Abstract
The diagnostic pathway for oral suspicious lesions usually starts with the clinical examination based on inspection and palpation of the oral mucosa. Such a phase is strongly related to the experience of the operator. Moreover, oral epithelial dysplasia and early oral carcinomas may already be present within areas of macroscopically intact oral mucosa. A great interest for techniques potentially improving the diagnostic accuracy has developed in several fields of surgical oncology in order to increase the specificity and sensitivity of the conventional diagnostic pathway. The development of noninvasive methods for real-time screening of neoplastic changes in oral cavity may be associated with the improvement of patients’ quality of life and survival rate. The analysis of tissue autofluorescence (AF) for improving sensitivity and specificity in cancer diagnosis has been proposed for different organs, including colon, lung, cervix, and esophagus. Particularly, there are several evidences supporting the effectiveness of this technique in head and neck cancer diagnosis. Autofluorescence shows high specificity and sensitivity for oral cancer and precancerous lesions: 72.4% and 63.79%, respectively. It can also provide valuable information for diagnosis, for planning of margin resection in surgical excision, and for monitoring the therapeutic response during follow-up. Direct visual fluorescence examination (DVFE) is based on the action of irradiation of specific wavelengths, between 375 and 440 nm, which excites some natural fluorochromes which show fluorescence in the range of the green color. The analysis of the lesions with AF tools must be performed in a dark environment to avoid the interference of white light wavelengths and to improve the quality of recorded images. Healthy oral mucosa emits fluorescence, detectable as green light. Cell and tissues within dysplastic and malignant lesions display modifications of the amount, distribution, and chemical–physical properties of the endogenous fluorophores. This results in an autofluorescence pattern variation that can be potentially used at diagnostic level. Loss of autofluorescence (LAF) seems to increase in correspondence to the progression of dysplasia, and altered tissue appears dark (brown to black). LAF in dysplasia and carcinoma seems to be connected to different mechanisms, such as altered metabolic activity of dysplastic keratinocytes, altered structure of subepithelial collagen, and absorbance of light by increased blood circulation due to inflammatory phenomena in dysplastic tissue and cancer. AF can be used for guiding incisional biopsy and in the excision to identify the resection margins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autofluorescence (AF)
- Fluorophores
- Oral cancer diagnosis
- Noninvasive tools for cancer diagnosis
- Potentially malignant oral disorder surgical treatment
- Biopsy of oral precancerous lesions
1 Noninvasive Methods in Diagnostics and Surgical Oncology
Oral cancer is a common malignant tumor. The 5-year survival rate of this neoplasm is low (approximately 50%), the delayed diagnosis being among the most important reasons. It is therefore important to emphasize the role of early diagnosis and treatment [1].
The diagnostic pathway for oral suspicious lesions usually starts with the clinical examination based on inspection and palpation of the oral mucosa. Such a phase is strongly related to the experience of the operator. Moreover, oral epithelial dysplasia and early oral carcinomas may already be present within areas of macroscopically intact oral mucosa.
A great interest for techniques potentially improving the diagnostic accuracy has developed in several fields of surgical oncology in order to increase the specificity and sensitivity of the conventional diagnostic pathway. The development of noninvasive methods for real-time screening of neoplastic changes in oral cavity may be associated with the improvement of patients’ quality of life and survival rate.
Principles underlying the functioning of noninvasive visual diagnostic tools for oral cancer and dysplastic lesions are very different, being based on diverse specific cellular and tissue characteristics. Most common tools within such a context are chemiluminescence (CL), toluidine blue (TL), and chemiluminescence associated with toluidine blue (CLTB). Among these, the use of autofluorescence (AF) has recently attracted the interest of researchers.
The analysis of tissue autofluorescence for improving sensitivity and specificity in cancer diagnosis has been proposed for different organs, including colon, lung, cervix, and esophagus. Particularly, there are several evidences supporting the effectiveness of this technique in head and neck cancer diagnosis. Autofluorescence shows high specificity and sensitivity for oral cancer and precancerous lesions: 72.4% and 63.79%, respectively. It can also provide valuable information for diagnosis, for planning of margin resection in surgical excision, and for monitoring the therapeutic response during follow-up [2].
2 Autofluorescence: Background
The extent and nature of structural and biochemical changes taking place during the transformation from normal to precancerous state to oral cancer are poorly understood.
