Abstract
Progression of oral mucosal lesions is generally marked by changes in the concentration of the intrinsic fluorophores such as collagen, nicotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide (FAD) and porphyrin present in the human oral tissue. In this study, we have probed the changes in FAD and porphyrin by exciting with 405 nm laser light on different sites (tongue, buccal mucosa, lip etc.) of the oral cavity. Testing has been done by an in-house developed fluorescence-based portable imaging device on oral squamous cell carcinoma (OSCC) patients, dysplastic patients and control (normal) group. Fluorescence images recorded from OSCC and dysplastic patients have displayed an enhancement in the red band (porphyrin) as compared to those from the normal volunteers. Porphyrin to FAD intensity ratio (IPorphyrin/IFAD), referred to red to green ratio (Ired/Igreen) has been taken as the diagnostic marker for classification among the groups. Receiver operating characteristic (ROC) analysis applied on IPorphyrin/IFAD is able to discriminate OSCC to normal, dysplasia to normal and OSCC to dysplasia with sensitivities of 100%, 81%, 92% and specificities of 100%, 93% and 92% respectively. Fluorescence imaging probe can capture a large area of oral lesions in a single scan and hence would be useful for initial scanning. On comparison with spectroscopy studies performed by our group, it is found that combining both spectroscopy and imaging as a device may be effective for the early detection of oral lesions. This clinical study was registered on the date 13/10/2017 in the clinical trials registry-India (CTRI) with registration number CTRI/2017/10/010102.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, oral malignancy is emerging as a major health issue, ranking as the sixth most prevalent cancer. In India, the severity of the disease is estimated with 1.7 million cases being projected in 2035 as compared to 1 million cases reported in 2012. High mortality, shown by a 5-year survival rate (≤ 50%), is posing as a big challenge for clinicians and scientists [1,2,3]. In India, this is mainly attributed to late diagnosis and lack of effective diagnostic tools. Early and real time diagnosis is needed to improve the present scenario of high morbidity and high mortality rates [4].
Screening methods used to identify different stages of oral lesions are conventional oral examination (COE), oral cytology, oral brush biopsy, staining (Toluidine Blue, Lugol’s Iodine, Methylene Blue) and light-based detection systems. But the confirmatory diagnosis of oral cancer is tissue biopsy which is an invasive procedure followed by histological examination [5,6,7]. Here, it is critical to find the precise area for biopsy as the oral lesion is an extensive spread. Also, the technique is invasive and time consuming. Optical techniques may be useful in such limitation. Various optical techniques (Raman, fluorescence, Stokes shift, diffuse reflectance spectroscopy and imaging, optical coherence tomography (OCT) etc.) have been studied by the research groups for diagnosis of several cancers [8,9,10,11,12,13,14,15,16,17]. Among them fluorescence spectroscopy has emerged as a strong diagnostic tool for in vivo detection of oral cancer. Fluorescence based techniques (spectroscopy and imaging) have the potential of early diagnosis of oral cancer since they are sensitive to subtle biochemical changes during disease progression [18,19,20,21,22,23,24].
For fluorescence studies, tissue is generally illuminated by UV–visible light and fluorescence signal is recorded by a spectrometer or charge couple device (CCD). The major fluorophores present in human oral tissue are tryptophan, collagen, nicotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide (FAD) and porphyrin. It is well established that the concentrations of these fluorophores change with the development of the disease and fluorescence-based devices are able to detect these biochemical changes at an early stage of the disease [14]. Several research groups have carried out fluorescence spectroscopic studies for oral cancer detection. In a study on oral neoplasia, Gillenwater et al. observed an enhancement in red fluorescence in abnormal tissue. They found sensitivity of 88% and specificity of 100% in differentiating normal mucosa to abnormal [25]. Inaguma M. and Hashimoto K. reported that 85% of oral malignancies showed porphyrin-like fluorescence [26]. Analysis of fluorescence spectroscopy and imaging by Betz et al. showed distinct differentiating features between normal and malignant oral tissues of buccal mucosa and spectral differences in 94.4% of cases [27]. Van Staveren et al. found sensitivity of 86% and a specificity of 100% in differentiation of abnormal tissues from normal tissues by applying neural network analysis on fluorescence spectra [28]. Majumdar et al. obtained 86% sensitivity and 63% specificity in their in-vivo study for oral cancer detection using fluorescence spectroscopy [29]. A handheld fluorescence-based device tested for early oral cancer detection by Lane et al. showed sensitivity of 98% and specificity of 100% [30]. Fluorescence spectroscopy with multivariate analysis method by Nazeer et al. for early detection of oral cancer showed a sensitivity of 60 to 100% and specificity of 76 to 100% was obtained [31]. Di Stasio et al. used a high-frequency (18 MHz) linear ultrasound probe for capturing tongue malignant lesions of oral cavity. They found that the ultrasound images correlate well with histopathology results and could distinguish a tumour from healthy tissue [32, 33]
Spectroscopic study has been conducted by our group for oral precancer detection using an in-house developed device. Using the Mahalanobis distance-based classifier; they were able to discriminate among the groups with significantly higher values of sensitivities and specificities [34].
