Abstract
Herein, we report the extraction of natural pigment curcumin from curcuma longa and their linear and third-order nonlinear optical (NLO) characteristics. The characterization techniques viz., UV–Visible absorption, FT-IR, Micro Raman and Gas Chromatography Mass Spectrum (GC–MS) are used to study the spectral characteristics of curcumin. Third-order NLO features of curcumin are studied using Z‒scan technique with a semiconductor diode laser working at 405 nm wavelength. The natural pigment exhibits negative nonlinear index of refraction resulting from self-defocusing and positive coefficient of absorption is the consequence of reverse saturable absorption (RSA). The order of nonlinear index of refraction (n2) and nonlinear coefficient of absorption (β) is measured to be 10−7 cm2/W and 10−2 cm/W, respectively. Third-order NLO susceptibility (χ(3)) and second-order hyperpolarizability (γ) of curcumin is measured to be 2.73 × 10‒7 esu and 1.67 × 10‒31 esu, respectively. A low optical limiting (OL) threshold of 0.71 mW is observed in the extracted pigment. The experimental results are supplemented by quantum mechanical calculations of the NLO parameters. The overall result finding is that curcumin extracted from curcuma longa has the potential to be novel optical candidates for photonics and optoelectronics applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonlinear optical (NLO) materials are extensively used in photonics and optoelectronics applications including optical switching and limiting, optical data storage, optical communication and 3D imaging [1,2,3,4]. A lot of research work has been devoted towards finding suitable material for NLO applications. Therefore, materials with significant NLO properties have a key role in the aforementioned applications [5]. Hitherto, different materials are studied and proved to be optically nonlinear under low power regime [6,7,8,9,10]. In the past three decades, chemical dyes have received notable consideration in NLO study [2, 11,12,13,14]. The natural dyes made from plant leaves, roots, vegetables, minerals, etc. are the best alternative to synthetic dyes [15]. The natural pigments have received special place in nonlinear optics because of its large susceptibility, chemical stability and significant hyperpolarizability [4]. The important reasons for using natural pigments in NLO studies are: abundance in nature, low cost, non-toxic, synthetic ease, etc. A large number of natural pigments have been involved in NLO study such as chlorophylls, carotenoids, anthocyanin, lycopene, Hibiscus Sabdariffa dye, chinese tea, betanin, and henna [16,17,18,19,20,21,22,23,24,25]. The nonlinear absorption (NLA) coefficient of these natural pigments is very attractive and found to be 10–10000 times better than some conventional materials such as CS2, bismuth borate glasses and sulphur rich compounds [26] etc. Curcumin is one among the available natural pigment extracted from turmeric (curcuma longa) which is a perennial herb plant belonging to the family of ginger [27]. The turmeric plant is widely cultivated in tropical parts of Southeast Asia. Curcuminoids are active compounds of Curcuma longa, which consists of curcumin, demethoxycurcumin and bis demethoxycurcumin [28]. Curcumin is an asymmetric compound named as (E,E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-1–6-heptadiene-3,5-dione, with chemical formula C21H20O6 and molecular weight 368.38 g/mol. Curcumin has three important functional groups: aromatic o-methoxy phenolic group, α, β-unsaturated β-diketo moiety, and seven carbon linkers. In recent time, Curcuma Longa is used as antioxidant, anti-inflammatory, antimicrobial and anticancer agent [29].
Various experimental techniques are used to quantify the third-order NLO features of the compounds such as degenerate four-wave and three-wave mixing, ellipse rotation, beam distortion, Z‒scan technique, etc. [30,31,32,33]. Among the available experimental techniques, Z‒scan is the most sensitive and simple tool to calculate the third-order NLO characteristics of the materials [16]. This technique has wide advantages including easy experimental procedure, sign and magnitude of the NLO index of refraction and NLO coefficient of absorption is simultaneously measured from closed and open aperture techniques, simple calculation, real and imaginary features of the sample is simultaneously measured from the experiments, etc.