The native cellular fluorescence is the innate capacities of tissues to absorb and transmit light. It has been well recognized since many years that several subcellular components, called fluorophores, are capable of emitting light of specific wavelengths different from that of an exciting radiation.
Fluorophores can be classified into endogenous and exogenous. Endogenous fluorophores, either intracellular or extracellular, are present in several biologic tissues, being responsible for the phenomenon of autofluorescence (AF). Autofluorescence is a peculiar visual property of some tissues depending on the concentration and distribution of specific fluorophores.
In the literature, the use of this property in differentiating normal from neoplastic oral mucosa is widely reported [3].
The technique of AF detection is based on illumination of suspicious lesions with monochromatic light, followed by the recording of fluorescent spectra emitted by endogenous tissue fluorophores. The presence of disease results in alteration in concentration of fluorophores as well as in alteration of light scattering and absorption properties of tissue, nuclear size changes and distribution, collagen content, and epithelial thickness, which leads to spectral variations [4].
It has been hypothesized that fluorophores of oral human tissues are some proteins (e.g., structural proteins, collagen, elastin; coat proteins, keratin) and several coenzymes involved in cellular metabolism, including nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD). Such molecules are stimulated by wavelengths between blue and violet/ultraviolet light.
The reduced form of NADH and the oxidized form of FAD are important fluorophores that are good indicators of cellular metabolism. It has been shown that fluorescence intensity due to NADH increases with dysplastic progression, while that of FAD decreases. Maximum NADH fluorescence occurs at 340-nm excitation and 45-nm emission, and FAD occurs at 450-nm excitation and 515-nm emission [5].
Another source of AF originates in the submucosal collagen cross-links which have been demonstrated to decrease in the immediate vicinity of malignant or premalignant lesions.
Loss of collagen fluorescence is generally attributed to changes in its biochemistry, possibly due to the breakdown of the extracellular matrix by dysplastic cells.
Collagen cross-links and basal lamina of tissue affected by cancer or epithelial dysplasia are destroyed, and glucose is highly consumed in malignant tissue even in an aerobic environment. On the contrary, the concentration of FAD decreases in epithelial dysplastic tissues [6].
One hypothesis is that matrix metalloproteinase (MMP) expression in stromal cells and the consequent remodeling of the extracellular matrix are induced by altered signaling from dysplastic epithelial cells. Collagen yields maximum fluorescence at 340-nm excitation and 420-nm emission and has significant fluorescence when excited between 410 and 470 nm [5].
3 Autofluorescence: A Diagnostic Support in Oral Cancer and Precancerous Lesions
Oral squamous cell carcinoma (OSCC) has an incidence of more than 500,000 cases per year worldwide.
The most important prognostic factor in influencing the disease-specific survival rate is the tumor stage at diagnosis.
The 5-year relative survival rate is 64.3%. However, survival rates for OSCC are highly stage-dependent, with 83.7% of people alive 5 years after diagnosis when a localized cancer is diagnosed and 64.2% and 38.5% of people alive 5 years after diagnosis when regional and distant metastases are diagnosed, respectively. Approximately 70% of all new cases are diagnosed at a late stage, underscoring the importance of early detection and prevention [7].
The diagnostic pathway for oral suspicious lesions usually starts with the conventional objective examination (COE) based on inspection and palpation of the oral mucosa with the support of an incandescent light available on the dental chair. It is well known that COE mainly depends on a subjective interpretation, which is a consequence of the experience of the operator.
Oral epithelial dysplasia (OED) is often observed in the tissue surrounding oral squamous cell carcinoma (OSCC), and it is reportedly associated with a malignant transformation rate of 2.2–38.1% [8].
Moreover, epithelial dysplasia and early oral cancer can be located within the context of oral potentially malignant disorders such as leukoplakia, erythroplakia, submucous fibrosis, and oral lichen planus as well in areas of apparently healthy mucosa.
The gold standard for the diagnosis of oral dysplastic and neoplastic malignant lesions is the histological examination. Incisional or excisional biopsy techniques are the most reliable methods to collect a surgical specimen suitable for microscopic evaluation. However, despite the little invasivity of such techniques, they still have some disadvantages in terms of morbidity and possible artifacts induced by the method of collection.