Fluorescence images produced by the endogenous fluorophores present in the oral tissue are captured by a charge coupled device (CCD). Oral cavity is largely heterogeneous, so large area scanning has the advantage of overcoming time-consuming point measurements of spectroscopy where one may miss crucial signatures. Many research groups have tested fluorescence imaging system for oral cancer detection [35,36,37,38,39,40]. Kulapaditharom and Boonkitticharoen achieved sensitivity of 100% and specificity of 73% for head and neck cancer detection using a fluorescence endoscope [36]. Betz et al. have shown much more clear differentiation between normal and malignant lesions in case of flat, epithelial tumours compared to exophytic tumours [37]. Paczona et al. observed the reduced intensity of green fluorescence and shift to reddish-blue for malignant tissues [38]. Using the ratio of red to green fluorescence, Rahman et al. classified the normal and cancerous tissues with a sensitivity of 90% and a specificity of 87% [39]. Roblyer et al. used quantitative fluorescence imaging to differentiate the cancerous samples and achieved 100% sensitivity and 91.4% specificity [40].
Here, we have reported in-vivo detection of oral mucosal lesions using fluorescence-based portable imaging device. Using the device, it is possible to capture an area of 1.0 cm2 of oral cavity lesions in a single scan. With 405 nm excitation wavelength, signatures of FAD and porphyrin are observed. The red band, which is attributed to porphyrin is found to increase in most of the OSCC and dysplastic groups than the control groups. For classification among the groups, receiver operating characteristic (ROC) analysis is applied on the ratio (IPorphyrin/IFAD) [41, 42]. In this study, we have also compared the imaging results with spectroscopy results. Spectroscopic study was performed by our group using an in-house developed device on OSCC, dysplastic and control patients and classification was done by using Mahalanobis distance classifier [34].
Materials and Methods
Instrumentation
The block diagram and photographs of the fluorescence-based imaging probe are shown in Fig. 1a, b, respectively. The imaging portable probe consists of a laser diode, collimating lens (CL), beam splitter (BS), camera lens, long-pass filter (LPF), band pass filters (BPFs), charge couple device (CCD) and connecting optical fibres. Laser light from a fibre coupled 405 nm diode laser (Diode Laser 405 nm, Model: ADR-1805) is irradiated onto the sample (oral tissue sites) via a beam splitter and by way of a collimating lens (UV/Visible collimating lens, 200-2000 nm). Reflected light falling on the oral cavity lesions captures an area of 1cm2. Fluorescence signal, transmitted through the beam splitter is recorded using a charge coupled device (PCO Pixelfly, digital 14 bit). LPF of 450 nm and BPFs of 500 nm and 635 nm are used in the device. These filters are replaced manually in the filter section of the device during the measurement. LPF (450 nm) allows the entire signal above the excitation λ (405 m) (i.e., signal due to FAD and porphyrin) and BPFs of 500 & 635 nm select only FAD and porphyrin bands respectively. Camware V3.17 Software is utilized for data acquisition. A disposable cap of teflon material is used at the tip of the probe while performing measurements on patients and volunteers. An optimal power of 2 milliwatts that falls on the oral cavity was enough to produce measurable fluorescence from patients and volunteers. Fluorescence spectral measurements were carried-out using an already existing in-house developed handheld device [34].