This paper reports the spectral properties of curcumin extracted from curcuma lango. The density functional theory (DFT) study is used to find the molecular polarizability of curcumin. Third-order NLO features of the extracted natural pigments are determined by Z‒scan technique with 405 nm wavelength and the OL study is carried out under same experimental condition.
Materials and Methods
Extraction of Curcumin from Curcuma Longa
The extraction technique applied in the current study is same as that of the reported work by Sahne et al. [34]. The pure turmeric is purchased from local market and dried in oven at 90 °C for 2 days. The dried turmeric is grinded using motor. The curcumin powder is highly soluble in ethanol which is purchased from Merck India. Soxhelt experiment [35] is used to extract the natural pigment curcumin from turmeric (curcuma longa). 20 g of turmeric powder is dissolved with 200 ml of diluted ethanol, which means that 190 ml ethanol mixed with 10 ml of water. The solution is placed in the Soxhelt apparatus and heated upto 80 °C for 8 h. Now, the ethanol is separated from the extract using rotary evaporator under vacuum at 40 °C. The residue is known as curcumin and further dried, stored in a refrigerator for future study.
Instrumentation
The FT-IR spectrum of curcumin is examined using a SHIMADZU, IRTRACER 100 FT-IR Spectrometer. The Micro Raman study of the sample is studied using HORIBA, LabRam HR evolution operating at 532 nm wavelength with resolution of 0.4 cm−1. The UV–Visible absorption spectrum of curcumin is studied by SHIMADZU, UV 3600 plus spectrophotometer. The gas chromatography mass spectrometry (GC–MS) of curcumin is studied by SHIMADZU, QP 2010 plus.
Z‒scan Technique
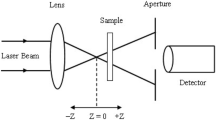
The Z–scan experimental method used in the present work is similar to that of our reported work [1]. A semiconductor diode laser with a CW output power of 5 mW operating at a wavelength of 405 nm is used for the studies. The beam intensity at the focus (I0) and beam waist (ω0) are measured to be 751 W/cm2 and 20.58 μm, respectively. A convex lens with 50 mm focal length is placed before the cuvette. A 1 mm thick cuvette is filled with the natural pigment is placed on the micrometer stage and translate from -Z to + Z positions. The closed aperture (CA) and open aperture (OA) techniques are used to measure the n2 and β of curcumin. To measure the beam transmittance, a power meter is positioned far from the source. The condition for thin sample is validated because the measured Rayleigh length is greater than sample length (ZR > > L). In the present study, the measured Rayleigh length is 3.28 mm.
Results and Discussion
UV–Vis Absorption Study
Figure 1 depicts the UV–Visible absorption spectrum of curcumin in ethanol as a solvent between 300 and 600 nm. The spectrum covers the whole visible region and the maximum absorbance was observed at 427 nm due to low energy π-π* transition of the extracted natural pigment. The result obtained in UV–Vis spectra holds good agreement with the reported work by Kim et al. [36]. This transition is due to the shift in wavelength at longer wavelength region is called bathochromic shift or red shift. The linear absorption coefficient of the curcumin is calculated from the relation is given by,
where A and d are the absorbance of the curcumin and thickness of the curcumin inside a cuvette, respectively.
FT-IR Study
The FT-IR analysis of curcumin is shown in Fig. 2. The frequency band at 3278 cm−1 is owing to alkyne C-H unit. The stretching band at 2972 cm−1 displays the existence of alkane C-H unit. Likewise, the stretching frequency respectively at 2345 and 2117 cm−1 seemed for aliphatic C-H stretching vibration. A weak band at 1552 cm−1 and 1529 cm−1 specifies the existence of C = C groups of aromatic moieties. The O–H bending vibration is observed at 1388 cm−1 and the C-O stretching vibration of the extracted pigment has observed at 1041 cm−1.