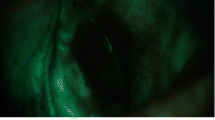
Direct visual fluorescence examination (DVFE) is based on the action of irradiation of specific wavelengths, between 375 and 440 nm, which excites some natural fluorochromes which show fluorescence in the range of the green color. The analysis of the lesions with AF tools must be performed in a dark environment to avoid the interference of white light wavelengths and to improve the quality of recorded images (Fig. 12.1). Healthy oral mucosa emits fluorescence, detectable as green light (Figs. 12.2, 12.3, 12.4, and 12.5). Cell and tissues within dysplastic and malignant lesions display modifications of the amount and distribution and chemical–physical properties of the endogenous fluorophores.
This results in an autofluorescence pattern variation that can be potentially used at diagnostic level. Loss of autofluorescence (LAF) seems to increase in correspondence to the progression of dysplasia, and altered tissue appears dark (brown to black) (Figs. 12.6 and 12.7). LAF in dysplasia and carcinoma seems to be connected to different mechanisms, such as altered metabolic activity of dysplastic keratinocytes, altered structure of subepithelial collagen, and absorbance of light by increased blood circulation due to inflammatory phenomena in dysplastic tissue and cancer [2].
According to some clinical experiences reported in the literature, red lesions are related to hypofluorescence, whereas white lesions are mostly related to hyperfluorescence, most probably because of keratin increase. There is a significant association of OED and carcinoma and autofluorescence alteration considering both hypo- and hyperfluorescence.
OED and carcinoma are particularly associated with hypofluorescence, excluding verrucous carcinomas, which appear hyperfluorescent because of intense keratinization.
AF analysis could be a valid adjunctive technique if associated with the clinician experience and knowledge. It may be used to spot lesions at risk, to identify suitable sites for incisional biopsies, and to define excision margins of the lesions.
4 Clinical Applications of Autofluorescence in Oral Surgery
AF can be used for guiding incisional biopsy and in the excision to identify the resection margins.
4.1 Autofluorescence-Guided Biopsy
For patients seeking care for suspicious lesions, immediate performance of a biopsy or referral to a specialist is an important recommendation in clinical practice.
Potentially malignant oral disorders are a group of clinically suspicious conditions, a small percentage of which will undergo malignant transformation. Dysplasia is the most well-established marker to distinguish high-risk lesions from low-risk lesions, and performing a biopsy to establish dysplasia is the diagnostic gold standard. Dysplasia is defined as the presence of specific epithelial architectural and cytologic changes, and it can be graded as mild, moderate, or severe based on the depth and severity of the cellular changes. It is frequently assumed that oral carcinogenesis involves oral premalignant disorders that undergo a gradual progression evolving through stages of mild dysplasia, moderate dysplasia, severe dysplasia, carcinoma in situ, and finally carcinoma after cellular invasion through the basement membrane [9]. However, oral premalignant disorders with dysplasia are considered non-obligate precursors of OSCC, indicating that not all dysplastic lesions will progress to invasive cancer [10].
COE is generally effective for lesion identification, but not always for the biopsy planning. Once the decision has been made to perform a biopsy lesion, clinicians must select a bioptic site, which should represent the area of the lesion most likely to contain dysplasia or carcinoma [11].
AF can be useful for simple incisional biopsies for homogeneous lesions or multiple biopsies for multifocal, large, or nonhomogeneous lesions. In particular, for the last type of lesions, it is difficult to indicate representative biopsy area.
In a surface with a diffuse and homogeneous green light aspect, some area of hypofluorescence (or in certain cases of hyperfluorescence) can be suspected, and the biopsy can be performed at these sites [12] (Figs. 12.8, 12.9, 12.10, 12.11, 12.12, and 12.13).
According to the literature, tissue AF imaging revealed a heterogeneous pattern of loss and increase of fluorescence in patients with actinic cheilitis (AC). Epithelial dysplasia was found in 93% of the cases, and most of the areas graded as moderate or severe were chosen for incisional biopsy with the aid of AF.
The advantages of AF in incisional biopsies include high sensitivity for dysplasia and cancer, capability to assess large areas of the oral mucosa at the point of care, nonrequirement of consumables, and noninvasiveness (Figs. 12.14, 12.15, 12.16, 12.17, and 12.18).
