Abstract
The natural occurrence, distribution (within a plant) and roles of four phenylbutanoid compounds (anisyl acetone, cue-lure, raspberry ketone and zingerone) are elucidated for the Asia-Pacific and Oceania regions. These phenylbutanoids may act individually or in combination to attract true fruit fly males belonging to a tribe Dacini of subfamily Dacinae (Diptera: Tepritidae). Of special interest are the mutualistic interactions between the Dacini fruit fly males and the tropical daciniphilous (attracting exclusively Dacini fruit flies) orchids – leading to cross pollination for the orchids and enchanced mating success for the flies. When offered to male flies, anisyl acetone and cue-lure are generally converted to raspberry ketone. Upon consumption, raspberry ketone and zingerone are individually sequestered in the male rectal (pheromonal) gland unchanged. Attracted male flies readily imbibe the phenylbutanoid(s) in the floral synomone to compliment the endogenously synthesized male sex pheromonal components – to enhance attraction of conspecific females during courtship as well as attract conspecific males to form ‘leks’. The phenylbutanoid(s) may also act as an allomone to deter vertebrate predators, especially geckos, besides possessing antimicrobial and antioxidant activities. Cue-lure, raspberry ketone and zingerone are important attractants/lures used in pest surveillance and mass trapping under the integrated pest management (IPM) program against quarantine Dacini fruit fly pest species, particularly Bactrocera tryoni and Zeugodacus cucurbitae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
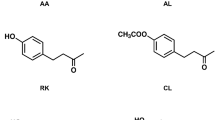
Four known natural phenylbutanoids, anisyl acetone (AA), cue-lure (CL), raspberry ketone (RK) and zingerone (ZN) (Fig. 1), are specific and potent attractants for many fruit fly species belonging to the genera Bactrocera, Dacus and Zeugodacus within the tribe Dacini of the subfamily Dacinae (Diptera: Tephritidae). These molecules possess a common 4-phenyl-2-butanone structure typically with substituents (e.g. -OH and/or -OCH3) on the benzene ring. AA and CL were first discovered as synthetic Dacini fruit fly attractants until recently when they were detected in plants, especially in Bulbophyllum (Asparagales: Orchidaceae: Epidendroideae) orchid flowers. It should be pointed out from the onset that the attractants may be released individually or in combination to influence the behavior of insects, especially Dacini fruit flies. Daciniphilous (attracting Dacini fruit flies) flowers, particularly certain Bulbophyllum orchids (the largest orchid genus with ca 2000 species; Frodin 2004), have an obligatory mutualistic interrelationship with the fruit fly males (Tan 2009; Ong et al. 2011; Tan and Nishida 2013; Nishida and Tan 2016), This group of flowers makes use of either phenylpropanoids or phenylbutanoids in their fragrances as floral synomones to exclusively attract male Dacini fruit flies for the sole purpose of pollination.
Traditionally, fruit fly species belonging to the Bactrocera, Dacus and Zeugodacus were categorized into three groups based on their specific response or non-response to two known male attractants CL and methyl eugenol: a) CL-responsive species (ca. 250 species (FAO/IAEA 2018) but now > 400 species, see below), including mango fruit fly, Bactrocera frauenfeldi (Schiner), Queensland fruit fly, B. tryoni (Froggatt), Zeugodacus caudatus (Fabricius), melon fly, Z. cucurbitae (Coquillett), and pumpkin fruit fly, Z. tau (Walker); or b) methyl eugenol (ME, a phenylpropanoid); ca. 60 responsive species, FAO/IAEA 2018), including carambola fruit fly, B. carambolae Drew & Hancock, guava fruit fly, B. correcta (Bezzi), Oriental fruit fly, B. dorsalis (Hendel), and peach fruit fly B. zonata (Saunders); or c) non-responsive (> 500 mostly non-pest species) to these aromatic male attractants (neither phenylbutanoids nor phenylpropanoids), but including several serious pests, such as Chinese citrus fly, B. minax (Enderline), Japanese orange fly, B. tsuneonis (Miyake), and solanaceous fruit fly, B. latifrons (Hendel), as serious pests. More key pest species under each of these groups have been listed in various publications (CDFA [California Department of Food and Agriculture], 2013; Nishida and Tan 2016; FAO/IAEA 2018; Doorenweerd et al. 2018). It is important to note at this juncture, that none of the known male phenylbutanoid attractants is as potent as ME for B. dorsalis (see review by Shelly et al. 2010). ME is found in over 450 plant species (Tan and Nishida 2012), especially as a component of floral synomone involved in cross pollination for certain daciniphilous Bulbophyllum species [e.g. B. cheiri Lindl. subspecies cheiri, Bu. macranthum Lindl. s. l. (ecotype ex. Philippines), Bu. sinapis J. J. Verm. & P. O'Byrne and Bu. vinaceum Ames & C. Schweinf.] (Tan et al. 2002, 2006; Nishida et al. 2004, 2022; Tan and Nishida 2015; Nakahira et al. 2018). In several cases, daciniphilous orchids produce/emit phenylbutanoid synomone as a mixture of specific ratios of AA, RK, and ZN and in rare cases also as a mixture with ME (discussed below).
This review is a sequel to a preceding review for the natural occurrence of ME (Tan and Nishida 2012). It also describes the origin and ecological/physiological functions of the phenylbutanoid attractants in association with the largest group of CL-responsive Dacini fruit fly species (> 400 species), most of which are endemic in the Asia-Pacific region (particularly in Southeast Asia and Oceania).
Semiochemicals of Dacini fruit flies
Semiochemicals of Dacine fruit flies have recently been reviewed (Segura et al. 2018, Scolari et al. 2021), and a review related to chemosensory mechanisms involving semiochemicals in fruit flies, especially serious pest species, was published by Ono et al. (2021). Our intentions are to a) update and clarify current knowledge for a large group of true fruit fly species in the genera Bactrocera, Dacus and Zeugodacus in relation to both intra- and inter-specific interactions with other organisms in the natural ecosystems and b) review the actual role(s) of each attractant as to whether it is a precursor or a booster or a component of the male sex pheromone. Traditionally, semiochemicals have been categorized based on intra- and/or inter-specific behavioral and/or physiological effects induced in an organism during its interactions with other individuals/organisms (Nordlund and Lewis 1976).
Figure 2 shows the classification of semiochemicals specifically for Dacini fruit flies. Semiochemicals are sub-divided into two groups - one with intra-specific activity (pheromone) and the other with inter-specific activity (allelochemicals). Pheromones and allelochemicals are further divided into the following (Nordlund and Lewis 1976):
-
1. Pheromones
-
A) Sex pheromone
-
Males of Bactrocera and Zeugodacus flies possess rectal pheromone glands and release characteristic "pheromone smoke" to attract conspecific females (See section IV-A).
-
-
B) Aggregation pheromone
-
Prior to courtship, Dacini males often form a lek. The pheromone emission signals conspecific males to aggregate to an arena where matings take place (See section IV-B).
-
-
C) Host-marking pheromone
-
-
2. Allelochemicals
-
A) Allomone (emitter benefits and detrimental to receiver)
-
a) In fruit fly-predator interactions, sex pheromonal components of B. dorsalis, e.g., 2-allyl-4,5-dimethoxyphenol, are moderate deterrents against Japanese sparrows Passer montanus Linn. (Nishida and Fukami 1990) and coniferyl alcohol against European starlings, Sturnus vulgaris Linn. (Jakubas et al. 1992). The chemicals produced by B. dorsalis males also have allomonal and hepatotoxic effects against lizard predators, Gekko monarchus (Wee and Tan 2001).
-
b) In fruit fly-host fruit interactions, oviposition by female B. carambolae in mango fruits which subsequently produce deterrent chemicals that repel other gravid conspecific females (Muryati et al. 2017).
-
-
B) Kairomone (receiver benefits and detrimental to emitter)
-
Fruits generally release volatiles or fragrant chemicals during ripening. The fruit volatiles act as a ‘fruit kairomone’ that attracts gravid female fruit flies for oviposition, which subsequently causes fruit damage, dropping and/or infestation. γ-Octalactone has been characterized as an oviposition stimulant of B. dorsalis and B. tryoni (Damodaram et al. 2014; Kempraj et al. 2019), and a number of cucumber volatiles attract females of B. cucumis (French) (Royer et al. 2014) and Z. cucurbitae (Jang et al. 2017).
-
-
C) Synomone (benefits to both emitter and receiver)
-
A floral volatile compound, such as ME, attracts fruit fly males to execute cross-pollination. In this mutual interaction, both organisms gain reproductive benefits, i.e., the flower gets pollinated while the male fly gains either sex pheromone or sex pheromonal precursor via feeding on the flower. ME as a component of a floral synomone has been shown to attract ME-responsive Bactrocera species as exclusive myophilous pollinators of Bulbophyllum cheiri subspecies cheiri and Bu. vinaceum (Tan et al. 2002, 2006; Nishida et al. 2004; Tan and Nishida 2015). Below, we discuss floral synomones that consist of an individual or a blend of phenylbutanoids.
-
-
From the onset, it is paramount to bear in mind that semiochemicals may often simultaneously act as pheromones (during intraspecific interactions) as well as allelochemicals (during interspecific interactions) in a natural ecosystem e.g., male sex pheromone of B. dorsalis as in Item 2A above.
As for the chemicals not released/secreted naturally, it is important to note that in a review of insect para-pheromones, Renou and Guerrero (2000) restrict the term ‘para-pheromones’ to “chemical compounds of anthropogenic origin not known to exist in nature”. This had led to a very potent male attractant/lure, ME, found in over 450 plant species (Tan and Nishida 2012) but thus far not detected in the pheromonal/rectal gland of male fruit flies being erroneously referred to as a ‘para-pheromone’ in many publications – similarly, for CL (as used by Bakthavatsalam 2016). Hitherto CL, like ME, has not been detected in any Dacini male pheromonal/rectal gland and never been shown to induce sex pheromonal activity (Tan and Nishida 1995; Nishida et al. 2022); and strictly, should also not be referred to as a ‘para-pheromone’ but as a male attractant/lure instead, when used in fruit fly control or surveillance programs. Similarly, potent synthetic analogues of RK – raspberry ketone formate (melolure) (Oliver et al. 2002), and raspberry ketone trifluoroacetate (Siderhurst et al. 2016) were found to be superior to CL, and should also be categorized as male attractants/lures.