Micro Raman Study
The Micro Raman spectrum of curcumin extracted from curcuma longa is depicted in Fig. 3. The vibrational assignment at 2586 cm−1 is owing to hydroxyl (-OH) unit. The asymmetric stretching C-H unit is observed with corresponding frequency at 2353 cm−1. The weak alkyne C-H unit is observed at 2175 cm−1. The C = O stretching unit is observed at 1070 cm−1. The aromatic rings observed in the sample with corresponding frequency at 922 cm−1.
Gas Chromatography Mass Spectrum (GC–MS) Study
The gas chromatography mass spectrum (GC–MS) of curcumin extracted from curcuma longa is shown in Fig. 4. From Fig. 4, the major curcumin compounds such as 2-Hydroxy-2-Methyl-4-Pentanone (14.37%), Ar-tumerone (38.27%), Tetradecanoic acid (28.35%), n-Hexadecanoic acid (100%), 9-Octadecenal (21.18%), 4-Butylbenzoic acid, 2-dimethylaminoethyl ester (44.20%) are present in the extracted sample.
Third-Order NLO Study
Third-order NLO properties of curcumin obtained from curcuma longa is assessed from CA and OA Z‒scan techniques. The NLO index of refraction is calculated from CA method, and NLO absorption coefficient is measured from OA method. The intensity dependent NLO absorption coefficient and refraction is given by [33],
where α0 is the linear absorption coefficient, Is is the saturation intensity which depends on the property of the sample and n0, n2 are the linear and nonlinear refractive index of the material.
Figure 5 shows the outcome of OA Z‒scan technique of curcumin extracted from curcuma longa. The absorption coefficient of curcumin shows negative nonlinearity due to RSA. RSA is the nonlinear absorption (NLA) phenomena which results from interaction of light source with curcumin at the focal point. A symmetric nature of the curve of Fig. 5 shows intensity reliant on absorption effect. The RSA is caused in curcumin may be due to the molecules in higher excited state has more absorption cross-section than the ground state. The five-level model consists of different energy level [5] is also used to explain the RSA property of the sample which is same as that of our reported work [5]. The normalized transmittance is given by [33],
where
where Leff and Zo are the effective and diffraction length of the sample. The β of curcumin is given by [33],
The sign and magnitude of nonlinear refractive index (NLR) is estimated from CA Z‒scan method. In CA, the aperture is used with measurable size and the beam transmittance is determined at far-field using power meter. The NLR is attained from CA technique encompasses both NLR and NLA. Hence, the pure NLR part is determined by dividing CA data to the OA data. Figure 6 shows the pure NLR curve of curcumin extracted from curcuma longa. The transmittance curve unveils pre focal peak followed by post focal valley transmittance is the result of self-defocusing. Self-defocusing effect is owing to thermal nonlinearity, which arises from continuous absorption of used light source. The difference in temperature inside the natural pigment is owing to the continuous absorption of laser irradiation, which leads to thermal lensing effect. The normalized transmittance of curcumin extracted from curcuma longa is given by [33],
where X = Z/Z0.
The nonlinear refractive index n2 of curcumin is calculated by using the relation
where \(\Delta {\varnothing }_{0}\) is the on-axis phase shift, λ is the wavelength of the light source and I0 be the intensity of the light beam at the focus. The measured n2 value of curcumin is tabulated in Table 1. The real and imaginary features of the third-order NLO susceptibility is given by [33],
where c is the velocity of light in vacuum and ε0 is the vacuum permittivity. The third-order NLO susceptibility is given by [33],
The calculated value of χ(3) of curcumin extracted from curcuma longa is tabulated in Table 1. It is observed from the Table 1 that, the natural pigment curcumin shows large third-order NLO susceptibility over some recently reported natural materials [37, 38]. The second-order hyperpolarizability (γ) of curcumin is related to the nonlinear susceptibility by the following relation [39],
where N is the number density of the molecules in cm–3 and L is the local field correction factor given by \([\nicefrac{\left({n}^{2}+2\right)}{3}]\), where n is the linear refractive index. The measured second order hyperpolarizability of curcumin is tabulated in Table 1.
