Abstract
We report here a study on human saliva tested as a diagnostic medium for oral cancer detection on three groups: oral squamous cell carcinoma (OSCC), oral sub mucous fibrosis (OSMF; precancer), and healthy controls (normal). Measurements have been conducted using fluorescence spectroscopy with 350 nm excitation and Stokes shift (SS) spectroscopy (SSS) with 120 nm shift from a total of 99 saliva samples. For classification, principal component analysis (PCA) and linear discriminant analysis (LDA) have been applied on the data sets. Linear discriminant (LD) scores of fluorescence spectra are able to differentiate OSCC to normal, OSMF to normal and OSCC to OSMF with sensitivities 91%, 92%, 91% and specificities 97%, 100%, 94% respectively, while LD scores of SS spectra differentiate respective groups with sensitivities 100%, 94%, 94% and specificities 97%, 100%, 94%. Cross-validation on the datasets of PC scores during LDA illustrates that sensitivity and specificity of SSS data are less affected than those of fluorescence data. Saliva is thus seen as a potential non-invasive and simple diagnostic medium, with SS spectroscopy as a better diagnostic tool for oral precancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A five year survival rate of oral cancer is known to be less than 45% due to lack of early symptoms and diagnosis at advance stage [1]. Early detection is thus imperative to reduce mortality rate. Oral premalignant lesion starts in the form of leukoplakia (white patches), erythroplakia (red patches), erythrolukoplakia (red and white patches) and oral submucosal fibrosis (OSMF) and finally these lesions convert into malignancy (such as squamous cell carcinoma) with different rates [2, 3]. In India, oral cancer is the most common cancer and ranked number one among males and third most in females. Globally it is 10 most common cancer [4,5,6].
Conventional biopsy techniques used for identifying oral premalignant and malignant lesions are visual inspection, use of toluidine blue, brush biopsy, cytology brushing and tissue biopsy by a pathologist, among which tissue biopsy with histopathology is gold standard [7, 8]. Tissue biopsy is not only an invasive method but an added problem with it is to decide on the best area for biopsy, since the lesions are spread over a large area of oral cavity. Different non-invasive techniques such as fluorescence spectroscopy, Raman spectroscopy, diffuse reflectance spectroscopy etc. have been extensively used for in vivo and in vitro study [9,10,11,12,13,14]. Among these techniques fluorescence spectroscopy is widely used by several researches for in vivo detection of oral cancer and has been established as a powerful diagnostic tool [15, 16] since it is simple to use, cheap and less time consuming. Among all these properties its high sensitivity also makes it more robust for detecting any subtle biochemical changes in tissue. Fluorescence spectroscopic studies for diagnostic were pioneered by Alfano and his group on human teeth and on malignant and normal human breast and lung tissues [17, 18]. However limitations appear for multi fluorophores with overlapping emission bands. Decoupling these bands is effectively done through Synchronous fluorescence (SF) spectroscopy (SFS) [19,20,21,22]. In conventional fluorescence spectroscopy, we scan emission signal for a fixed excitation wavelength and in excitation fluorescence spectroscopy scan of excitation signal for a fixed emission wavelength are taken. However, in SF spectroscopy, simultaneous scan of emission and excitation signals with constant wavelength interval (Δλ) are recorded. This simultaneous scan makes it unique in resolving multiple component systems such as overlapping natural fluorophores in human tissue and thus offers better specificity of target fluorophores. Tuan Vo-Dinh had shown that SF spectroscopy has the potential to resolve complex systems [19]. R. R Alfano et al. used Stokes shift spectroscopy (SSS) on mixture solution of fluorescent molecules (tryptophan, NADH, FAD) as well as normal breast tissue at different fixed wavelength intervals and were able to resolve and achieve signatures of maximum number of fluorophores in a single scan [23]. J Ebenezar et al. used SF technique for cervical cancer detection in normal and abnormal tissues. Using 20 nm offset, they were able to differentiation different grades of cancer with 100% sensitivity and specificity [24]. Yang Pu et al. have also utilized SS spectroscopy for breast cancer detection on cancerous and normal tissues as well as on mixed solution of fluorophores tryptophan, NADH and FAD. They obtained the optimal Stokes shift for cancer detection in human tissue and achieved sensitivity of 80% and specificity of 86.7% [25]. During the progress of cancer various changes occur in the tissue of oral cavity, such as nuclear size distribution, blood concentration, fluorophore concentration [12]. Unlike the change in human oral tissue, changes are also perceived in human saliva during development of oral cancer. It is well known that biochemical changes are reflected in biofluids such as blood and urine [26, 27]. Similarly, human saliva consists of many biomarkers like amino acids (tryptophan, tyrosin etc.), proteins, amylase, deoxyribonucleic acids (DNA), ribonucleic acids (RNA), coenzymes (NADH, FAD etc.), porphyrin and metabolites etc. [28, 29]. As the disease progresses, changes in these biomarkers also occur [30, 31]. During in vivo detection of oral cancer, it is sometimes found that the lesions are spread deep inside the oral cavity like tonsillar fossa, near the neck and inside the neck as well as symptoms of difficulty in opening of the mouth are also seen. For such cases, measurements was tedious to perform. Here saliva may be an appropriate medium for detection. Daily production of saliva is between 1 and 1. 