Patient Selection and Data Collection
Spectroscopic measurements were conducted on 55 tissue sites of 24 OSCC patients, 40 tissue sites of 17 dysplastic patients and 54 tissue sites of 23 normal volunteers. Imaging measurements were performed on 50 tissue sites of 25 patients (OSCC = 7, dysplastic = 18) and 41 tissue sites of 16 normal volunteers. The control groups who consumed tobacco and smoke cigarettes occasionally were also included in the study. The average age of OSCC and dysplastic patients was 45 ± 11 and 36 ± 8. However, the average age of control group was 30 ± 7. The above mentioned detail is summarized in Table 1. The patient selection was based on certain features (ulcer, white patches, red patches, mixture of white and red patches, lump etc.) observed by the medical doctor. The control group in our study were volunteers without any sign and symptoms. Another criterion of selection was the age of patients and volunteers (≥ 20 years). After completion of all these prior steps, fluorescence measurements were carried out in the presence of the doctor. Subsequently, patients were sent for biopsy.
For the clinical study, ethical clearance was obtained with IEC communication number IITK/IEC/2015–16/2/10 and clinical trials registry-India (CTRI) with registration number CTRI/2017/10/010102. Prior to the testing in patients and volunteers, benefits of the imaging device over the conventional devices as well as its working were explained to all participating patients and volunteers. Purpose to inform was to make them comfortable and then informed consents were taken. All the necessary details of patients and volunteers for example age, family background, life style, habits of smoking cigarette/bidi, etc. were taken in a questionnaire form.
Analysis Method
To analyse the data, receiver operating characteristic analysis (ROC) is employed on the intensity ratio values (IPorphyrin/IFAD). ROC is a statistical method to check the performance of a test. In ROC analysis, binary data sets among the three groups are classified by computing the cut-off values. While applying ROC analysis in a diagnostic test, a two-dimensional curve is generated, known as ROC curve. It is a plot of sensitivity and specificity. Sensitivity and specificity are the probabilities of identifying unhealthy and healthy groups as positive and negative respectively. Area under the ROC curve is also a parameter to check the performance of a given diagnostic test. Sensitivity and specificity is defined as
Results and Discussion
Fluorescence Spectra of the Oral Cavity
Averaged fluorescence spectra and the typical spectra recorded from OSCC, dysplastic and control groups in the scan range of 450 – 800 nm are shown in Fig. 2a, b respectively. Averaged spectra involve 55 sites of 24 OSCC patients, 40 sites of 17 dysplastic patients and 54 sites of 23 normal volunteers. Fluorescence spectra show a FAD band and porphyrin bands (major and minor) in all three groups. FAD band, also referred to as green band, is peaked around 500 nm and porphyrin bands, referred to as red bands, are peaked at 634, 676 and 703 nm respectively. It can be seen in the averaged spectra that intensity of porphyrin band is significantly higher than the FAD band in the OSCC group. In dysplastic group, intensity of porphyrin band is slight lower than the FAD band. However, in the control cases, porphyrin bands are almost non-existent compared to the FAD band. Figure 2b, which displays the typical spectra, shows that FAD band is dominant over the porphyrin in OSCC. However, in dysplastic cases porphyrin dominates over the FAD band. Such behavior was observed only in few cases.
Florescence Images of Oral Cavity
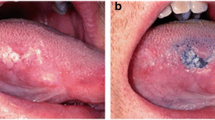
Typical fluorescence images recorded from an OSCC patient, a dysplastic patient and from a normal volunteer are displayed in Fig. 3Aa–c. Images displayed in figure are the original images (true images). These images were recorded using 450 long pass filter (LPF) in the device. Fluorescence images consist of RGB (red, green and blue) colours/bands. From the images, it is clear that OSCC has higher contrast of red colour and very much localized, dysplastic has lower contrast of red colour and normal does not show any signature visually. Red colour in the RGB appears due to porphyrin while blue and green colours appear due to NADH and FAD respectively. Extracted RGB images from the true images of OSCC, dysplastic and control group are shown in Fig. 3Ba–c.