Optical Limiting Study
Optical limiting materials are widely used for eye and sensor protection from the harmful high intense lasers [40,41,42,43]. The low power lasers even power less than 1 mW can also damage the retina of human eye when it is directly exposed [44]. In order to reduce the intensity of the lasers OL are used to safeguard the devices. OL are the materials with low optical limiting threshold can act as perfect optical limiters. In the present work, curcumin obtained from curcuma longa in ethanol as a solvent is placed at focal point of the lens. The intensity of the beam is varied by a neutral density filter and the beam transmittance at far-filed is measured by photo detector. Figure 7 depicts the optical limiting property of curcumin. A low optical limiting threshold of 0.71 mW is observed and concluded that the natural pigment curcumin extracted from curcuma longa is suitable candidates for applications in optical limiting devices. The observed optical limiting threshold results compared with some reported natural pigments obtained from different plants and vegetables and noticed that the curcumin has better optical limiting threshold than reported materials [45,46,47,48].
Quantum Mechanical Calculations
The enol-form of curcumin which is found to be more stable than its keto form due to the extended conjugation [49] is taken for the Density Functional Theory (DFT) calculations using Gaussian16 [50]. Among the three isomers possible [51] with respect to the relative arrangement of the methoxy group and the enol group, the one with methoxy groups lying trans to the enol group has the highest dipole moment (Table 2) and is therefore employed for computing the NLO properties. The molecule was optimised at CAM-B3LYP/6–311 + + G** level of theory [52] and vibrational analysis is done at the same level of theory to characterize the nature of the stationary point. It is found to be a minimum on the potential energy surface (PES) with the lowest frequency of 9.97 cm−1. Computations under ethanol as a solvent is done using the self-consistent reaction field (SCRF) incorporated into the Polarizable Continuum Model (PCM) [53]. The optimised molecule with symmetry Cs is shown in Table 2 along with NLO associated parameters calculated at the same level of theory.
Solvation energy of curcumin in ethanol is found to be -11.96 kcalmol−1 and is a minimum on the PES with the lowest frequency of 12.25 cm−1. The optical properties of curcumin also see a significant enhancement in ethanol solution with dipole moment increasing from 7.5 Debye to 10.05. Accordingly, the associated polarizability and hyperpolarizabilities are also amplified. The gas-phase and the solvation-modelled calculations are done under both static limit as well as at the single-photon absorption wavelength of 405 nm which is employed in the experimental set-up. Nonlinearity of the optical properties of curcumin at 405 nm is prominent as evident from the computed hyperpolarizabilities. Calculated γ-values at ω = 405 nm in the order of 10–31 is 0.133 esu which increases to 1.441 esu in ethanol as a solvent and is very close to the observed experimental value (1.67 esu). So far, hyperpolarizabilities were found to be highly sensitive towards the choice of the basis-set [54, 55].
Conclusions
The natural pigment curcumin was extracted from curcuma longa using Soxhelt experiment and their spectral, third-order NLO features and optical limiting were studied. The functional group present in the extracted pigment was studied by FT-IR and Micro Raman spectrometer. The mass spectrum image confirmed the compounds present in curcumin. Third-order NLO characteristics of curcumin were examined via Z‒scan technique using CW diode laser working at 405 nm wavelength with total power of 5 mW. The natural pigments displayed self-defocusing and RSA based optical nonlinearities. The order of real and imaginary part of χ(3) was measured to be 10‒7 esu. The order of third-order NLO susceptibility and second-order hyperpolarizability of curcumin extracted from curcuma longa was found to be 10‒7 esu and 10‒31 esu, respectively. DFT calculations correlate well with the experimental observations and support the choice of ethanol as the solvent to enhance the NLO properties of the compound. A low optical limiting threshold was observed in curcumin dye dissolved in ethanol solvent and suggests that the natural pigment is an opted candidate for optical limiting applications.
Availability of Data and Materials
All the data available with the authors.