Natural Occurence, Distribution and Role of Individual Phenylbutanoids
Anisyl acetone [4-(4-methoxyphenyl)-2-butanone (CAS RN: 104-20-1)] (other names: p-methoxybenzylacetone; raspberry ketone methyl ether)
Anisyl acetone (AA) was the first synthetic fruit fly male attractant/lure discovered via mass screening of synthetic chemicals for the melon fly, Z. cucurbitae (Barthel et al. 1957). It was then used in Queensland, Australia from 1959 to 1962 (until the discovery of a new synthetic and more potent attractant, see cue-lure below) for trapping of eleven Dacini fruit fly species (unpublished data c/f Royer et al. 2020).
To date, AA has been detected in ten plant species belonging to eight families (Table 1). It is generally detected as a trace or minute/minor component in plants, except in flowers of Bu. hahlianum Ames & C. Schweinf. (Fig. 3) (Nishida et al. 2022). The natural role of AA as a constituent in plants, besides possessing some anti-oxidant properties (Yang et al. 2012), is not fully understood. Nonetheless, as a component in floral fragrance, AA in combination with RK and/or ZN is an important floral synomone component in enhancing cross-pollination of certain daciniphilous Bulbophyllum (Orchidaceae) species, such as, Bu. hahlianum (Nishida et al. 2022) and Bu. macranthoides Kraenzl. subspecies tollenoniferum (J.J. Sm.) J.J. Verm. (Katte et al. 2020).
Fruit fly field captures in Cairns, Australia, showed that B. frauenfeldi males were most responsive to CL, equally to RK and AA, and poorly to ZN (Royer et al. 2020). Furthermore, comparison of flies captured in PNG using three phenylbutanoid lures - AA, RK and ZN - showed that AA captured the highest number of individuals for B. atramentata, B. bryoniae and B. fraeuenfeldi (Nishida et al. 2022). AA, when offered to B. frauenfeldi males, was converted to RK and 4-(4-methoxyphenyl)-2-butanol (AL) for storage in the rectal (pheromonal) gland, while consumed RK is sequestered unchanged in the gland (Wee et al. 2020). Additionally, AA has also been detected in whole body extracts of B. atramentata and B. fraeuenfeldi males collected from flowers of Bu. hahlianum (Nishida et al. 2022).
The presence of AA in anise, Pimpinella anisum L. (Apiaceae), depends on cultivated locations. It is detected in the Alberta anise oil but not in Michigan anise oil. It gives the Alberta oil “a sweet floral and slightly fruity, cherry preserve-like odor”, and it consituted ca. 1% in seed oil and 0.3% in whole plant oil (Embong et al. 1977). Similarly, agarwood, Aquilaria crassna Pierre ex. Lecomte (Thymelaeaceae), showed significant variation in AA content cultivated in three provinces in Vietnam, with 2.27, 0.68 and 0.31 % peak area of plant essential oils, respectively (Thuy et al. 2019).
From a pharmacological perspective, AA when inhaled induced good sedative effects against mice (Miyoshi et al. 2013). AA also possesses strong antimicrobial properties (Yang et al. 2010), especially against multidrug resistant bacteria - Pseudomonas aeruginosa (Schröter) Migula and Acinetobacter baumannii Bouvet and Grimont (Osoro et al. 2013). Extracts of Illicium verum, Chinese star anise (Schisandraceae), used as traditional Chinese medicinal herbs, revealed a strong synergistic antibacterial activity of AA, anisyl alcohol (4-methoxybenzyl alcohol), anisyl aldehyde (4-methoxybenzaldehyde) and (E)-anethole, against sixty-seven clinical antibiotic-resistant isolates; and thereby, demonstrating that the compounds might be the active ingredients against many species of bacteria (Yang et al. 2010).
Cue-lure [4-(4-acetoxyphenyl)-2-butanone) (CAS RN: 3572-06-3)] (other names: cuelure, cue lure)
Cue-lure (CL) was first discovered as a synthetic attractant and identified as a highly potent male attractant for Z. cucurbitae through mass screening of synthetic chemicals (Beroza et al. 1960). It was assumed for a long time to be solely a synthetic male attractant (Metcalf 1990). To date, a total of 410 CL-responsive Dacini species of which 206 are Bactrocera, 83 Dacus and 121 Zeugodacus species have been reported (Supplementary Table) (Doorenweerd et al. 2018).
After five decades of successful usage as a lure in surveillance, monitoring and control of certain pest fruit flies (see review by Vargas et al. 2010), CL was detected in two plant species. Trace quantities of CL were detected in flowers of an orchid species Bulbophyllum hortorum J.J. Verm. et al. (Orchidaceae) (Table 2, Fig. 4; Tan et al. 2014; Nishida and Tan 2016; Katte et al. 2020) and as a minor floral component in passion fruit, Passiflora maliformis L. (Passifloraceae) (Park et al. 2020) (Table 2).
Flowers of Bulbophyllum hortorum possessing cue-lure (CL) as a trace component together with AA, RK and ZN. [Bar scale = 1 cm]. A - A freshly bloomed flower with Dacus maculipterus male probing on the medial sepal;. B - A characteristic second day flower (reopened after partial closing on first night – petals unspread) with a melon fly, Zeugodacus cucurbitae, male bearing a pollinarium (white arrow - from a different Bulbophyllum species, probably from Bu. macranthum or its sibling species) on its abdominal dorsum. N.B. Bu. hortorum pollinarium when removed by a fly adheres to the thoracic dorsum – see (Tan et al. 2023)
In the orchid species, Bu. hortorum, traces of CL were detected in some whole flower samples (Katte et al. 2020). All floral parts possess three major floral phenylbutanoid components, AA, RK and ZN, plus their respective alcohol analogues: AL, 4-(4-hydroxyphenyl)-2-butanol (rhododendrol) (RL), and 4-(3-hydroxy-4-methoxyphenyl)-2-butanol (zingerol) (ZL) as minor components. However, at this juncture, the actual role of floral CL, besides aiding other phenylbutanoids in enhancing pollination by attracted CL-responsive Dacini fruit flies, is unknown. In passion fruit, P. maliformis, the combination of fruit fly attractants RK, CL, ZN and ZL was detected as trace components in petals and sepals but not in all floral parts (Park et al. 2020) (Table 2).
More males of Z. cucurbitae were attracted to CL in the morning [09:00-10:00 h] than mid-day [12:00-13:00 h] or late afternoon [15:00-16:00 h] (Manoukis and Jang 2013). This diurnal rhythm is corroborated by an earlier field trapping conducted by Nakamori and Soemori (1985), who showed the male melon flies were trapped at highest numbers between 07:00 to 09:00 h, with a steep decline of fly numbers at other day-time periods.
To date, CL has never been detected in fruit flies, especially in the male rectal/pheromonal gland. But when synthetic CL (with high purity - >95%) was offered to males of Z. cucurbitae and B. tryoni, it was rapidly hydrolysed to RK, probably in the crop, that was eventually sequestered for storage in the rectal gland (Nishida et al. 1990; Tan and Nishida 1995). Likewise, consumed CL was converted to RK for storage in the rectal gland of B. melanotus (formerly B. melanota) (Coquillett) (Fletcher and Kitching 1995). Additionally, in the case of B. tryoni, a trace quantity of CL was biotransformed to and stored as RL (Kumaran et al. 2014a). Therefore, CL would potentially act as an exogenous male sex pheromonal precursor for CL-responsive species.
Males of Z. cucurbitae exposed to CL on the day of or a day prior to testing mated more frequently than unexposed males; but for a relatively short period of two days; additionally, CL might further enhance mating performance via increased male wing-fanning activity (Shelly and Villalobos 1995).
Previously, CL was reported to capture two times more number of Z. cucurbitae males than RK (Beroza et al. 1960) and ca. 1.5 times more B. tryoni males than RK (Monro and Richardson 1969). A recent quantitative study conducted in Queensland, Australia, confirmed that CL is a more potent attractant than RK in the attraction of Dacini fruit fly species that are specifically responsive to phenylbutanoid attractants (Royer et al. 2020). This might reflect a higher release rate and vapour pressure of CL, ca. five fold that of RK (Metcalf 1990), and also possible chemo-structural differences that affect ligand-receptor binding (Royer et al. 2020). Furthermore, CL has been assumed for many years to be hydrolysed to RK – the true attractant for CL-responsive species (Drew 1974; Metcalf 1990) - until it was shown by Park et al. (2016) that CL remains intact in the atmosphere as a male attractant/lure.
Regarding capture probability of released males, it is very low for Z. cucurbitae in CL-baited traps when compared with B. dorsalis in ME-baited traps (Shelly et al. 2010). The capture percentage at a central trap from release points of males at 25, 50 and 100 m distance from the CL-baited trap was 5.29, 4.66 and 0.40 %, respectively. This study conducted in California by Shelly and co-workers (2010) showed that the capture probability of released sterile males, particularly for CL-responsive species, drops drastically when the distance between release-point and trap is beyond 50 m.
From a pest management perspective, traps baited with ‘dorsalure’ (a commercial mixture of CL and ME) attracted significantly fewer B. dorsalis males than traps baited with ME alone (Tan and Lee 1982). Combining CL and ME (either as liquid mixture or physically apart each in separate cotton rolls within each trap) as baits in traps captured significantly fewer B. dorsalis and B. umbrosa males than ME-baited traps (Tan 1983). This was further corroborated by Vargas et al. (2000) who showed that CL-ME baited traps captured similar numbers of the CL-responsive pest species Z. cucurbitae as traps baited with CL alone but significantly fewer ME-responsive species B. dorsalis than traps baited with ME alone. Additionally, field studies in Australia and PNG showed that combining CL and ME in equal parts placed in each Steiner trap significantly reduced captures of most species of Dacini fruit flies (Royer and Mayer 2018). These studies have shown that the presence of CL in the lure-mixture interfered in the capture of fruit flies, especially those of ME-responsive species when used as simultaneous control programs for both CL- and ME-responsive fruit fly pest species, particularly against the notorious invasive and quarantine species Z. cucurbitae and B. dorsalis, respectively. This phenomenon is contrasted by a study in Hawaii, in which the CL-ME mixture was replaced by the RK-ME mixture, and no significant differences in captures of either Z. cucurbitae or B. dorsalis males between traps baited with a single lure and the mixture (Shelly and Kurashima 2016). The cause of reduction in trap captures of both the CL- and ME-responsive pest species due to the presence of CL in bait mixtures warrants in-depth investigations.