In the extracted images, intensity of red band is higher in both OSCC and dysplastic cases than the control group. In the control case, intensity of green band is much higher than the red band. Red and green images were also collected using band pass filters (500 and 635 nm) on few patients and volunteers and their ratios are taken. Ratio between the Red to green bands was very close to the as obtained by using 450 nm LPF.
Data Analysis of Spectroscopic and Imaging Data
ROC analysis is applied on the ratio (IPorphyrin/IFAD) to determine the performance of diagnostic test. From the spectra shown in Fig. 2a, maximum values of intensities at 500 nm and 634 nm are taken from all the three groups and the ratio I (634)/I(500) are estimated. Scatter plot of these ratios in log scale are shown in Fig. 4a. ROC is employed on the log of the ratio values and cut-off values are obtained. Corresponding to these cut-off values, lines (solid, dash, dash with dot) are drawn in the scatter data plot. ROC curves are obtained as shown in Fig. 4b. OSCC to normal, dysplasia to normal and OSCC to dysplasia are differentiated with sensitivities of 89% (49/55), 83% (33/40), 80% (44/55) and specificities of 100% (54/54), 98% (53/54), and 69% (27/40) respectively.
Similarly, ROC analysis is applied on the imaging data. Here, maximum intensity values of red and green bands are extracted and their ratios are computed for each patient and volunteer. Scatter plots of OSCC, dysplastic and normal groups are shown in Fig. 5a. ROC curves are obtained and displayed in Fig. 5b. ROC differentiates among the groups with sensitivities of 100% (14/14), 81% (30/37), 92% (13/14) and specificities of 100% (37/37), 93% (38/41) and 92% (34/37) with the overall accuracies of 100%, 87% and 92% respectively. Patients (M/F) having different lesion types reported in the histopathology report and ratio of fluorescence intensity values with the standard deviation (SD) are summarized in Table 2. For better comparison of the results, sensitivity, specificity and accuracy values obtained in spectroscopic and imaging measurements are summarized in Table 3. These methods are useful mainly to the experts in oral medicine and pathology also because the same could give a false-positive result in the face of even a pseudo lesion [43].
Conclusion
This study is a comparison of spectroscopy and imaging techniques for the detection of different grades of oral lesions. To perform this work, portable device was fabricated and measurements were carried out on patients and volunteers. Biomarkers FAD and porphyrin were found in oral tissue cavity on patients and volunteers and taken as a diagnostic marker. Porphyrin contribution was found significantly higher in OSCC and dysplastic groups than the control group. Porphyrin to FAD ratio was chosen as a discriminator and using ROC on the ratio variables, sensitivity and specificity values were estimated. In both methods, accuracy values were found higher compare to the other techniques reported [27,28,29,30,31], especially in discriminating OSCC to normal and dysplasia to normal. Spectroscopy is a point measurement technique. The advantage of the imaging technique is that it demarcates the affected lesion. Once analysis is done with imaging technique, further testing with spectroscopy can be performed for confirmation and better demarcation of the cancerous region to find the best biopsy specimen. In conclusion, it can be said that autofluorescence spectroscopy and imaging both are equally promising as diagnostic tools for the detection of oral malignancies at early stage. Combination of both spectroscopy and imaging techniques in one handheld device may be the best way for robust diagnosis of oral cancer.
Availability of Data and Material
Authors do not have any objection to provide data. Data is available with the corresponding author.