References
Jeyaram S (2021) Intermolecular charge transfer in donor-acceptor substituted triarylmethane dye for NLO and optical limiting applications. J Mater Sci Mater Elect 32:9368–9376
Vinitha G, Ramalingam A (2008) Single beam Z─scan measurement of the third-order optical nonlinearities of triarylmethane dyes. Laser Phys 18:1176–1182
Jamshidi-Ghaleh K, Salmani S, Majles Ara MH (2007) Nonlinear response and optical limiting behavior of fast green FCF dye under a low power CW He-Ne laser irradiation. Opt Commun 271:551–554
Zongo S, Sanusi K, Britton J, Mthunzi P, Nyokong T, Maaza M, Sahraoui B (2015) Nonlinear optical properties of natural laccaic dye studied using Z─scan technique. Opt Mater 46:270–275
Jeyaram S, Geethakrishnan T (2017) Third-order nonlinear optical properties of acid green 25 dye by Z-scan method. Opt Laser Technol 89:179–185
Dhanuskodi S, Sabari Girisun TC, Bhagavannarayana G, Uma S, Phillip J (2011) Mechanical, thermal and laser damage threshold analyses of II group metal complexes of thiourea. Mater Chem Phys 126:463–469
Motiei H, Jafari A, Naderali R (2017) Third-order nonlinear optical properties of azo dyes by using nonlinearity parameter and Z-scan technique. Opt Laser Technol 88:68–74
Sreeja VG, Vinitha G, Reshmi R, Anila EI, Jayaraj MK (2017) Effect of reduction time on third-order optical nonlinearity of reduced graphene oxide. Opt Mater 66:460–468
Sudha N, Surendran R, Jeyaram S (2022) Synthesis, characterization, linear and nonlinear optical features of novel organic compound pyridylcarboxamide chalcone for nonlinear optical applications. Opt Mater 131:112668
Sakthi sabarimoorthi A, Martin Brotto Dhas SA, Jose M (2018) Nonlinear optical properties of Ag@SiO2 core-cell nanoparticles investigated by continuous wave He-Ne laser. Mater Chem Phys 212:224–229
Sreenath MC, Hubert Joe I, Rastogi VK (2018) Third-order optical nonlinearities of 1,-diaminoanthraquinone for optical limiting applications. Opt Laser Technol 108:218–234
Alsous MB, Zidan MD, Ajji Z, Allahham A (2014) Z-scan measurements of optical nonlinearity in acid blue 29 dye. Optik 125:5160–5163
Pramodini S, Poornesh S (2014) Effect of conjugation length on nonlinear optical properties of anthraquinone dyes investigated using He-Ne laser operating in CW mode. Opt Laser Technol 62:12–19
Jeyaram S (2022) Nonlinear optical responses in organic dye by Z-scan method. J Opt 51:666–671
Jeyaram S, Geethakrishnan T (2020) Spectral and third-order nonlinear optical characteristics of natural pigment extracted from coriandrum sativum. Opt Mater 107:110148
Jeyaram S, Geethakrishnan T (2019) Linear and nonlinear optical properties of chlorophyll-a extracted from Andrographis paniculata leaves. Opt Laser Technol 116:31–36
Jeyaram S (2022) Spectral, third-order nonlinear optical and optical switching behavior of β-carotenoid extracted from phyllanthus niruri. Indian J Phys 96:1655–1661
Jeyaram S, Geethakrishnan T (2020) Vibrational spectroscopic, linear and nonlinear optical characteristics of anthocyanin extracted from blueberry. Results Opt 1:100010
Numan N, Jeyaram S, Kaviyarasu K, Neething P, Sackey J, Kotsedi CL, Akbari M, Morad R, Mthunzi-kufa P, Sahraoui B, Maaza M (2022) On the remarkable nonlinear optical properties of natural tomato lycopene. Sci Rep 12:9078
Diallo A, Zongo S, Mthunzi P, Rehman S, Alqaradawi SY, Soboyejo W, Maaza M (2014) Z-scan and optical limiting properties of Hibiscus Sabdariffa dye. Appl Phys B 117:861–867