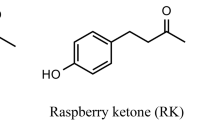
Raspberry ketone [4-(hydroxyphenyl)-2-butanone (CAS RN: 5471-51-2)] (other names: p-hydroxybenzylacetone; frambinone; oxyphenylon; rasketone; rheosmin)
Raspberry ketone (RK), known as the most characteristic aromatic flavor of raspberry fruit, Rubus idaeus (Rosaceae), has been detected in 18 plant species from 9 families, including Actinidiaceae (1 species), Asteraceae (1), Ericaceae (2), Lamiaceae (1), Orchidaceae (7), Pinaceae [Gymnosperm] (2), Polygonaceae (1), Rosaceae (2), and a Gymnosperm family Taxaceae (1) (Table 3). All the seven orchid species listed have floral RK that attracts males of CL-responsive species belonging to the three Dacini genera - Bactrocera, Dacus and Zeugodacus.
Among orchid species, floral RK was first detected in all three color (purple, white and mixed of the two colors) varieties of Dendrobium superbum (Nishida et al. 1993). Nevertheless, the actual role of RK in the three floral-color varieties of this Dendrobium species is still uncertain as attracted fruit fly males apparently lick the petal area but approach neither the floral column nor the inner surface of the large immovable lip where the pollination would occur. Furthermore, the stiff and relatively large lip does not have a mechanism to tip a fly in to the column cavity to remove or deposit pollinia (Nishida et al. 1993). Therefore, in this particular case, attracted fruit fly males do not act as pollinators, and RK in the floral fragrance of D. superbum may attract other pollinator species, such as bees, and/or deter florivores (see below).
RK is a major component of floral synomones released to attract many CL-responsive species of the Dacini fruit flies to assist in the pollination of several daciniphilous Bulbophyllum species, such as Bu. ecornutum J. J. Sm (syn. Bu. apertum) (Fig. 5A), Bu. gerlandianum, Kraenzl. (syn. Bu. emiliorum Ames & Quisumb.) (Fig. 5B), Bu. hahlianum Schltr. (Fig. 3), Bu. hortorum J.J Verm., P. Oyrne & A.L. Lamb (Fig. 4), Bu. macranthoides Kraenzl. subspecies tollenoniferum (J.J. Sm.) J.J. Verm. and Bu. praetervisum J. J. Verm. (Fig. 5C), (Table 3). The whole process of cross-pollination of Bu. praetervisum flowers – pollinarium removal and pollinia deposition by two of the CL-responsive Dacini fruit fly species, namely B. fraeunfeldi and Z. caudatus - has been reported by Tan and Tan (2018). It is interesting to note that there is intraspecific variation in morphological characters and even in the floral chemical composition of phenylbutanoid components acting as synomone in the interactions between the daciniphilous orchids and the Dacini fruit flies. Although RK is the major floral component of Bu. praetervisum (Fig. 5C) in Malaysia, there are three different ecotypes based on major synomone components – a) RK + RL, b) RK + RL + ZN, and c) RK + ME + RL + ZN (Nakahira et al. 2018). Also, in Bu. ecornutum subspecies verrucatum, solitary flowers of Nabawan-ecotype emit a characteristic fragrance of RK along with smaller amounts of RL (Tan and Nishida 2005), while those of Apin-Apin-ecotype emit ZN and ZL as major components (Tan et al. 2021).
Bulbophyllum flowers emitting raspberry ketone (RK) as a major floral synomone component. [Bar scale = 1 cm]. A. Bulbophyllum ecornutum (syn. B. apertum) subsp. verrucatum (Nabawan-ecotype) with a male Zeugodacus caudatus feeding on the small lip. B. Bulbophyllum gerlandianum (syn. B. emiliorum) with two male fruit flies, Bactrocera frauenfelidi, on medial sepal (orange arrow), and a Zeugodacus caudatus on lateral sepals (green arrow). C. Bulbophyllum praetervisum
RK when consumed remained unchanged and ultimately sequestered in the rectal gland of Z. tau (Nakahira et al. 2018). RK was detected from male body extracts of five Dacini species (B. atramentata, B. bryoniae, B. frauenfeldi, B. pseudodistincta and Z. cucurbitae) captured while feeding on flowers of Bu. hahlianum, which contain RK as the major floral volatile. This observation strongly suggests that RK may have a role as an allomone and/or sex pheromone (Nishida et al. 2022) as discussed below.
RK generally attracts the same species that are responsive to CL belonging to the genera Bactrocera, Dacus and Zeugodacus (FAO/IAEA 2018; Doorenweerd et al. 2018). Attracted Bactrocera and Zeugodacus males readily consume and sequester RK to be incorporated as one of the male sex pheromonal components (Nishida et al. 1993; Tan and Nishida 2005; Wee et al. 2020; Tan et al. 2021). Z. cucurbitae males release sex and aggregation pheromone at dusk during courtship (Kuba and Sokei 1988). The aggregation pheromone attracts other conspecific males to form ‘leks’ (Kuba et al. 1984), while the sex pheromone attracts conspecific females during courtship (Nishida et al. 1990; Tan 1983, Tan 2000a).
Another important role of RK in Z. cucurbitae males is acting as an allomone for defence against vertebrate predators (Tan 2000b). The Asian house gecko, Hemidactylus frenatus Duméril & Bibron, consumed significantly fewer houseflies (Musca domestica L.) topically treated with RK (5 µg /fly) than controls (untreated houseflies) (Tan 2000b).
Affinity of fruit fly males to RK seems to be selected by the conspecific female's preference towards RK-scented males via sexual selection, as in the case of B. dorsalis in that males acquired ME as sex pheromone and females prefer to mate with males signaling with ME-metabolites (Tan and Nishida 1996; Shelly 2007; Kumaran et al. 2014b; Nishida 2014).The bird's nest fungus, Nidula niveo-tomentosa (Hennings) Loyd (Agaricaceae) has RL and RK as major and minor components, respectively, in submerged cells of the basidiomycete (Zorn et al. 2003). The ratio of RK: RL in this fungus is completely different from that found in floral synomone, in which RL is always present in minute quantity relative to that of RK. RK is also detected in minute quantities (<1 µg/kg) from brewed coffee (Coffea arabica L.) (Akiyama et al. 2007, 2008); and in petals and sepals of passion fruit as trace quantities (Park et al. 2020). The quantity of RK in raspberries is extremely low (1-4 mg/kg); and natural RK is very expensive for flavouring industries (Milke et al. 2020). To improve production and reduce cost of natural RK, bioengineered candidate genes from raspberry and other plants into bacterial and yeast expression systems yielded significant production of natural RK, up to 5 mg/L (Beekwilder et al. 2007). The biosynthesis of RK in raspberry fruits and its tissue cultures has been investigated, and characterized a RK/ZN synthase which catalyzes the NADHP-reduction of 4-hydroxybenzalacetone to RK and ZN, respectively (Koeduka et al. 2011). Synthesis of natural RK was further improved using a bioengineered strain of a Gram-positive bacterium, Corynebacterium glutamicum (Kinoshita et al.) Abe et al., which was able to accumulate up to 99.8 mg/L from p-coumaric acid as supplement (Milke et al. 2020). More recently Koeduka et al. (2021) demonstrated the production of RK by redirecting the metabolic flux using tobacco plants, Nicotiana tabacum.
Natural anti-oxidant properties of RK have been demonstrated through a) increased total anti-oxidant capacity; b) upregulated anti-oxidant enzymes, e.g. superoxide dismutase and catalase; and c) improved lipid peroxidation. These activities are directly or indirectly linked to RK’s other various physiological activities (see review by Lim and Choi 2021). RK prevents obesity and fatty liver in mice (Morimoto et al. 2005); and it enhanced metabolic activity in adipose tissue plus impeded small-intestinal absorption of lipids (Beekwilder et al. 2007). Additionally, RK and hydroxycitric acid from Garcinia gummi-guta (L.) N. Robson (syn. G. camboagia (Gaertn.) Desr.) have anti-obesity properties in rats; and “nutraceutical agents are extensively promoted and used over the counter to manage obesity” (Attia et al. 2019). Nevertheless, there is inadequate scientific evidence in support of RK being actually an anti-obesity chemical for human to counter obesity; and supplementary-diet containing high RK content “should be consumed with restraint” (Lee 2016).
Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone (CAS RN: 122-48-5)] (other names: 4-hydroxy-3-methoxybenzylacetone; vanillylacetone)
At the turn of the new millennium, ZN was first identified as the attractive component in the flowers of the Bulbophyllum orchids e.g. Bu. patens King (Fig. 6A), Bu. macranthoides Kraenzl subsp. tollenoniferum, Bu. macranthum sensu stricto (ecotypes ex. Malaysia) (Fig. 6B), Bu. baileyi F. Muell. (Suppl. Fig. A), Bu. cheiri subsp. subuliferum (Schltr.) J.J. Verm., P. O'Byrne & A.L. Lamb (Suppl. Fig. B) and Bulbophyllum macranthoides subsp. tollenoniferum (J.J. Sm.) J.J. Verm. (Suppl. Fig. C) (Tan & Nishida 2000, 2007; Nakahira et al. 2018; Katte et al. 2020). To date, natural ZN is known to exist mainly in certain orchid flowers and is an important floral synomone component in seven daciniphilous Bulbophyllum species (Table 4). It specifically enhances cross-pollination of these orchids by Dacini fruit fly pollinators. Additionally, ZN has also been detected in non-orchid flowers, e.g., Passiflora maliformis L. (Passifloracea) and Semecarpus australiensi (Anacardiaceae) Engl. (Park et al. 2020, 2022) (Table 4).
Zingerone (ZN) as a major floral synomone component in daciniphilous Bulbophyllum flowers. [Bar scale = 1 cm]. A. Bulbophyllum patens with a Bactrocera dorsalis (bearing a pollinarium on a lateral sepal - white arrow) which would eventually moved to the see-saw lip (pink arrow) prior to depositing the pollinia when tipped in to the column cavity. B. Bulbophyllum macranthum (ex Indonesia) with a Bactrocera dorsalis (bearing the former’s pollinarium on the abdominal dorsum (white arrow) on tips of lateral sepals
The discovery of ZN as a major floral synomone component of Bu. patens by Tan and Nishida (2000) was an important landmark in research related to male fruit fly attractants, because ZN’s chemical structure has both the functional moieties of RK (with a phenylbutanone) and ME (with a methoxybenzene) and attracts certain species unresponsive to either of the two established attractants. Hence, a new group of ZN-responsive species was identified and to date includes 24 species of Dacini fruit flies attracted exclusively to ZN (Table 5). Furthermore, a group of CL- and ME-responsive species was found to respond, though weakly or moderately, to ZN (Table 6).
