. Laser Phys. 24, 055402 (2014)
S. Pramodini, P. Poornesh, Effect of conjugation length on nonlinear optical parameters of anthraquinone dyes investigated during He-Ne laser operating in CW mode. Opt. Laser Technol. 62, 12 (2014)
S. Jeyaram, T. Geethakrishnan, Low power laser induced NLO properties and optical limiting of an anthraquinone dye using Z-scan technique. J. Mater. Sci. Mater. Elect. 28, 9820 (2017)
S. Zafar, Z.H. Khan, M.S. Khan, Experimental and theoretical investigations of nonlinear optical properties of 1, 4-diamino-9, 10-anthraquinone. Spectrochim. Acta Part A 114, 164 (2013)
Q. Meng, W. Yan, M. Yu, D. Huang, A study of third-order nonlinear optical properties for anthraquinone derivaties. Dyes Pigm. 56, 145 (2003)
S. Jeyaram, Study of third-order NLO properties of organic dye for optoelectronics applications. Optoelectron. Adv. Mat. Rapid Commun. 15, 62 (2021)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeyaram, S. Nonlinear optical responses in organic dye by Z-scan method. J Opt 51, 666–671 (2022). https://doi.org/10.1007/s12596-022-00834-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-022-00834-y