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan. Int J Cancer 136(5):359–386
Chaturvedi AK, Anderson WF, Tieulent JL et al (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31(36):4550–4559
El-Naggar AK, Chan JKC, Takata T, Grandis JR, Slootweg PJ (2017) The 4th edition of the head and neck World Health Organization blue book: editors’ perspectives. Hum Pathol 66:10–12
Coelho KR (2012) Challenges of the oral cancer burden in India, J Cancer Epidemiol, Volume 2012. Article ID 701932:17
Omar E (2015) Current concepts and future of noninvasive procedures for diagnosing oral squamous cell carcinoma-a systematic review. Head Face Med 11
Scully C, Bagan JV, Hopper C, Epstein JB (2008) Oral cancer: Current and future diagnostic techniques. Am J Dent 21:199–209
Koenig K, Schneckenburger H (1994) Laser-induced autofluorescence for medical diagnosis. J Fluoresc 4:17–40
Ramanujam N, Mitchell MF, Mahadevan-Jansen A, Thomson SL, Staerkel G, Malpica A, Wright T, Atkinson N, Richardas-Kortum R (1996) Cervical precancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths. Photochem Photobiol 64:720–735
Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J (2000) Non-invasive in vivo characterization of breast tumours using photo migration spectroscopy. Neoplasia 2:26–40
Majumder SK, Gupta PK (2000) Synchronous luminescence spectroscopy for oral cancer diagnosis. Lasers Life Sci 9:143–152
Hillemanns P, Reiff J, Stepp H, Soergel P (2007) Lymph node metastasis detection of ovarian cancer by porphyrin fluorescence photodetection: case report. Lasers Med Sci 22:131–135
De Veld DC, Bakker Schut TC, Skurichina M, Witjes MJ, Van der Wal JE, Roodenburg JL, Sterenborg HJ (2005) Autofluorescence and Raman microspectroscopy of tissue sections of oral lesions. Lasers Med Sci 19:203–209
Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, Yeoh KG, So JB, Huang Z (2011) Combining near-infrared-excited autofluorescence and Raman spectroscopy improves in vivo diagnosis of gastric cancer. Biosens Bioelectron 26:4104–4110
Alfano RR (2012) Advances in ultrafast time resolved fluorescence physics for cancer detection in optical biopsy. AIP Adv 2:011103
Singh SP, Deshmukh A, Chaturvedi P, Murali KC (2012) In vivo Raman spectroscopic identification of premalignant lesions in oral buccal mucosa. J Biomed Opt 17105002
Devi S, Panigrahi PK, Pradhan A (2014) Detecting cervical cancer progression through extracted intrinsic fluorescence and principal component analysis. J Biomed Opt 19:127003
DeCoro M, Wilder-Smith P (2010) Potential of optical coherence tomography for early diagnosis of oral malignancies. Expert Rev Anticancer Ther 10:321–329
De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL (2005) The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol 41:117–131
Patton LL, Epstein JB, Kerr AR (2008) Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc 139:896–905
Farwell DG, Meier JD, Park J, Sun Y, Coffman H, Poirier B, Phipps J, Tinling S, Enepekides DJ, Marcu L (2010) Time- resolved fluorescence spectroscopy as a diagnostic technique of oral carcinoma. Arch Otolaryngol Head Neck Surg 136:126–133
Amelink A, Sterenborg HJ, Roodenburg JL, Witjes MJ (2011) Non-invasive measurement of the microvascular properties of non-dysplastic and dysplastic oral leukoplakias by use of optical spectroscopy. Oral Oncol 47:1165–1170
Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, Berean KW, Ng S, Tseng OL, MacAulay C et al (2006) Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res 12(22):6716–6722
Paderni C, Compilato D, Carinci F, Nardi G, Rodolico V, Lo Muzio L, Spinelli G, Mazzotta M, Campisi G (2011) Direct visualization of oral-cavity tissue fluorescence as novel aid for early oral cancer diagnosis and potentially malignant disorders monitoring. Int J Immunopathol Pharmacol 24:121–128
Kumar P, Singh A, Kanaujia SK, Pradhan A (2018) Human saliva for oral precancer detection: a comparison of fluorescence & stokes shift spectroscopy. J Fluoresc 28:419–426
Gillenwater A et al (1998) Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Arch Otolaryngol Head Neck Surg 124:1251–1258
Inaguma M, Hashimoto K (1999) Porphyrin-like fluorescence in oral cancer: in vivo fluorescence spectral characterization of lesions by use of a near-ultraviolet excited autofluorescence diagnosis system and separation of fluorescent extracts by capillary electrophoresis. Cancer 86:2201–2211
Betz CS, Mehlmann M, Rick K, Stepp H, Grevers G, Baumgartner R et al (1999) Autofluorescence imaging and spectroscopy of normal and malignant mucosa in patients with head and neck cancer. Lasers Surg Med 25:323–334