Field tests conducted in Penang, Malaysia, showed that ZN was found to weakly attract several CL-responsive species [B. frauenfeldi Meijere, Z. caudatus Fabricius, Z. cucurbitae, Z. tau (Walker)] and ME-responsive species [B. carambolae Drew & Hancock, B. dorsalis, B. indonesiae Drew & Hancock, and B. umbrosa (Fabricius)] (Tan and Nishida 2000, 2007; Tan et al. 2006). We were initially puzzled as to why we never detected any Dacini fruit fly species, strictly ZN-responsive species, attracted specifically to flowers of local endemic orchids that release floral synomone containing ZN as a major component, until recently. The attraction of B. pendleburyi (Perkins), a species known to respond exclusively to ZN, to a daciniphilous Bulbophyllum flower, Bu. cheiri subspecies subuliferum (Schltr.) J. J. Verm. et al. was observed in 2022 in an orchard in Kajang, Selangor, Malaysia, (Fig. 6D – Ong, personal communication; also Table 5). Further field tests in Queensland, Australia, showed ZN to be very attractive to a minor pest species, B. jarvisi (Tryon) which is previously known to be a CL-responsive species (Fay 2012). As a result of several recent field studies and area-wide surveys using ZN along with CL and ME as male attractants, there is now a new group of species that are attracted exclusively to ZN (Table 5). It should be noted that all the ZN-responsive species listed in Table 6 belong to the two genera, Bactrocera and Dacus, without any species from the genus Zeugodacus. We are of the opinion that more ZN-responsive Dacini fruit fly species will be found when further in-depth and large scale area-wide surveys are conducted in other tropical and subtropical countries, especially within the Asia-Pacific region, where some endemic plant species whose flowers are known to specifically release ZN as a major floral synomone component.
The fact that both CL-and ME-responders are attracted to ZN may provide circumstantial evidence that ZN might represent the divergent point in i) the evolution of the Dacini fruit fly’s responsiveness to the phenylpropanoids and phenylbutanoids, especially ME and RK, respectively; and ii) the co-evolution between daciniphilous Bulbophyllum species and the Dacini fruit fly species. The hypothetical perspectives are possibly i) ZN may have evolved to attract both types of flies, convergently; or ii) ZN could be a template or an ancestral compound from which ME and RK would have evolved divergently - through a runaway process, by manipulating the phenylpropanoid/phenylbutanoid synthetic pathway (Tan and Nishida 2000), thereby, need more in depth investigations.
In terms of biological functions related to consumption of ZN by fruit flies, males of B. dorsalis biotransform ZN to ZL for temporary storage in the rectal gland, while males of Z. cucurbitae sequester ZN unchanged into the rectal gland (Tan and Nishida 2000). The respective compounds stored in the male rectal gland are released during courtship to attract conspecific females (Khoo and Tan 2000). Response of B. jarvisi males to ZN is at nanogram levels (an ED50 of 179 ng), with an olfactory threshold >1600x than to RK (Wee et al. 2018). ZN-feeding, but not RK-feeding, by Z. tau males was shown to improve mating performance through enhancement of male courtship activity and sexual signalling (Shamshir and Wee 2019). Likewise, B. jarvisi females, when given a choice, preferred to mate with ZN-fed than RK-fed males (Wee and Clarke 2020). However, ZN-fed males of B. tryoni in comparison to CL-fed males did not show enhanced mating success (Kumaran et al. 2014a).
From a pharmaceutical perspective, ZN is only found in dried or cooked ginger and Zingiber rhizomes oils (Ahmad et al. 2015). The oils with anti-oxidant and anti-microbial properties have been commonly used for preserving foods against microbial spoilage and autoxidation (Singh et al. 2008; El-Baroty et al. 2010; Bellik 2014). The anti-microbial potential of Zingiber plant extracts has been demonstrated against both Gram-negative (Escherichia coli (Migula) Castellani & Chalmers, Salmonella typhi Lignières, Pseudomonas aeruginosa (Schröter) Migula, Klebsiella pneumonia (Schroeter) Trevisan); and Gram-positive (Bacillus cereus Frankland & Frankland, Staphylococcus aureus Rosenbach) bacteria (Kumar et al. 2011). The essential oils also possess anti-fungal properties against Candida glabrata (H.W. Anderson) S.A. Mey. & Yarrow, and C. albicans (C.-P. Robin) Berkhout. ZN also has potent anti-inflammatory, anti-diarrhoeic, anti-diabetic, anti-lipolytic, anti-spasmodic properties (see review by Ahmad et al. 2015).
Combination of Phenylbutanoids in Natural Sources
There are a number of other 4-phenyl-2-butanone type compounds, known as natural products, besides AA, CL, RK, and ZN. Initially, among more than 1000 synthetic compounds under screening, Z. cucurbitae males were found to be attracted to benzylacetone (BA, 4-phenyl-2-butanone) (Fig. 1), although BA was proven to be less attractive than AA in field tests (Barthel et al. 1957). Flath and Ohinata (1982) identified BA by a headspace collection of a melon fly-attracting D. superbum blossoms before Nishida et al. (1993) characterized RK as the main component to attract Z. cucurbitae males. BA has been shown to attract certain potential nocturnal pollinators in coyote tobacco (Nicotiana atenuata) flowers (Baldwin et al. 1997).
As noted above, AA, RK and ZN in dacinphilous orchid flowers are often accompanied by their corresponding 2-hydroxy derivatives, namely 4-(4-methoxyphenyl)-2-butanol (AL), 4-(4-hydroxyphenyl)-2-butanol (rhododendrol) (RL), and 4-(4-hydroxy-3-methoxyphenyl)-2-butanol (zingerol) (ZL), respectively, as minor components. AL, RL and ZL were shown to be attractive to Z. cucurbitae males to a much lesser degree when compared with the corresponding ketones (i.e. AA, RK and ZN, respectively) (Katte et al. 2020). Furthermore, 4-(4-hydroxy-3,5-dimethoxyphenyl)-2-butanone (proposed common name, syringerone (SN) (Fig. 1) was characterized from a daciniphilous orchid, Bu. macranthoides subsp. tollenoniferum as a minor component (together with AA, RK and ZN), and this compound did not induce any attractive responses from Z. cucurbitae males (Katte et al. 2020). Nonetheless, the individual role of the accompanying minor components in floral fragrances needs further investigation.
Pheromonal Function of Phenylbutanoids in the Sexual Interactions of Fruit Flies
Sex Pheromonal Function
Major components of Z. cucurbitae male rectal gland secretions were derivatives of pyrazines and aliphatic amides (Baker et al. 1982), while several aliphatic compounds were detected in the smoke-like fume emitted by males as sex pheromone during courting (Ohinata et al. 1982). The endogenously produced rectal volatiles in a Malaysian strain have six volatiles with ethyl 4-hydroxybenzoate as the major endogenous component; RK was accumulated from feeding on synthetic CL (Nishida et al. 1990). In addition, it was shown that Z. cucurbitae males accumulate RK from D. superbum flowers for more than six days in the pheromonal gland (Nishida et al. 1993). RK and ZN enhance sexual performance as Z. cucurbitae males fed CL-/RK- or ZN-fed attracted significantly more conspecific females than lure-deprived males in the laboratory (Khoo and Tan 2000). Thus, the exogenous source of phenylbutanoids, RK or ZN, plays a vital role in affecting the mating success of Z. cucurbitae. Similarly, consumption of ZN also significantly increased male mating success in another CL-responsive species, Z. tau (Wee et al. 2020).
In B. tryoni, the pheromonal constituents recovered from the rectal gland of lure-deprived flies are six aliphatic amides (Bellas and Fletcher 1979). More females responded to squashed rectal glands extracted from CL-fed males than to glands from control (CL-deprived) males. Furthermore, more females responded to the pheromone of calling CL-fed males than to control males (Kumaran et al. 2014a). However, ZN-fed B. tryoni males did not attract more females when compared with ZN-deprived males (Kumaran et al. 2014a). This phenomenon of a male attractant incorporated in to the endogenously produced sex pheromonal components probably indicates that B. tryoni is strictly a CL-/RK- responsive species, and the weak response to ZN may be an ancestral trait in the effectiveness of male sex pheromone.
Aggregation Pheromone and Lek Formation
Leks in Dacini fruit fly species generally consist of 2-10 males (Shelly 2018) as reported for B. dorsalis (Shelly and Kaneshiro 1991) and Z. cucurbitae (Iwahashi and Majima 1986; Mir and Mir 2016). For B. carambolae males, ME is converted to E-coniferyl alcohol; while for B. dorsalis, ME is converted mainly to 2-allyl-4,5-dimethoxyphenol and E-coniferyl alcohol, for storage in the pheromonal gland. E-coniferyl alcohol attracts conspecific males of the two species, while the dimethoxyphenol is a strong male attractant for B. dorsalis males, as potent as ME (Nishida et al. 1988; Tan and Nishida 1996). Thus, when released as pheromonal components, they act as an aggregation pheromone to attract other males, especially males deprived of ME to form a lek in a field cage (Wee et al. 2007). Furthermore, a previously deprived and marked B. carambolae male was shown to approach the anal section of a ME-fed male that had just begun copulating, after releasing sex pheromone with an attracted conspecific female (Wee et al. 2007). Likewise, although not much research has been conducted on CL-responsive species, males generally sequester phenylbutanoid attractants in the pheromonal gland in the form of either RK or ZN (as mentioned above for the potent natural phenylbutanoid attractants). For Z. cucurbitae, when released as a component in the aggregation pheromone, either RK or ZN would attract other conspecific males to form a lek. As mentioned previously, rectal volatiles are released by males during courtship at dusk that act as a sex and aggregation pheromone (Kuba and Sokei 1988), and the aggregation pheromone attracts other conspecific males to form a ‘lek’ (Kuba et al. 1984). A nonresourced-based lek mating system was presumed to occur in the Queensland fruit fly, B. tryoni (Ekanayake et al. 2017). To clarify and confirm that lek formation in Dacini fruit fly species, for CL-responsive species particularly B. tryoni and Z. cucurbitae, is probably the result of a resource-based lek mating system, i.e. to obtain phenylbutanoid(s) to compliment male sex pheromonal component(s) synthesized endogenously. Further investigations need to be conducted to determine i) males display lek behavior dependent or independent of obtaining the relevant phenylbutanoid attractant (RK/ZN); and ii) the actual pheromonal components released by males (to compare RK-/ZN-fed and deprived) during courtship and/or male fanning activity e.g. via headspace-gas chromatography-mass spectrometry (GC/MS) analyses.