Lin HH, Korpel A, Mehrl D, Anderson DR (1989) Optical nonlinearities of tea studied by Z-scan and four-wave mixing techniques. Opt News 15:55
Thankappan A, Thomas S, Nampoori VPN (2013) Solvent effect on the third order optical nonlinearity and optical limiting ability of betanin natural dye extracted from red beet root. Opt Mater 35:2332–2337
Henari FZ (2012) Optical Nonlinear Properties and Optical Switching of Henna (Lawson) Films. Int J Thin Film Sci Tec 2:55–60
Jeyaram S, Jeancy Rany D (2023) Extraction of natural pigment from ocimum tenuiflorum using different polar solvents and their nonlinear optical characteristics. J Fluoresc 33:287–295
Abdullah M, Aziz MS (2022) Ganesan Krishnan, Fauzan Ahmad, Sabah M. Mohammad, Ibrahim Abdul Razak, Sulaiman Wadi Harun, Large third-order optical nonlinear susceptibility in natural dye derived from Clitoria ternatea petal. Optik 256:168752
Zongo S, Kerasidou AP, Sone BT, Diallo A, Mthunzi P, Illopoulos K, Nkosi M, Maaza M, Sahraoui B (2015) Nonlinear optical properties of poly(methyl methacrylate) thin films doped with Bixa Orellana dye. Appl Sur Sci 340:72–77
Henari FZ, Cassidy S, Jasim KE, Dakhel AA (2013) Nonlinear refractive index measurement of curcumin with CW laser. J Nonlinear Opt Phys Mater 2:1350017
Elias RS, Hassan QMA, Sultan HA, Al-Asadi AS, Saeed BA, Emshary CA (2018) Thermal nonlinearities for three curcuminoids by diffraction ring patterns and Z-scan under visible CW laser illumination. Opt Laser Technol 107:131–141
Sultan HA, Hassan QMA, Al-Asadi AS, Elias RS, Bakr H, Saeed BA (2018) Far-field diffraction patterns and optical limiting properties of bisdemethoxycurcumin solution under CW laser illumination. Opt Mater 85:500–509
Maker PD, Terhune RW, Savage CM (1964) Intensity-dependent changes in the refractive index of liquids. Phys Rev Lett 12:507–509
Veduta AP, Kirsanoy BP (1968) Variation of the refractive index of liquids and glasses in a high intensity field of a ruby laser. J Exp Theor Phys 27:736–738
Buchalter B, Meredith GR (1982) Third-order optical susceptibility of glasses determined by third harmonic generation. Appl Opt 21:3221–3224
Sheik- Bahae M, Said AA, Wei T, Hagan DJ, Van Stryland EW (1990) Sensitive measurement of optical nonlinearities using a single beam. IEEE J Quant Elect QE 26:760–769
Sahne F, Mohammadi M, Najafpour GD (2016) Ali Akbar Moghadamnia, Extraction of bioactive compound curcumin from turmeric (curcuma longa L) via different routes: A comparative study. Pak J Biotechnol 13:173–180
Weggler BA, Gruber B, Teehan P, Jaramillo R, Dorman FL (2020) Inlets and sampling. Sep Sci Technol 12:141–203
Kim YJ, Lee HJ, Shin Y (2013) Optimization and validation of high-performance liquid chromatography method for individual curcuminoids in turmeric by heat-refluxed extraction. J Agric Food Chem 61:10911
Priyadarshani N, Sabari Girisun TC, Venugopal Rao S (2019) Improved femtosecond third-order nonlinear optical properties of thin layered Cu3Nb2O8. Opt Mater 88:586–593
Praseetha KP, Divyasree MC, Nimmy John V, Chandrasekaran K, Varghese S (2019) Enhanced optical nonlinearity in nematic liquid crystal on doping with CdSe quantum dot. J Mol Liq 273:497–503
Mathew E, Salian VV, Hubert Jeo I, Narayanan B (2019) Third-order nonlinear optical studies of two novel chalcone derivatives using Z-scan technique and DFT method. Opt Laser Technol 120:105697
Ramya M, Nideep TK, Vijesh KR, Nampoori VPN, Kailasnath M (2018) Synthesis of stable ZnO nanocolloids with enhanced optical limiting properties via simple solution method. Opt Mater 81:30–36