5 L and so, easy to collect. Since collection of saliva is easy and non-invasive for patients/volunteers of any age group, this makes it an unique bio-fluid for diagnostic purpose. Human saliva has been a promising disease detection bio-fluid studied by various groups over the past 10 to 15 years for different purposes using different techniques. Nikolaos S. Soukos et al. have reported its use for forensic purposes such as sexual assault and child abuse cases by using fluorescence spectroscopy [32]. It has also been utilize to examine drug abuse cases and to diagnose HIV infected patients [33]. There are reports of its application in breast cancer detection too [34]. Xiaozhou Li et al. have reported its use for lung cancer detection by using surface enhanced Raman spectroscopy and differentiated cancer from normal with sensitivity of 78% and specificity of 83% [35]. M. Yuvaraj et al. have detected oral cancer by fluorescence spectroscopy (emission and excitation) with sensitivity of 85.7% and specificity of 93.3% [36]. A bimodal detection by high performance liquid chromatography (HPLC)-along with laser-induced fluorescence (LIF) spectroscopy has been studied by Patil et al. for oral precancer detection using human saliva. They were able to differentiate the respective groups with 79% sensitivity and 78% specificity [37]. Concentration based studies have been performed by Rafael Nagler et al. for oral cancer detection between cancer and normal group and found sensitivity and specificity of 71% and 75% [38]. Diagnostic results for oral cancer with fluorescence spectroscopy have thus shown promise. SS spectroscopy is a specific diagnostic tool compared to conventional fluorescence spectroscopy and as per our knowledge, has not been used for precancer detection with human saliva as diagnostic medium. We report a comparison of fluorescence and SS spectroscopy of saliva for efficiently detecting oral precancer and cancer.
As a background study, we had used various Δλ values, specifically 20, 70, 90 and 120 nm and spectral data for these had been analyzed. Δλ = 120 nm, which is the Stokes shift for NADH, effectively captures the changes from most of the fluorophore bands (tryptophan, NADH, FAD and porphyrin) and displays difference in intensities among the groups (OSCC, OSMF and control). Whereas other Δλ values were not able to resolve all these fluorophores well enough. In a systematic data analysis (PCA and LDA) for these Δλ values, 120 nm has provided the maximum discrimination among the groups. None of the excitation wavelengths (325 nm, 405 nm and 450 nm) could discriminate the different grades as well as 350 nm could. We have thus reported a systematic study and comparison of fluorescence at 350 nm excitation and SS spectroscopy for 120 nm Stokes shift for oral cancer detection through human saliva.
Materials and Methods
Sample Collection
Measurements has been performed on a total of 99 human saliva samples, which were collected from 34 oral squamous cell carcinoma patients (OSCC), 35 oral sub mucous fibrosis patient (OSMF) and 30 from normal volunteers. The average age of OSCC patients was 48 ± 16, for OSMF patients was 41 ± 17 and for normal volunteers, it was 33 ± 9. Patients reporting to clinicians for treatment were asked not to consume food and drink water if required, so that they can generate saliva. It was also suggested to clean their oral cavity with water so that effect of food on saliva could be minimized. Normal volunteers who have given saliva were completely free from any habits and completely free from any disease. Ethical clearance was obtained with IEC communication number IITK/IEC/2015-16/2/10 and written consent was taken from all participating patients and volunteers. All the details of the patient such as age, occupation, family background, life style and habits (cigarate/bidi smoking, alcohol consumption etc.) were noted in the form in questionnaire. Saliva samples were collected in small sterile containers. Patients generally take 5 to 7 min to generate 2 to 3 ml of saliva. Collection of saliva samples was done in Hallet hospital affiliated to GSVM medical college, Kanpur. After collection of saliva samples, patients were sent for biopsy. A small piece of tissue from the affected area of the oral cavity was removed and sent for histopathology. The saliva samples in small sterile containers were brought in an ice box to IIT Kanpur campus. Collection of samples and bringing it to IIT Kanpur campus takes approximately two to three hours. The container of saliva was placed at room temperature (25 °C) for half an hour, after which saliva was poured into a quartz cuvette of size (1cm × 1cm × 5 cm) and measurements were taken. Histopathology reports of oral tissue samples were later obtained and correlated with the optical results.