Allomonal Function of Phenylbutanoids in Prey-Predator Interactions
Asian house gecko, H. frenatus consumed significantly fewer houseflies topically treated with RK (at 5 µg/fly) than controls (untreated houseflies) (Tan 2000b). In contrast, ME imbibed by B. dorsalis males is converted to two ME-analogues which strongly deter vertebrate predation (mentioned under allomone in item I above). Although, a major rectal pheromone gland component of Z. cucurbitae, 1,3-nonanediol, produced endogenously, is a potent allomone against vertebrate predator (Tan 2000b). The quantity of the sex pheromonal component stored in the pheromonal (rectal) gland increased significantly with age, beginning with sexual maturity about two weeks after adult eclosion (Nishida et al. 1990, 1993). Synthetic 1,3-nonanediol when applied topically to the thorax of the common housefly, Musca domestica L, at a dosage of 80-320 ng/fly, significantly reduced consumption by the Asian house gecko (Tan 2000b). Therefore, Z. cucurbitae males, after consumption of RK, probably induced a synergistic effect for the endogenously synthesized pheromonal components to deter predation by lizards. Whether this allomonal phenomenon also occurs in other phenylbutanoid-responsive species after consuming either RK and/or ZN (a known natural pungent tasting irritant – Ahmad et al. 2015) may be more significant in the natural ecosystem needs further investigations.
Synomonal Function of Phenylbutanoids in Fruit Fly-Orchid Interactions
Flowers of a number of orchid species in the genus Bulbophyllum selectively attract certain Dacini fruit fly species for pollination with specific fragrances in the tropical rain forests of Asia-Pacific and Oceanian regions. These synomonal components are either phenylpropanoids (mainly ME, see I-2C) or phenylbutanoids (AA, RK and ZN, often acompanied by their corresponding analogs, AL, RL and ZL respectively) as listed in Tables 1, 3 and 4, respectively. The relative concentrations of the synomonal component(s) are usually highest in the lip to lure a pollinator fly into a right alignment prior to conducting pollination.
Three important points related to the role of phenylbutanoid attractants in the mutualistic interactions between Dacini fruit flies and daciniphilous Bulbophyllum flowers are:
-
A)
As each flower produces only one pollinarium (bearing four pollinia for most daciniphilous Bulbophyllum species), regardless of the number of attracted flies to a flower, only one of the few flies initially attracted, if not the first fly, would eventually remove the only pollinarium. The fly would probe on the dynamic floral lip or slip on to the lip (for species belonging to the Bu. macranthum species complex while probing on the lateral sepals) and eventually be catapulted into the floral column, and ultimately, would remove the floral pollinarium to initiate pollination.
-
B)
The placement of pollinarium on either abdominal or thoracic dorsum of a potential fruit fly pollinator does not depend entirely on the type of floral attractant/synomone but the placement is entirely dependent on the floral structure and posture.
-
C)
Generally, for daciniphilous Bulbophyllum flowers the floral emission of synomone occurs, over a relatively short period (1-3 days), during the day-time when the male flies are most active in foraging for specific attractant(s).
Pertaining to the above item (B), the placement of pollinarium on an attracted fly is entirely dependent on the floral structure and dynamic lip mechanism to temporarily trap a fly - i.e. depending on floral natural posture either as a non-resupinate or a resupinate flower (Tan and Nishida 2015). The entrapment lip mechanism plays an essential and vital role in pollinarium removal. When an attracted male fly is forcibly tipped in to the column cavity a) head first, then pollinarium would invariably be stuck to the thoracic dorsum e.g. Z. cucurbitae male removed from Bu. patens (Figs. 6A and 7A); and Z. cucurbitae and Z. hochii males removed pollinarium from Bu. hortorum (Tan et al. 2023); and b) backward abdomen first, then pollinarium would certainly be attached to the abdominal dorsum when removed e.g. a male B. dorsalis attracted to floral ZN of, and removing pollinarium from Bu. macranthum sensu stricto (Fig. 7B) (Nakahira et al. 2018); and males of two other CL-responsive Dacini species (mentioned above) removing pollinarium from Bu. praetervisum that releases RK as floral synomone (Tan and Tan 2018)
Pollinarium (arrow) attachment on potential pollinators - either on thoracic or abdominal dorsum of Dacini fruit flies. [Bar scale = 1 cm]. A. Pollinarium of Bulbophyllum patens (with zingerone as floral synomone) on thoracic dorsum of a melon fly, Zeugodacus cucurbitae. B. Pollinarium of Bulbophyllum macranthum (with zingerone as floral synomone) on the abdominal dorsum of an oriental fruit fly, Bactrocera dorsalis (a methyl eugenol responsive species but weakly attracted to zingerone)
Natural Phenylbutanoids in Pest Management of Dacini Fruit Fly Pest
Detection, monitoring, surveillance. trapping plus control/management techniques, especially male annihilation technique (MAT), for Dacini fruit fly pests, particularly Z. cucurbitae and B. tryoni, have been reviewed via using mainly CL/RK baited traps (Vargas et al. 2010, Shelly et al. 2014, Tan et al. 2014). Recently, sterile B. tryoni males when fed with RK prior to release showed a) significantly reduced captures in CL-baited traps and b) higher post-release survival in the field when compared with unfed (control) males (Khan et al. 2017). Furthermore, sterile males, previously fed with RK at the sexually-immature adult stage, showed reduced attraction to CL probably due to a consequence of modified expression of genes responsible for chemoreception (Khan et al. 2021). These findings may allow simultaneous application of the sterile insect technique (SIT) and the MAT, which may be more effective than sequential use of the two techniques (Barclay and Hendrichs 2014) – both the techniques are inherently incompatible management tools, thereby, considered as an effective integrated management program against B. tryoni (Khan et al. 2017).
Conclusion
The natural occurrence of each of the phenylbutanoid attractants - AA, CL, RK and ZN – in a relatively small number of plant species under several families is listed separately in this review. The distribution of the phenylbutanoids varies greatly within a species according to growth stage or locations or physiological stage as well as time of day, especially among floral parts. The natural role of the phenylbutanoids as antimicrobial and pollinating agents have been highlighted. Particularly, their vital role as floral synomones to exclusively attract Dacini fruit fly males as potential pollinators for many daciniphilous orchid species. More research should be conducted to fully understand the biochemical, physiological and chemoecological basis, mediated by individual or combination of phenylbutanoid attractants, so that we can improve the pest management techneques of those destructive pest species. It will further enlighten the true mutualistic interactions between plants and insects, especially between certain Dacini fruit fly species, pest and/or non-pest, and the daciniphilous Bulbophyllum species, often endangered due to deforestation, in the natural tropical forest/jungle ecosystems.
Data Availability
No datasets were generated or analysed during the current study.
References
Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S, Mir MR (2015) A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone. Sci World J 816364:6 https://doi.org/10.1155/2015/816364
Ahmad AF, Youssef MSH (2015) Chemical composition and bioactive properties of Illicium verum (star-anise) extracts – Prepared by different methods. J Chem Biol Physi Sci Sec A 5:1160–1170
Akiyama M, Murakami K, Ikeda M, Iwatsuki K, Wada A, Tokuno K, Onishi M, Iwabuchi H (2007) Analysis of the headspace volatiles of freshly brewed Arabica coffee using solid-phase microextraction. J Food Sci 72:C388–C396
Akiyama M, Murakami K, Hirano Y, Ikeda M, Iwatsuki K, Wada A, Tokuno K, Onishi M, Iwabuchi H (2008) Characterization of headspace aroma compounds of freshly brewed Arabaica coffees and studies on a characteristic aroma compound of Ethiopian coffee. J Food Sci 73:C335–C346
Attia RT, Abdel-Mottaleb Y, Abdallah DM, El-Abhar HS, El-Maraghy NN (2019) Raspberry ketone and Garcinia cambogia rebalanced disrupted insulin resistance and leptin signalling in rats fed high fat fructose diet. Biomed Pharmacother 110:500–509
Ayinampudi SD, Domala R, Merugu R, Bathula S, Janaswamy MR (2012) New icetexane diterpenes with intestinal α-glucosidase inhibitory and free-radical scavenging activity isolated from Premna tomentosa roots. Fitoterapia 83:88–92
Baek SW, Kim ER, Kim JW, Kim YC (2011) Chemical constituents of Abies koreana leaves with inhibitory activity against nitric oxide production in BV2 microglia cells. Nat Prod Sci 17:175–180
Baker R, Herbert RH, Lomer RA (1982) Chemical components of the rectal gland secretions of male Dacus cucurbitae, the melon fly. Experientia 38:232–233
Bakthavatsalam N (2016) Semiochemicals. In: Ecofriendly Pest Managememt for Food Security, Omkar (ed.) Chapter 19, Elsevier. p 563-611
Baldwin IT, Preston C, Euler M, Gorham D (1977) Patterns and consequences of benzyl acetone floral emissions from Nicotiana attenuate plants. J Chem Ecol 23:2327–2343
Barclay HJ, Hendrichs J (2014) Modelling Trapping of fruit flies for detection, suppression, or eradication. In: Shelly TE, Epsky N, Jang EB, Reyes-Flores J, Vargas RI (eds) Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies. Springer, New York, NY, pp 379–420. https://doi.org/10.1007/978-94-017-9193-9
Barthel WF, Green N, Keiser I, Steiner LF (1957) Anisylacetone, synthetic attractant for male melon fly. Science 126:654
Beekwilder J. Van, der Meer IM, Sibbbesen O, Broekgaarden M, Qvist I, Mikkelsen JD, Hall RD (2007) Microbial production of natural raspberry ketone. Biotechnol J 2:1270–1279