Van Staveren HJ, van Veen RL, Speelman OC, Witjes MJ, Star WM, Roodenburg JL (2000) Classification of clinical autofluorescence spectra of oral leukoplakia using an artificial neural network: A pilot study. Oral Oncol 36:286–293
Majumdar SK et al (2000) A pilot study on the use of autofluorescence for diagnosis of the cancer of human oral cavity. Curr Sci 79:1089–1094
Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh C, Ng S, Williams M, Zhang L, Rosin M, MacAulay CE (2006) Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt 11024006
Nazeer SS et al (2014) Noninvasive assessment of the risk of tobacco abuse in oral mucosa using fluorescence spectroscopy: a clinical approach. J Biomed Opt 19:057013
Di Stasio D, Lauritano D, Paparella R, Franco R, Montella M, Serpico R, Lucchese A (2017) Ultrasound imaging of oral fibroma: a case report. J Biol Regul Homeost Agents 31(1):23–26
Di Stasio D, Montella M, Romano A, Colella G, Serpico R, Lucchese A (2022) High-definition ultrasound characterization of squamous carcinoma of the tongue: a descriptive observational study. Cancers 14(3):564
Kumar P, Kanaujia SK, Singh A, Pradhan A (2019) In vivo detection of oral precancer using a fluorescence-based, in-house-fabricated device: a Mahalanobis distance-based classification. Lasers Med Sci 34:1243–1251
Onizawa K, Okamura N, Saginoya H, Yoshida H (2003) Characterization of autofluorescence in oral squamous cell carcinoma. Oral Oncol 39:150–6
Kulapaditharom B, Boonkitticharoen V (2001) Performance characteristics of fluorescence endoscope in detection of head and neck cancers. Ann Otol Rhinol Laryngol 110:45–52
Betz CS, Stepp H, Janda P, Arbogast S, Grevers G, Baumgartner R et al (2002) A comparative study of normal inspection, autofluorescence and 5-ALA-induced PPIX fluorescence for oral cancer diagnosis. Int J Cancer 97:245–252
Paczona R, Temam S, Janot F, Marandas P, Luboinski B. (2003) Autofluorescence videoendoscopy for photodiagnosis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 260(10):544-548
Rahman MS, Ingole N, Roblyer D, Stepanek V, Richards-Kortum R, Gillenwater A et al (2010) Evaluation of a low-cost, portable imaging system for early detection of oral cancer. Head Neck Oncol 2:10
Roblyer D, Kurachi C, Stepanek V, Williams MD, El-Naggar AK, Lee JJ et al (2009) Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev Res (Phila) 2:423–431
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874
Sah AN, Kumar P, Pradhan A (2021) Fluorescence-based handheld imaging device for in-vivo detection of oral precancer. Proc of SPIE-OSA 11919:119190J
della Vella F, Lauritano D, Lajolo C, Lucchese A, Di Stasio D, Contaldo M, Serpico R, Petruzzi M. (2019) The pseudolesions of the oral mucosa: Differential diagnosis and related systemic conditions. Appl Sci 9(12):2412
Acknowledgements
Author is thankful to Dr. Surendra Kumar Kanaujia (ENT specialist at GSVM Medical College Kanpur) for providing samples. Special thanks to all participating patients and volunteers.
Funding
We would like to acknowledge IMPRINT India for financial support.
Author information
Authors and Affiliations
Contributions
All authors have contributed to execute this clinical work and preparing the manuscript. Spectroscopic data collection and data analysis was done by [Pavan Kumar] and imaging data collection and analysis was done by [Amar Nath Sah]. Few sections of the first draft of the manuscript was written by [Pavan Kumar] and few by [Amar Nath Sah]. Final manuscript was corrected by [Asima Pradhan].
Corresponding author
Ethics declarations
Ethics Declaration Statement
To conduct this study, ethical clearance was taken. It was approved by GSVM Medical College Kanpur and IIT Kanpur committee members. Clearance is accordance to declaration of Helsinki.
Consent to Participate
Consent was taken from the participating patients and volunteers before testing.
Consent of Publication
We all authors are permitting to publish this work.
Conflict of Interest/Competing Interests
The authors have not any relevant financial interests in this article and no potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sah, A.N., Kumar, P. & Pradhan, A. In-vivo Testing of Oral Mucosal Lesions with an In-house Developed Portable Imaging Device and Comparison with Spectroscopy Results. J Fluoresc 33, 1375–1383 (2023). https://doi.org/10.1007/s10895-023-03152-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03152-z