Measurement Techniques
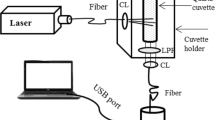
Fluorescence spectral measurements were taken on a spectrofluorimeter (Fluorolog 3, Model FL3-22). For all fluorescence spectroscopic measurements, slit width was 2 nm for both monochromators (excitation and emission) and spectra acquisition was performed with 0.1 s integration time. For SSS measurements, slit width was fixed at 1 nm for both monochromators and spectra acquisition were taken in 0.1 s integration time. Fluorescence signals were collected at angle 22.5 degree from the incident light to avoid specular reflection and recorded in the range from 380 to 650 nm for 350 nm excitation wavelength and for SSS scan, spectra were recorded in the range 250 to 550 nm for 120 nm Stoke shift.
Analysis Methods
Principal component analysis (PCA) has been applied on the fluorescence spectra in the range 380 to 650 nm with dimensions 271 and in SS spectra in the range 250 to 550 nm with dimensions 301. These higher dimensional data sets have been reduced to lower dimensional data sets by computing the principle components of correlation matrix. The correlation maps are then plotted with both axes representing wavelength shift of emission from the excitation wavelength 350 nm in the fluorescence data and a shift from 250 nm in SSS data. After computing principal components of correlation matrix, principle component scores are calculated by projecting the original data along the principle components. To obtain a better discrimination among the groups, further reduction of dimension was performed by using linear discriminate analysis (LDA) model. PC scores obtained from fluorescence spectra and SS spectra were loaded sequentially in LDA model and LD vectors have been computed. Kernel probability density functions have been plotted to show the distribution of LD scores [39,40,41].
Results and Discussion
Fluorescence Spectroscopy
Averaged fluorescence spectra and typical spectra of OSCC patients, OSMF patients and normal volunteers at excitation wavelength 350 nm in the scan range 380–650 nm are shown in the Fig. 1a, b respectively. Major NADH band and a minor porphyrin band are observed in the spectra. An FAD band (520 nm) may be masked within the broad band. Mean peak positions of NADH bands for OSCC patients, OSMF patients and normal volunteers with their standard deviation are 439 ± 7 nm, 435 ± 6 nm and 432 ± 6 nm respectively. Peak at 398 nm of a minor band is distinct in normal and OSMF but shows up as a subtle hump for OSCC. This is confirmed as a water Raman peak. Averaged fluorescence peak intensities of the three groups show significant differences, though the standard deviation displays some overlap. In 90% of cases, it was observed that fluorescence intensities of OSCC and OSMF patients were much higher than normal volunteers, while in more than half of the cases, fluorescence intensity of OSCC patients were higher than OSMF patients. OSCC to normal, OSMF to normal, and OSCC to OSMF by taking peak value of intensities are classified with sensitivities of 94%, 91%, 70% and specificities of 93%, 87%, 86% respectively. It is interesting to note here that though the FAD band is masked in the spectra, such bands are clearly visible as domains in the correlation maps seen in Fig. 2a-c, more distinctly in the OSCC cases. There is clear difference in correlation maps among the groups and three fluorescence domains along with a minor Raman domains are observed in the three groups which appear very distinct in OSCC.