Bellas TE, Fletcher BS (1979) Identification of the major components in the secretion from the rectal pheromone glands of the queensland fruit flies Dacus tryoni and Dacus neohumeralis (Diptera: Tephritidae). J Chem Ecol 5:796–803
Bellik Y (2014) Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale Roscoe. Asian Pac. J. Trop. Med. 4:40–44. https://doi.org/10.1016/S2222-1808(14)60311-X
Beroza M, Alexander BH, Steiner LF, Mitchell WC, Miyashita DH (1960) New synthetic lures for the male melon fly. Science 131:1044–1045
Borejsza-Wysocki W, Hrazdina G (1994) Biosynthesis of p-hydroxyphenylbutan-2-one in raspberry fruits and tissue cultures. Phytochemistry 35:673–678
CDFA [California Department of Food and Agriculture] (2013) Insect trapping guide. Gilbert AJ, Bingham RR, Nicolas MA, Clark RA (eds). 13th edition. CDFA, Sacramento, CA, USA
Damodaram KJP, Kempraj V, Aurade RM, Venkataramanappa RK, Nandagopal B, Verghese A, Bruce T (2014) Oviposition site selection by Bactrocera dorsalis is mediated through an innate recognition template tuned to γ-octalactone. PLOS One 9:1–6
Deifel A (1989) 4-(4-Hydroxyphenyl)-2-butanone (raspberry ketone). Review of natural occurrence and biogenesis Z. Lebensm. Unters For 188:330–332
Doorenweerd C, Leblanc L, Norrbom AL, San Jose M, Rubinoff D (2018) A global checklist for the 932 fruit fly species in the tribe Dacini (Diptera: Tephritidae). Zookeys 730:19–56. https://doi.org/10.3897/zookeys.730.21786
Doorenweerd C, Ekayanti A, Rubinoff D (2020) The Dacini fruit fly fauna of Sulawesi fits Lydekker’s line but also supports Wallacea as a biogeographic region (Diptera, Tephritidae. Zookeys 973:103–122. https://doi.org/10.3897/zookeys.973.55327
Drew RAI (1974) The responses of fruit fly species (Diptera: Tephritidae) in the South Pacific area to male attractants. J Aust Entomol Soc 13:267–270
Ebadollahi A, Sendi JJ, Aliakbar A, Razmjou J (2014) Chemical composition and acaricidal effects of essential oils of Foeniculum vulgare Mill. (Apiales: Apiaceae) and Lavandula angustifolia Miller (Lamiales: Lamiaceae) against Tetranychus urticae Koch (Acari: Tetranychidae). Psyche 2014:1-6 https://doi.org/10.1155/2014/424078
Ekanayake WMTD, Jayasundara MSH, Peek T, Clarke AR, Schutze MK (2017) The mating system of the true fruit fly Bactrocera tryoni and its sister species Bactrocera neohumeralis. Insect Sci 24:478–490. https://doi.org/10.1111/1744-7917.12337
El-Baroty GS, El-Baky HA, Farag RS, Saleh MA (2010) Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. African J Biochem Res 4:167–174
Embong M, Hadziyev D, Molnar S (1977) Essential oils from spices grown in Alberta. Anise oil (Pimpinella anisum). Can J Plant Sci 57:681–688
FAO/IAEA (2018) Trapping guidelines for area-wide fruit fly programmes, Second edition. Enkerlin WR, Reyes-Flores J (eds). Rome, Italy. pp 65. Licence: CC BY-NC-SA 3.0 IGO
Fay HAC (2012) A highly effective and selective male lure for Bactrocera jarvisi (Tryon) (Diptera: Tephritidae). Aust J Entomol. 51:189–197
Flath RA, Ohinata K (1982) Volatile components of the orchid Dendrobium superbum Rchb. f. J Agric Food Chem 30:841–842
Fletcher MT, Kitching W (1995) Chemistry of fruit flies. Chem Rev 95:789–828. https://doi.org/10.1021/cr00036a001
Frodin DG (2004) History and concepts of big plant genera. Taxon 53:753–776
Fronza G, Fuganti C, Guillou C, Reniero F, Joulain D (1998) Natural abundance 2H nuclear magnetic resonance study of the origin of raspberry ketone. J Agr Food Chem 46:248–254
Fronza G, Fuganti C, Pedrocchi-Fantoni G, Serra S, Zucchi G, Fauhl C, Guillou C, Reniero F (1999) Stable isotope characterization of raspberry ketone extracted from Taxus baccata and obtained by obtained by oxidation of the accompanying alcohol (betuligenol). J Agr Food Chem 47:1150–1155
Hirvi T, Honkanen E (1984) The aroma of the fruit of sea Buckthorn, Hippophae rhamnoides L. Z. Lebensm. Unters For 179:387–388
Hirvi T, Honkanen E, Pyysalo T (1981) The aroma of cranberries. (in German) Z Lebensm. Unters For 172:365–367
Hokkanen E, Pyysalo T, Hirvi T (1980) The aroma of Finnish wild raspberies, Rubus idaeus L. Z. Lebensm. Unters For 171:180–182
Hokkanen J, Mattila S, Jaakola L, Pirttilä AM, Tolonen A (2009) Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L.) leaves. J Agr Food Chem 57:9437–47
Iwahashi O, Majima T (1986) Lek formation and male-male competition in the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). Appl Entomol Zool. 21:70–75. https://doi.org/10.1303/aez.21.70
Jakubas WJ, Shah PS, Mason JR (1992) Avian repellency of coniferyl and cinnamyl derivatives. Ecol Appl 2:147–156
Jang EB, Carvalo LAFN, Chen CC, Siderhurst MS (2017) Cucumber lure trapping of Zeugodacus cucurbitae (Diptera: Tephritidae) in Hawaii and Taiwan: Logevity and nontargets captures. J Econ Entomol 110:201–207. https://doi.org/10.1093/jee/tow268
Katte T, Tan KH, Su ZH, Ono H, Nishida R (2020) Floral fragrances in two closely related fruit fly orchids, Bulbophyllum hortorum and B. macranthoides (Orchidaceae): assortments of phenylbutanoids to attract tephritid fruit fly males. Appl Entomol Zool 55:55–64. https://doi.org/10.1007/s13355-019-00653-x
Kempraj V, Park SJ, Taylor PW (2019) γ-Octalactone, an effective oviposition stimulant of Bactrocera tryoni. J Appl Entomol 143:1205–1209
Khan MA, Manoukis NC, Osborne T, Barchia IM, Gurr GM, Reynolds OL (2017) Semiochemical mediated enhancement of males to complement sterile insect technique in management of the tephritid pest Bactrocera tryoni (Froggatt). Sci Rep-UK 7:13366 https://doi.org/10.1038/s41598-017-13843-w
Khan MAM, Deshpande NP, Shuttleworth LA, Osborne T, Collins D, Wilkins MR, Gurr GM, Reynolds OL (2021) Raspberry ketone diet supplement reduces attraction of sterile male Queensland fruit fly to cuelure by altering expression of chemoreceptor genes. Sci Rep-UK 11:17632 https://www.nature.com/articles/s41598-021-96778-7
Khoo CCH, Tan KH (2000) Attraction of both sexes of melon fly, Bactrocera cucurbitae to conspecifie males – a comparison after pharmacophagy of cue-lure and a new attractant – zingerone. Entomol Exp Appl 97:317–320. https://doi.org/10.1046/j.1570-7458.2000.00745.x
Koeduka T, Watanabe B, Suzuki S, Hiratake J, Mano J, Yazaki K (2011) Characterization of raspberry ketone/zingerone synthase, catalyzing the alpha, beta-hydrogenation of phenylbutenones in raspberry fruits. Biochem Bioph Res Co 412:104–108. https://doi.org/10.1016/j.bbrc.2011.07.052
Koeduka T, Takarada T, Fujii K, Sugiyama A, Yazaki K, Nishihara M, Matsui K (2021) Production of raspberry ketone by redirecting the metabolic flux to the phenylpropanoid pathway in tobacco plants. Metab Eng Co 13:e00180
Krosch MN, Schutze MK, Armstrong KF, Graham GC, Yeates DK, Clarke AR (2012) A molecular phylogeny for the Tribe Dacini (Diptera: Tephritidae): Systemaatic and biogeographic implications. Mole Phylogenet Evol 64:512–523. https://doi.org/10.1016/j.ympev.2012.05.006
Kuba H, Sokei Y (1988) The production of pheromone clouds by spraying in the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). J Ethol 6:105–110
Kuba H, Koyama J, Prokopy RJ (1984) Mating behavior of wild melon flies, Dacus cucurbitae Coquillett (Diptera: Tephritidae) in a field cage. Appl Entomol Zool 19:367–373
Kumar G, Karthik L, Rao KB (2011) A review on pharmacological and phytochemical properties of Zingiber officinale Roscoe (Zingiberaceae). J Pharma Res 4:2963–2966
Kumaran N, Hayes RA, Clarke AR (2014a) Cue-lure but not zingerone make the sex pheromone of male Bactrocera tyoni (Tephritidae: Diptera) more attractive to females. J Insect Physiol 68:36–43
Kumaran N, Prentis PJ, Mangalan KP, Schutze MK, Clarke AR (2014b) Sexual selection in true fruit flies (Diptera: Tephritidae): transcriptome and experimental evidences for phytochemicals increasing male competitive ability. Mol Ecol 23:4645–4657. https://doi.org/10.1111/mec.12880
Kumaran N, Van der Burg CA, Qin Y, Cameron SL, Clarke AR, Prentis PJ (2018) Plant-mediated female transcriptomic changes post-mating in a tephritid fruit fly Bactrocera tryoni. Genome Biol Evol 10:94–107
Larsen M, Poll L, Callesen O, Lewis M (1991) Relations between the content of aroma compounds and the sensory evaluation of 10 raspberry varieties (Rubus idaeus L). Acta Agr Scand 41:447–454
Leblanc L, Doorenweerd C, San Jose M, Pham HT, Rubinoff D (2018a) Descriptions of four new species of Bactrocera and new country records highlight the high biodiversity of fruit flies in Vietnam (Diptera, Tephritidae, Dacinae). Zookeys 197:87–110
Leblanc L, Doorenweerd C, San Jose M, Sirisena UGAI, Hemachandra KS, Rubinoff D (2018b) Description of a new species of Dacus from Sri Lanka, and new country distribution records (Diptera, Tephritidae, Dacinae). Zookeys 795:105–114
Leblanc L, Tsatsia F, Doorenweerd C (2021) Novel lures and COI sequences reveal cryptic new species of Bactrocera fruit flies in the Solomon Islands (Diptera, Tephritidae, Dacini). Zookeys 1057:49–103
Lee J (2016) Further research on the biological activities and the safety of raspberry ketone is needed. NFS J 2:15–18
Lim SH, Choi C-I (2021) Potentials of raspberry ketone as a natural antioxidant. Antioxid 10:482. https://doi.org/10.3390/antiox10030482
Lin, Y.L. and Chow, C.J. 1984. Studies on the constituents of aerial parts of Scutellaria rivularis wall. Kuo Li Chung-Kuo 1 Yao Yen Chiu So Yen Chiu Pao Kao141–165
Manoukis NC, Jang EB (2013) The diurnal rhythmicity of Bactrocera cucurbitae (Diptera: Tephritidae) Attraction to cue-lure: insights from an interruptable lure and computer vision. Ann Entomol Soc Am 106:136–142. https://doi.org/10.1603/AN12095
Marco JA, Barbera O, Rodriguez S, Domingo C, Adell J (1988) Flavonoids and other phenolics from Artemisia hispanica. Phytochemistry 27:3155–3159
Meier M, Kohlrnberg B, Braun NA (2003) Isolation of anisyl acetone from Agrwood oil. J Essent Oil Res 15:54–56