Unfortunately, the application of AF can be limited by false-positive results: inflammatory benign lesions, infectious stomatitis, and vascular diseases often exhibit a loss of fluorescence (Figs. 12.19 and 12.20). Keratin is autofluorescent, and hyperkeratinized high-risk diseases such as proliferative verrucous leukoplakia may not show LOF even in the presence of dysplasia or cancer (Figs. 12.21 and 12.22). AF may have clinical utility for risk assessment during longitudinal monitoring of patients with known high-risk potentially malignant oral disorders (such as proliferative verrucous leukoplakia, oral lichenoid lesions, and oral lichen planus) or previous history of cancer of the upper aerodigestive tract [13].
4.2 Autofluorescence-Guided Excision
Excisional biopsy can be performed for smaller lesions and could prevent sampling bias, but the risk of incomplete excision of malignant lesions exists, and the procedure may be not indicated in case of benign lesions.
Because the statistically significant reported risk factors for malignant transformation of leukoplakia include the presence of epithelial dysplasia, we support the opinion that surgical resection with adequately radical margins including the area of epithelial dysplasia would be effective in preventing malignant transformation.
Residual epithelial dysplasia after surgical treatment of oral cancer is an important risk factor for poor prognosis, and precise detection of affected areas with epithelial dysplasia prior to surgical resection of malignant lesions is important to prevent local recurrence [14].
AF can be used to delineate margins during surgical resection of OSCC to reduce recurrence rates. LOF frequently extends beyond the visible borders of a lesion, and such extension often shows dysplasia and loss of heterozygosity (Figs. 12.23, 12.24, 12.25, and 12.26).
Recent experiences reported in the literature indicate that the molecular profile of oral potentially malignant disorders changes with divergence away from the center of the lesion and that autofluorescence determined margins are superior to the white light margin in achieving a clear molecular margin when excising an OPMD [15].
Some leukoplakias show clinically invisible extensions during histopathological examination and AF. Most of leukoplakias are not surrounded by green light but by dark areas, indicating LOF with a mean size of 66%, exceeding the clinically visible margins of the disease. This technique enables clinicians to measure the extent of lesions beyond their visible margins [16].
Surgical operations for advanced stages of oral cancer are invasive with poor prognosis. On the contrary, a minimal and faster intervention under local anesthesia is useful both for compliance of the patients and for the success of the treatment. Early detection of suspicious lesions through AF and less invasive surgery performed with different lasers (Er:YAG, CO2, diode, Nd:YAG) guided by AF for healthy margin detection may determine a complete mucosal healing containing the risk of spread of the disease and reduce the risk of cancerization [17,18,19,20].
5 Conclusions
The usefulness of autofluorescence for oral tissue examination, especially within an oral medicine secondary care facility, before performing a biopsy and in monitoring oral lesions is confirmed by several studies [21]. In patients with clinically evident, potentially malignant, or seemingly malignant lesions, clinicians should perform a biopsy of the lesion. Autofluorescence represents an important support to conventional objective examination and palpation. For general dental practitioners, devices using AF are potentially useful for screening purposes. Furthermore, referral to a specialist for a biopsy (clinicians with training in oral and maxillofacial surgery, oral and maxillofacial pathology, oral medicine, and otolaryngology–head and neck surgery) is indicated when clinicians are not trained adequately to perform a biopsy of precancerous or cancerous lesions.
References
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16.
Giovannacci I, Vescovi P, Manfredi M, Meleti M. Non-invasive visual tools for diagnosis of oral cancer and dysplasia: a systematic review. Med Oral Patol Oral Cir Bucal. 2016;21(3):e305–15.
Nazeer SS, Asish R, Venugopal C, Anita B, Gupta AK, Jayasree RS. Noninvasive assessment of the risk of tobacco abuse in oral mucosa using fluorescence spectroscopy: a clinical approach. J Biomed Opt. 2014;19(5):057013.
Jayanthi JL, Mallia RJ, Shiny ST, Baiju KV, Mathews A, Kumar R, et al. Discriminant analysis of autofluorescence spectra for classification of oral lesions in vivo. Lasers Surg Med. 2009;41(5):345–52.
Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11(2):024006.
Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005;11:227–56.