Stokes Shift Spectroscopy (SSS)
Averaged SS spectra with typical spectra of OSCC patients, OSMF patients and normal volunteers at 120 nm Stokes shift are shown in the Fig. 3a, b. Stokes shift of 120 nm gives two major bands and some minor bands are noticed in the typical spectra. Mean peak positions of first major band for OSCC patients, OSMF patients and normal volunteers with their standard deviation are 274 ± 4.7 nm, 272 ± 2.8 nm and 270 ± 1.9 nm respectively, while those of the second major band are 340 ± 7 nm, 345 ± 7.7 nm and 348 ± 11 nm respectively. The bands are sharper and more distinct than the fluorescence bands and are attributed to tryptophan and NADH. Averaged SS spectra show significant difference in intensity of the second major band, among all the groups. Mean peak intensities taken around 330–360 nm from OSCC, OSMF and normal groups with their standard deviation display overlap among groups with values (1.24 ± 1.16)*104 nm, (5.86 ± 4.46)*103 nm and 1868 ± 752 nm respectively. Minor bands also appear in SS spectra in normal, OSMF and OSCC groups seen more distinctly in typical spectra, while averaged spectra do not show these bands clearly. In the typical spectra, minor bands observed at 420 nm and 525 nm are attributed to FAD to porphyrin respectively. It may be noted that though tryptophan in not excited with 350 nm in the fluorescence spectra, it appears very distinctly in the SS spectrum. Well defined domains appear in correlation maps as seen in Fig. 4a-c, which are more distinct than in the fluorescence case, as expected. Clear differences are noticed in the correlation plots of different groups.
Statistical Analysis (PCA and LDA)
As described in the Materials and Methods section, PCA has been applied to the fluorescence and SS spectra. The first four PCs (PC1 to PC4) of fluorescence spectra and first six PCs (PC1 to PC6) of SS spectra with variance ≥ 98% are chosen. Figure 5a, b display scatter plots of the first three PC scores obtained from fluorescence and SS spectra respectively. Clusters formed by the normal groups in both cases are well separated from the overlapped clusters formed by OSMF and OSCC groups. However overlap for these three PC scores between OSCC and OSMF group is higher in SS spectra compared to fluorescence spectra.
The first two LD vectors captured information with 99.99% variance for PCs of fluorescence and SS spectra. Corresponding to these LD vectors, LD scores have been obtained. Kernel probability density functions of linear discriminant scores (LD1 & LD2) extracted from PC scores of fluorescence and SS spectra of the three groups have been plotted to show how first and second LD scores of each group are separated from one another. It is evident in Fig. 6a that first LD score of fluorescence spectra clearly discriminates only OSCC from the other two, while second LD score decouples the OSMF and normal, as seen in Fig. 6b. Together LD1 and LD2 scores discriminate all the groups. A similar trend is seen in SSS probability density plots in Fig. 7a, b, where OSMF is discriminated from OSCC and normal in (a) and normal is discriminated from OSMF and OSCC in (b). The probability densities of the different groups for fluorescence display more overlap than for SS case. Overlap is indicated by the shaded regions in Figs. 6 and 7. For fluorescence, 23% and 53% of normal and OSMF groups overlap with one another as can be seen in Fig. 6a and 34% and 17% of normal and OSCC groups overlap as shown in Fig. 6b. Lower overlaps of 26% and 47% from normal and OSCC groups and 25% and 2% from OSMF and OSCC groups are observed in SSS illustrated by the shaded regions in Fig. 7a, b. Since both LD1 and LD2 contribute to discriminating all three groups, LD1 versus LD2 are plotted in Fig. 8a, b respectively and three distinct clusters are seen.
To differentiate among the clusters, cut off lines passing through zero have been drawn and sensitivity and specificity have been calculated. Sensitivity and specificity of fluorescence and SS spectroscopy with and without cross-validation has been shown in Table 1. Fluorescence spectroscopy differentiates OSCC/normal, OSMF/normal and OSCC/OSMF with high sensitivities and specificities at 95% confidence level (p < 0.05), while SS spectroscopy differentiates the respective groups with higher sensitivities and specificities. The cross-validation on fluorescence spectra results in changes up to 8%, but only up to 5% in SS spectra.