Menon AN, Padmakumari KP, Kutty BS, Sumathikutty MA, Sreekumar MM, Arumugham C (2007) Effects of processing on the flavor compounds of Indian fresh ginger (Zingiber officinale Rosc.). J Essent Oil Res 19:105–110
Metcalf RL (1990) Chemical ecology of Dacinae fruit flies (Diptera: Tephritidae). Ann Entomol Soc Am 83:1017–1030
Milke L, Mutz M, Marienhagen J (2020) Synthesis of the character impact compound raspberry ketone and additional flavoring phenylbutanoids of biotechnological interest with Corynebacterium glutamicum. Microb Cell Fact 19:92. https://doi.org/10.1186/s12934-020-01351-y
Mir SH, Mir GM (2016) Lekking behaviour and male-male rivalry in the melon fly Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J Insect Behav 29:379–384
Miyoshi T, Ito M, Kitayama T, Isomori S, Yamashita F (2013) Sedative effects of inhaled benzylacetone and structural features contributing to its activity. Biol Pharma Bull 36:1474–1481. https://doi.org/10.1248/bpb.b13-00250
Monro J, Richardson NL (1969) Traps, male lures, and a warning system for Queensland fruit fly, Dacus tryoni (Frogg.) (Diptera, Trypetidae). Aust J Agr Res 20:325–338
Morimoto C, Satoh Y, Hara M, Inoue S, Tsujitae T, Okuda H (2005) Anti-obese action of raspberry ketone. Life Sci 77:194–204
Muryati M, Trisyono YA, Wijaksono W, Wahyono W (2017) Deterrent of Bactrocera carambolae resulted from eggs deposition on mango. Agrivita J Agr 39:201–213. https://doi.org/10.17503/agrivita.v39i2.1097
Nakahira M, Ono H, Wee SL, Tan KH, Nishida R (2018) Floral synomone diversification of Bulbophyllum sibling species (Orchidaceae) in attracting fruit fly pollinators. Biochem Syst Ecol 81:86–95. https://doi.org/10.1016/j.bse.2018.10.002
Nakamori H, Soemori H (1985) Diurnal changes in the attraction of the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae), in different habitats. Japanese J Appl Entomol Zool 293:216–222 (in Japansese with English summary)
Nishida R (2014) Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci Biotechnol Bioch 78:1–13
Nishida R, Fukami H (1990) Sequestration of distasteful compounds by some pharmacophagous insects. J Chem Ecol 16:151–164. https://doi.org/10.1007/BF01021276
Nishida R, Tan KH, Serit M, Lajis NH, Sukari AM, Takahashi S, Fukami H (1988) Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis. Experentia 44:534–536
Nishida R, Tan KH, Takahashi S, Fukami H (1990) Volatile components of male rectal glands of the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). Appl Entomol Zool 25:105–112. https://doi.org/10.1303/aez.25.105
Nishida R, Iwahashi I, Tan KH (1993) Accumulation of Dendrobium (Orchidaceae) flower fragrance in the rectal glands by males of the melon fly, Dacus cucurbitae (Tephritidae). J Chem Ecol 19:713–722. https://doi.org/10.1007/BF00985003
Nishida R, Tan KH, Wee SL, Hee AKW, Toong YC (2004) Phenylpropanoids in the fragrance of the fruit fly orchid, Bulbophyllum cheiri, and their relationship to the pollinator, Bactrocera papayae. Biochem Syst Ecol 32:245–252. https://doi.org/10.1016/S0305-1978(03)00179-0
Nishida R, Tan KH (2016) Search for new fruit fly attractants from plants: a review, pp. 249–262. In: Sabater-Munoz B, Vera T, Pereira R, Orankanok W (eds.), Proceedings, 9th International Symposium on Fruit Flies of Economic Importance, Bangkok, Thailand
Nishida R, Howcroft N, Tan KH, Su Z-H, Ono H (2022) Floral synomone components of fruit fly-attracting orchids, Bulbophyllum sinapis and B. hahlianum, in Papua New Guinea. Biochem Syst Ecol 105. https://doi.org/10.1016/j.bse.2022.104481
Nordlund DA, Lewis WJ (1976) Terminology of chemical releasing stimuli in intraspecific and interspecific interactions. J Chem Ecol 2:211–220
Ohinata K, Jacobson M, Kobayashi M, Chambers DL, Fujimoto MS, Higa H (1982) Oriental fruit fly and melon fly: Biology and chemical studies of smoke production by males. J Environ Sci Health A17:197–216
Oliver JE, Casana-Giner V, Jang EB, McQuate GT, Carvalho L (2002) Improved attractants for the melon fly, Bactrocera cucurbitae. Proceedings of 6th International Fruit Fly Symposium, Stellenbosch, South Africa. p 283-290
Ong PT, Hee AKW, Wee SL, Tan KH (2011) The attraction of flowers of Bulbophyllum section Sestochilus to Bactrocera fruit flies (Diptera: Tephritidae). Males Orchid J 8:93–102
Ono H, Hee AKW, Jiang HB (2021) Recent advancements in studies on chemosensory mechanisms underlying detection of semiochemicals in Dacini fruit flies of economic importance (Diptera; Tephritidae). Insects 12:106. https://doi.org/10.3390/insects12020106
Osoro EK, Yong ZH, Ndagijimanab A, Imbenzia PS (2013) A review on phenolic compounds in Illicium species and their pharmacological effects. Der Pharmacia Sinica 4:17–30
Park SJ, Siderhurst SM, Jamie I, Taylor PW (2016) Hydrolysis of Queensland fruit fly, Bactrocera tryoni (Froggatt), attractants: Kinetics and implications for biological activity. Austr J Chem 69:1162–1166
Park SJ, De Faveri SG, Cheesman J, Hanssen BL, Cameron DMS, Jamie IM, Taylor PM (2020) Zingerone in the flower of Passiflora maliformis attracts an Australian fruit fly, Bactrocera jarvisi (Tryon). Molecules 25:2877. https://doi.org/10.3390/molecules25122877
Park SJ, Hanssen B, De Faveri S, Cheesman J, Royer J, Cameron D, Jamie J, Jamie I, Taylor P (2022) Plant-based attractant, zingerone and its analogs reveal structural requirements for attraction of Jarvis’s fruit fly. S9-0153 p. 166 Book of Abstract, 2022 ISCE-APACE Joint Meeting of the 3th Annual Meeting of the International Society of Chemical Ecology and the 11th Asia-Pacific Association of Chemical Ecologists Conference, Kuala Lumpur, Malaysia
Purnomo H, Jaya F, Widjanarko SB (2010) The effects of type and time of thermal processing on ginger (Zingiber officinale Roscoe) rhizome antioxidant compounds and its quality. Int Food Res J 17:335–347
Royer JE (2015) Responses of fruit flies (Tephritidae: Dacinae) to novel male attractants in north Queensland, Australia, and improved lures for some pest species. Austral Entomol 54:411–426
Royer JE, Mayer DG (2018) Combining cue-lure and methyl eugenol in traps significantly decreases catches of most Bactrocera, Zeugodacus and Dacus Species (Diptera: Tephritidae: Dacinae) in Australia and Papua New Guinea. J Econ Entomol 111:298–303. https://doi.org/10.1093/jee/tox334
Royer JE, De Faveri SG, Lowe GE, Wright CL (2014) Cucumber volatile blend, a promising female-bias for Bactrocera cucumis (French 1907) (Diptera: Tephritidae: Dacinae), a pest fruit fly that does not respond to male attractants. Austral Entomol 53:347–352. https://doi.org/10.1111/aen.12083
Royer JE, Agovaua S, Bokosou J, Kurika K, Mararuai A, Mayer DG, Niangu B (2017) Responses of fruit flies (Diptera: Tephritidae) to new attractants in Papua New Guinea. Austral Entomol 57:40–49. https://doi.org/10.1111/aen.12269
Royer JE, Mille C, Cazeres S, Brinon J, Mayer DG (2019) Isoeugenol, a more attractive male lure for the cue-lure-responsive pest fruit fly Bactrocera curvipennis (Diptera: Tephritidae: Dacinae), and new records of species responding to zingerone in New Caledonia. J Econ Entomol 112:1502–1507. https://doi.org/10.1093/jee/toz034
Royer JE, Tan KH, Mayer DG (2020) Comparative trap catches of male Bactrocera, Dacus and Zeugodacus fruit flies (Diptera: Tephritidae) with four floral phenylbutanoid lures (anisyl acetone, cue-lure, raspberry ketone and zingerone) in Queensland. Australia Environ Entomol 49:815–822. https://doi.org/10.1093/ee/nvaa056
Samadi Andzagi G, Yaghoubi H, Fardin M (2017) Investigation of compositions and effects of local herbal Silybum marianum and Foeniculum vulgare extractions on hospital acquired infections (HAI) and cell line of liver cancer (HepG2) by MTT assays. J Herb Drug 7:275–282
Scolari F, Valerio F, Benelli G, Papadopoulos NT, Vaníčková L (2021) Tephritid fruit fruit fly semiochemicals: Current knowledge and future perspectives. Insects 12:408 (https://www.mdpi.com/2075-4450/12/5/408)
Segura DF, Belliard SA, Vera MT, Bachmann GE, Ruiz MJ, Jofre-Bartud F, Fernandez PC, Lopez ML, Shelly TE (2018) Plant chemicals and the sexual behaviour of male Tephritid fruit flies. Ann Entomol Soc Am 111:239–264. https://doi.org/10.1093/aesa/say024
Shamshir RA, Wee SL (2019) Zingerone improves mating performance of Zeugodacus tau (Diptera: Tephritidae) through enhancement male courtship activity and sexual signaling. J Insect Physiol 119 https://doi.org/10.1016/j.jinsphys.2019.103949
Shelly TE (2010) Effects of methyl eugenol and raspberry ketone/cue lure on the sexual behavior of Bactrocera species (Diptera: Tephritidae). Appl Entomol Zool 45:349–361
Shelly TE (2018) Sexual selection on leks: A fruit fly primer. J Insect Sci 18:1–16. https://doi.org/10.1093/jisesa/iey048
Shelly TE, Kaneshiro KY (1991) Lek behaviour of the oriental fruit fly in Hawaii. J Insect Behav 20:453–472
Shelly TE, Kurashima RS (2016) Mixing male lures results in an effective multispecies bait for trapping Bactrocera (Diptera: Tephritidae) fruit flies. Fla Entomol 99:318–320. https://doi.org/10.1653/024.099.0229
Shelly TE, Villalobos EM (1995) Cue lure and the mating behaviour of male melon flies (Diptera: Tephritidae). Fla Entomol 78:473–482
Shelly T, Nishimoto J, Diaz A, Leathers J, War M, Shoemaker R, Al-Zubaidy M, Joseph D (2010) Capture probability of released males of two Bactrocera species (Diptera: Tephritidae) in detection traps in California. J Econ Entomol 103:2o42-2051 https://doi.org/10.1603/EC10153
Shelly T, Epsky N, Jang EB, Reyes-Flores J, Vargas R (2014) Trapping and the detection, control, and regulation of Tephritid fruit flies. Springer: Dordrecht Heidelberg, NY London, p 638. https://doi.org/10.1007/978-94-017-9193-9
Siderhurst MS, Park SJ, Buller CN, Jamie IM, Manoukis NC, Jang EB, Taylor PW (2016) Raspberry ketone trifluoroacetate, a new attractant for the Queensland fruit fly, Bactrocera tryoni (Froggatt). J Chem Ecol 42:156–162
Singh G, Kapoor IPS, Singh P, de Heluani CS, de Lampasona MP, Catalan CAN (2008) Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem Toxicol 46:3295–3302 https://doi.org/10.1016/j.fct.2008.07.017.