Lingen MW, Abt E, Agrawal N, et al. Evidence-based clinical practice guideline for the evaluation of potentially malignant disorders in the oral cavity. J Am Dent Assoc. 2017;148:712–27.
Yamamoto N, Kawaguchi K, Fujihara H, Hasebe M, et al. Detection accuracy for epithelial dysplasia using an objective autofluorescence visualization method based on the luminance ratio. Int J Oral Sci. 2017;9:e2. https://doi.org/10.1038/ijos.2017.37. Online publication 10 Nov 2017.
Warnakulasuriya S, Johnson NW, Van Der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–80.
Patel KJ, De Silva HL, Tong DC, Love RM. Concordance between clinical and histopathologic diagnoses of oral mucosal lesions. J Oral Maxillofac Surg. 2011;69:125–33.
Epstein JB, Güneri P, Boyacioglu H, Abt E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J Am Dent Assoc. 2012;143:1332–42.
Rana M, Zapf A, Kuehle M, Gellrich NC, Eckardt AM. Clinical evaluation of an autofluorescence diagnostic device for oral cancer detection: a prospective randomized diagnostic study. Eur J Cancer Prev. 2012;21(5):460–6.
Takahama Junior A, Kurachi C, Cosci A, Pereira Faustino IS, Camisasca DR, da Costa Fontes KB, Pires FR, Azevedo RS. Usefulness of tissue autofluorescence imaging in actinic cheilitis diagnosis. J Biomed Opt. 2013;18(7):76023. https://doi.org/10.1117/1.JBO.18.7.076023.
Yang EC, Tan MT, Schwarz RA, Richards-Kortum RR, Gillenwater AM, Vigneswaran N. Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: current limitations and future directions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018. pii: S2212-4403(18)30092-0.; https://doi.org/10.1016/j.oooo.2018.02.020.
Farah CS, Kordbacheh F, John K, Bennett N, Fox SA. Molecular classification of autofluorescence excision margins in oral potentially malignant disorders. Oral Dis. 2017;24:732. https://doi.org/10.1111/odi.12818. [Epub ahead of print].
Elvers D, Braunschweig T, Hilgers RD, Ghassemi A, Möhlhenrich SC, Hölzle F, Gerressen M, Modabber A. Margins of oral leukoplakia: autofluorescence and histopathology. Br J Oral Maxillofac Surg. 2015 Feb;53(2):164–9.
Monteiro L, Barbieri C, Warnakulasuriya S, Martins M, Salazar F, Pacheco JJ, Vescovi P, Meleti M. Type of surgical treatment and recurrence of oral leukoplakia: a retrospective clinical study. Med Oral Patol Oral Cir Bucal. 2017;22(5):e520–6.
Nammour S, Zeinoun T, Namour A, Vanheusden A, Vescovi P. Evaluation of different laser-supported surgical protocols for the treatment of oral leukoplakia: a long-term follow-up. Photomed Laser Surg. 2017;35(11):629–38.
Del Corso G, Gissi DB, Tarsitano A, Costabile E, Marchetti C, Montebugnoli L, Foschini MP. Laser evaporation versus laser excision of oral leukoplakia: a retrospective study with long-term follow-up. J Craniomaxillofac Surg. 2015;43(6):763–8. https://doi.org/10.1016/j.jcms.2015.04.009. Epub 2015 Apr 17.
Huang Z, Wang Y, Liang Q, Zhang L, Zhang D, Chen W. The application of a carbon dioxide laser in the treatment of superficial oral mucosal lesions. J Craniofac Surg. 2015;26(3):e277–9.
Paderni C, Compilato D, Carinci F, Nardi G, Rodolico V, Lo Muzio L, Spinelli G, Mazzotta M, Campisi G. Direct visualization of oral-cavity tissue fluorescence as novel aid for early oral cancer diagnosis and potentially malignant disorders monitoring. Int J Immunopathol Pharmacol. 2011;24(2 Suppl):121–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vescovi, P., Giovannacci, I., Meleti, M. (2020). Laser Applications and Autofluorescence. In: Stübinger, S., Klämpfl, F., Schmidt, M., Zeilhofer, HF. (eds) Lasers in Oral and Maxillofacial Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-29604-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-29604-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29603-2
Online ISBN: 978-3-030-29604-9
eBook Packages: MedicineMedicine (R0)