Conclusion
In this study, human saliva has been used as a diagnostic medium for oral precancer detection using fluorescence and SS spectroscopy as diagnostic tools. Both methods show significantly higher intensity in OSCC, OSMF compared to the control samples. In the fluorescence spectra of all the three groups the major band is due to presence of NADH and a sharp peak at 398 nm is confirmed as the Raman scattering by water present in human saliva [42]. Presence of porphyrin is noticed at 625 nm. Interestingly a FAD domain is distinct in the correlation map, while it is masked in the broad fluorescence spectra. Domains indicating presence of NADH, porphyrin are observed in correlation maps of fluorescence data, more distinctly in OSCC group. A tryptophan band, not seen in the fluorescence spectra, is observed in the SS spectra at around 270 nm, while the second major band around 340 nm is due to NADH.42 The SS correlation maps display very well defined domains of tryptophan, NADH, FAD and porphyrin. The number of domains increases with grades of cancer. This indicate that as the precancer develops to cancer, there may occur enhanced emission from other fluorescent molecules such as FAD and porphyrin. The 120 nm Stokes shift effectively captures the changes from most of these fluorophore bands and displays difference in intensities among the groups. The importance of using SS spectroscopy is illustrated through the fluorescence from tryptophan which does not appear in the fluorescence spectrum. This suggest that the contribution of all four fluorophores tryptophan, NADH, FAD and porphyrin play an important role in discriminating the precancer and cancer from control group. To classify the groups, PCA and LDA have been applied. Almost 100% variance is obtained with first two LD vectors and their scores clearly discriminate the three groups from one another. K-fold cross-validation during LDA also illustrates the robustness of SSS since sensitivity and specificity of its data are less affected than those of fluorescence data. There is more overlap of the probability density in the case of fluorescence as compared to SSS. The analysis thus finally indicates that SS spectroscopy may be a better diagnostic tool for oral precancer detection, using saliva as the diagnostic medium.
References
Neville BW (2002) Oral cancer and precancerous lesions. CA Cancer J Clin 52:195–215
Yardimci G, Kutlubay Z, Engin B, Tuzun Y (2014) Precancer lesions of oral mucosa. World J Clin Cases 2(12):866–872
Hazarey VK, Erlewad DM, Mundhe KA, Ughade SN (2007) Oral submucous fibrosis: study of 1000 cases central India. J Oral Path Med 36:7–12
Varshitha A (2015) Prevalence of oral cancer in India. J Pharm Sci Res 7(10):845–848
Dikshit R, Gupta PC, Ramasundarahettige C et al (2012) Cancer mortality in India: a nationally representative survey. Lancet 379:1807–1816
Chaturvedi AK, Anderson WF, Tieulent JL et al (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31(36):4550–4559
Scully C, Bagan JV, Hopper C, Epstein JB (2008) Oral cancer: Current and future diagnostic techniques. Am J Dent 21(4):199–209
Fedele S (2009) Diagnostic aids in the screening of oral cancer. Head Neck Oncol 1:1–5
Wang CY, Chiang HK, Chen CT, Chiang CP, Kuo YS, Chow SN (1999) Diagnosis of oral cancer by light-induced auto fluorescence spectroscopy using double excitation wavelengths. Oral Oncol 35:144–150
Omar EA (2015) Current concepts and future of noninvasive procedures for diagnosing oral squamous cell carcinoma-a systematic review. Head Face Med 11:6
Pichardo JL, García OB, Franco MR, Juarez GG (2007) Raman spectroscopy and multivariate analysis of serum samples from breast cancer patients. Lasers Med Sci 22:229–236
De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL (2005) The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol 41:117–131
Schantz SP, Kolli V, Savage (1998) In vivo cellular fluorescence and histological characteristics of head and neck cancer. Clin Cancer Res 4:1177–1182
Ramanujam N, Mitchell MF, Jansan AM et al (1996) Cervical precancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths. Photochem Photobiol 64(4):720–735
Lane PM, Gilhuly T, Whitehead P et al (2006) Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt 11(2):024006
Inaguma M, Hashimoto K (1999) Porphyrin-like fluorescence in oral cancer. Cancer 86(11):2201–2211
Alfano RR, Lam W, Zarrabi HJ, Alfano MA, Cordero J, Tata DB, Swenberg CE (1984) Human teeth with and without caries studied by laser scattering, fluorescence, and absorption spectroscopy. IEEE J Quantum Electron 20(12):1512–1516