Qusay M (2018) Hassan, Study of nonlinear optical properties and optical limiting of acid green 5 in solution and solid film. Opt Laser Technol 106:366–371
Sultan HA, Hassan QMA, Al-Asadi AS, Elias RS, Bakr H, Saeed BA, Emshary CA (2018) Far-field diffraction patterns and optical limiting properties of bisdemethoxycurcumin solution under CW laser illumination. Opt Mater 85:500–509
Sajna MS, Perumbilavil S, Prakashan VP, Sanu MS, Joseph C, Biju PR, Unnikrishnan NV (2018) Mater Res Bull 104:227–235
Jeyaram S, Geethakrishnan T (2017) Low power laser induced NLO properties and optical limiting of an anthraquinone dye using Z–scan technique. J Mater Sci Mater Elect 23:9820–9827
Rashidian M, Dorranian D, Ahmadi Darani S, Saghafi S, Ghoranneviss M (2009) Nonlinear responses and optical limiting behavior of Basic Violet 16 dye under CW laser illumination. Optik 120:1000–1006
Dhanuskodi S, Sabari Girisun TC, Vinitha S (2011) Optical limiting behavior of certain thiourea metal complexes under CW laser excitation. Curr Appl Phys 11:860–864
Sharafudeen KN, Adithya A, Vijayakumar S, Sudheesh P, Kalluraya B, Chandrasekharan K (2011) Multiphoton absorption process and self-focusing effect in coumarin derivative doped PMMA films by z-scan and optical limiting studies. Curr Appl Phys 11:1089–1093
Kavitha P, Christopher Jeyaseelan S, Sabari Girisun T.C (2022) Two photon absorption induced optical limiting action of L-Aspartic acid monohydrate. Opt Mater 134:113134
Chatterjee P, Dutta SS, Chakraborty T (2022) Tautomers and Rotamers of Curcumin: A Combined UV Spectroscopy, High-Performance Liquid Chromatography, Ion Mobility Mass Spectrometry, and Electronic Structure Theory Study. J Phys Chem A 126:1591
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT
Kolev TM, Velcheva EA, Stamboliyska BA, Spiteller M (2005) DFT and experimental studies of the structure and vibrational spectra of curcumin. Int J of Quantum Chem 102:1069
Yanai T, Tew D, Handy N (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51
Tomasi J, Mennucci B, Cammi R (2005) Quantum Mechanical Continuum Solvation Models. Chem Rev 105:2999
Margar SN, Sekar N (2016) Nonlinear optical properties of curcumin: solvatochromism-based approach and computational study. Mol Phys 114:1
Muhammad S, Shehzad RA, Iqbal J, Al-Sehemi AG, Saravanabhavan M, Khalid M (2019) Benchmark study of the linear and nonlinear optical polarizabilities in proto-type NLO molecule of para-nitroaniline. J of Theor and Comp Chem 18:1950030
Author information
Authors and Affiliations
Contributions
Conceptualization-S. Deepa, S. Madhu, S. Devasenan, Methodology- S. Jeyaram, M. Maaza, G. Murali Validation- M. Maaza, K. Kaviyarasu, Pattath. D. Pancharatna Writing-review and editing- Pattath. D. Pancharatna, S. Deepa, S. Jeyaram Supervision‒S. Jeyaram.
Corresponding author
Ethics declarations
Ethics Approval
The submitted work should be original and should not have been published elsewhere in any form or language.
Informed Consent
Not applicable.
Consent to Participate
Yes.
Consent for Publication
Yes granted.
Research Involving Human Participants and/or Animals
Research involving human participants.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deepa, S., Madhu, S., Devasenan, S. et al. Extraction of Natural Pigment Curcumin from Curcuma Longa: Spectral, DFT, Third-order Nonlinear Optical and Optical Limiting Study. J Fluoresc 34, 1885–1892 (2024). https://doi.org/10.1007/s10895-023-03421-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03421-x