Alfano RR, Tang GC, Pradhan A, Lam W, Choy DSJ, Opher E (1987) Fluorescence spectra from cancerous and normal human breast and lung tissues. IEEE J Quantum Electron 23(10):1806–1811
Vo-Dinh T (1978) Multicomponent analysis by synchronous luminescence spectroscopy. Anal Chem 50(3):396–401
Majumder SK, Gupta PK (2000) Synchronous luminescence spectroscopy for oral cancer diagnosis. Lasers Life Sci 9:143–152
Rajasekaran R, Aruna P, Koteeswaran D, Baludavid M, Ganesan S (2014) Synchronous luminescence spectroscopic characterization of urine of normal subjects and cancer patients. J Fluoresc 24(4):1199–1205
Devi S, Ghosh N, Pradhan A (2015) A technique for correction of attenuations in synchronous fluorescence spectroscopy. J Photochem Photobiol 151:1–9
Alfano RR, Yang Y (2003) Stokes shift emission spectroscopy of human tissue and key molecules. IEEE J Sel Top Quantum Electron 9:148–153
Ebenezar J, Aruna P, Ganesan S (2010) Synchronous fluorescence spectroscopy for the detection and characterization of cervical cancers in vitro. J Photochem Photobiol 86:77–86
Pu Y, Wang W, Yang Y, Alfano RR (2012) Stokes shift spectroscopy highlights of cancerous and normal human tissues. Optical Letters 37(16):3360–3362
Schmidt B, Engel E, Carstensen T, Weickmann S, John M, Witt C (2005) Quantification of free RNA in serum and bronchial lavage: a new diagnostic tool in lung cancer detection? Lung Cancer 48:145–147
Bryzgunova OE, Skvortsova TE, Kolesnikova EV et al (2006) Isolation and comparative study of cell-free nucleic acids from human urin. Ann NY Acad Sci 1075:334 – 40
Markopoulos AK, Michailidou EZ, Tzimagiorgis G (2010) Salivary markers for oral cancer detection. Open Den J 4:172–178
Wu JY, Chen Y, Chung HR et al (2010) Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol 46:226–231
Harris AT, Lungari A, Needham CJ et al (2009) Potential for Raman spectroscopy to provide cancer screening using a peripheral blood sample. Head Neck 1:34
Kuznetsov A, Frorip A, Maiste A, Rosenberg MO, Sünter A (2015) Visible auto-fluorescence in biological fluids as biomarkers of pathological process and new monitoring tool. J Innov Opt Health Sci 8(3):1541003
Soukos NS, Crowley K, Bamberg MP, Gillies R, Doukas AG, Evans R, Kollias N (2000) A rapid method to detect dried saliva stains swabbed from human skin using fluorescence spectroscopy. Forensic Sci Int 114:133–138
Virkler K, Lednev IK (2009) Analysis of body fluids for forensic purposes: From laboratory testing to non-destructive rapid confirmatory identification at a crime scence. Forensic Sci Int 188:1–17
Hossein FA, Dizgah IM, Rahimi A (2009) Correlation of serum and salivary CA15–3 levels in patients with breast cancer. Med Oral Patol Oral Cir Bucal 14(10):521–524
Xiaozhou L, Yang T, Lin J (2012) Spectral analysis of human saliva for detection of lung cancer using surface-enhanced Raman spectroscopy. J Biomed Opt 17(3):037003
Yuvaraj M, Udayakumar K, Jayanth V et al (2014) Fluorescence spectroscopic characterization of salivary metabolites of oral cancer patients. J Photochem Photobiol 130:153–160
Patil A, Choudhari KS, Unnikrishnan VK et al (2013) Salivary protein markers: a noninvasive protein profile-based method for the early diagnosis of oral premalignancy and malignancy. J Biomed Opt 18(10):101317
Nagler R, Bahar G, Shpitzer T, Feinmesser R (2006) Concomitant analysis of salivary tumor markers – A new diagnostic tool for oral cancer. Clin Cancer Res 12(13):3979–3984
Devi S, Panigrahi PK, Pradhan A (2014) Detecting cervical cancer progression through extracted intrinsic fluorescence and principal component analysis. J Biomed Opt 19(12):127003
Mazanec J, Melisek M, Pavlovicova J (2008) Support vector machines, PCA and LDA in face recognition. J of Electrical Engineering 59(4):203–209
Silverman BW (1986) Density estimation for statistics and data analysis. J Appl Probab Stat 30(7):175
Al-Thunayan MA (2006) Cancer diagnosis by synchronous fluorescence spectra of blood and urine components. Phd thesis, King Saud University
Acknowledgements
We thank to Dr. Shivani Nautiyal and Dr. Kumar Ashutosh for helping in saliva collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial interests in this article and no potential conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Kumar, P., Singh, A., Kumar Kanaujia, S. et al. Human Saliva for Oral Precancer Detection: a Comparison of Fluorescence & Stokes Shift Spectroscopy. J Fluoresc 28, 419–426 (2018). https://doi.org/10.1007/s10895-017-2203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2203-2