Sota H, Yasuo T, Hideaki M, Taro I (2006) Study on the volatile compounds of various gingers. Koryo, Terupen oyobi Seiyu Kagaku ni kansuru Toronkai Koen Yoshishu 50:10–12 (in Japanese)
Tan KH (2000a) Behaviour and chemical ecology of Bactrocera flies. In: Tan KH (ed.) Area-wide control of fruit flies and other insect pests. Joint proceedings of the international conference on area-wide control of insect pests, 28 May-2 June, 1998 and the Fifth International Symposium on Fruit Flies of Economic Importance, Penang, Malaysia, 1-5 June, 1998. p 647–656
Tan KH (2000b) Sex pheromone components in defense of melon fly, Bactrocera cucurbitae against Asian house gecko, Hemidactylus frenatus. J Chem Ecol 26:697–704. https://doi.org/10.1023/A:1005480206023
Tan KH (2009) Fruit fly pests as pollinators of wild orchids. Orchid Digest 73:180–187
Tan KH, Lee SL (1982) Species diversity and abundance of Dacus (Diptera: Tephritidae) in five ecosystems of Penang, West Malaysia. Bull Entomol Res 72:709–716. https://doi.org/10.1017/S0007485300008737
Tan KH, Nishida R (1995) Incorporation of raspberry ketone in the male rectal glands of the Queensland fruit fly, Bactrocera tryoni Froggatt (Diptera: Tephritidae). Appl Entomol Zool 30:494–497. https://doi.org/10.1303/aez.30.494
Tan KH, Nishida R (1996) Sex pheromone and mating competition after methyl eugenol comsumption in the Bactrocera dorsalis complex. In: McPheron BA, Steck GJ (eds) Fruit Fly Pests — A World Assessment of Their Biology and Management. St. Lucie Press, Florida, pp 147–153
Tan KH, Nishida R (2000) Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J Chem Ecol 26:533–546. https://doi.org/10.1023/A:1005477926244
Tan KH, Nishida R, Toong YC (2002) Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. J Chem Ecol 28:1161–1172. https://doi.org/10.1023/A:1016277500007
Tan KH, Tan LT, Nishida R (2006) Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination. J Chem Ecol 32:2429–2441. https://doi.org/10.1007/s10886-006-9154-4
Tan KH, Vermeulen JJ, Katte T, Ono H, Nishida R (2021) Diversification in both the floral morphology and chemistry in two daciniphilous orchid ecotypes in Borneo. Arthro-Plant Interact 15:447–455. https://doi.org/10.1007/s11829-021-09821-9
Tan KH, Ong PT, Tan LT (2023) Morphology and movement of Bulbophyllum hortorum (Orchidacea) flowers enable selection of optimal-sized Dacini fruit fly males. Arthropod-Plant Interact 17:647–660. https://doi.org/10.1007/s11829-023-09987-4
Tan KH, Nishida R (2005) Synomone or Kairomone? - Bulbophyllum apertum (Orchidaceae) flower releases raspberry ketone to attract Bactrocera fruit flies. J Chem Ecol 31:509-519 https://doi.org/10.1007/s10886-005-2023-8
Tan KH, Nishida R (2007) Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochem Syst Ecol 35:334-341 https://doi.org/10.1016/j.bse.2007.01.013
Tan KH, Nishida R (2012) Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci 12:56
Tan KH, Nishida R (2015) Pollination of bactrocerophilous Bulbophyllum orchids. In Elliott J, Kuraweil HF, O’Byrne P, Tan KW, van Schans AS, Wong SM, Yam TW (eds.). Proceedings of the 20th World Orchid Conference, Singapore, 201. p 273-279. https://www.researchgate.net/publication/275834142_Pollination_of_bactrocerophilous_Bulbophyllum_Orchids
Tan KH, Tan LT (2018) Movements of floral parts and roles of the tooth on column wall of Bulbophyllum praetervisum (Orchidaceae) flower for pollination by Dacini fruit flies (Diptera: Tephritidae). J Poll Ecol 24: 157-163. https://www.pollinationecology.org/index.php?journal=jpe&page=article&op=view&path%5B%5D=500
Tan KH, Nishida R, Jang EB, Shelly TE (2014) Pheromones, male lures, and trapping of tephritid fruit flies. In: Shelly TE, Epsky N, Jang EB, Reyes-Flores J, Vargas RI (eds.). Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies. Springer:New York, NY. p 15–74. https://doi.org/10.1007/978-94-017-9193-9
Tan, K.H. 1983. Response of Dacus (Diptera: Tephritidae) to Ocimum sanctum (Linn.) extracts and different synthetic attractants in Penang, Malaysia. In: Cavalloro R (ed.) Proceedings of the CEC/IOBC International Symposium, Athens, “Fruit flies of economic importance”. p 513–520
Thamrin S, Rauf A, Purwatiningsih, Ratna ES (2017) Identification of kairomonal compounds from host plants aattractive to melon fly, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). J Enbtomol 14:216–227
Thuy DTT, Tran TT, Thuy TTT, Pham THM, Quoc TT, Pham QL, Duy CN, Long GB, Nyuyen QC (2019) Isolation process and compound identification of agarwood essential oils from Aquilaria crassna cultivated at three different locations in Vietnam. Processes 2019(7):432. https://doi.org/10.3390/pr7070432
Vargas RI, Stark JD, Kido MH, Ketter HM, Whitehand LC (2000) Methyl eugenol and cue-lure traps for suppression of male oriental fruit flies and melon flies (Diptera: Tephritidae) in Hawaii: effects of lure mixtures and weathering. J Econ Entomol 93:81–87
Vargas RI, Shelly TE, Leblanc L, Piñero JC (2010) Recent advances in methyl eugenol and cue-lure technologies for fruit fly detection, monitoring, and control in Hawaii. Vitam Horm 83: 575-595 https://doi.org/10.1016/S0083-6729(10)83023-7.
Wee SL, Clarke A (2020) Male-lure type, lure dosage, and fly age at feeding all influence male mating success in Jarvis’ fruit fly. Sci Rep-UK 10:15004. https://doi.org/10.1038/s41598-020-72209-x
Wee SL, Tan KH (2001) Allomonal and hepatotoxic effects following methyl eugenol consumption in Bactrocera papayae male against Gekko monarchus. J Chem Ecol 27:953–964. https://doi.org/10.1023/A:1010387020135
Wee SL, Tan KH, Nishida R (2007) Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J Chem Ecol 33:1272–1282. https://doi.org/10.1007/s10886-007-9295-0
Wee SL, Peek T, Clarke AR (2018) The responsiveness of Bactrocera jarvisi (Diptera: Tephritidae) to two naturally occurring phenylbutanoids, zingerone and raspberry ketone. J Insect Physiol 109:41–46. https://doi.org/10.1016/j.jinsphys.2018.06.004
Wee SL, Royer JE, Herring J, Meyer DG, Tan KH (2020) Relative response of male Bactrocera frauenfeldi (Diptera: Tephritidae) to phenylbutanoid phytochemicals: implications for fruit fly control and plant-insect interactions. Chemoecology 30:305–314. https://doi.org/10.1007/s00049-020-00320-6
Winter M (1961) Odeur et constitution XIX. Sur des homologues et analogues de la p-hydroxyphényl-1-butanone-3 («cétone de framboise»). Helv Chim Acta 44:2110–2121
Yang JF, Yang CH, Chang HW, Yang CS, Wang SM, Hsieh MC, Chuang LY (2010) Chemical composition and antibacterial activities of Illicium verum against antibiotic-resistant pathogens. J Med Food 13:1254–1262
Yang CH, Chang FR, Chang HW, Wang SM, Hsieh MC, Chuang LY (2012) Investigation of the antioxidant activity of Illicium verum extracts. J Med Plants Res 6:314–324
Yu X, Cheng B (1986) Studies on the chemical constituents of the essential oil from Limnophila rugose. Acta Bot. Yunnan. 8:103–106
Zhang H (2014) Research progress in chemical synthesis of raspberry ketone. Acetaldehyde Acetic Acid Chemistry and Industry 16:12–19
Zhang YX, Li JS, Chen LH, Peng WW, Cai BC (2012) Simultaneous determination of five gingerols in raw and processed ginger by HPLC. Chinese Pharma J 47:471–474
Zorn H, Fischer-Zorn M, Berger RG (2003) A labeling study to elucidate the biosynthesis of 4-(4-hydroxyphenyl)-butan-2-one (raspberry ketone) by Nidula niveo-tomentosa. Appl Environ Microbiol 69:367–372
Acknowledgements
We gratefully thank T.E. Shelly (APHIS, USDA, Waimanalo) for his assistance in reviewing this manuscript; and thank L. LeBlanc and M.K. Schutze for providing updated information pertaining to the zingerone-responsive species.
Author information
Authors and Affiliations
Contributions
Keng Hong Tan and Ritsuo Nishida wrote the text in the review article, prepared figures 1-7, and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors sincerely declare that there is no conflict of interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, K.H., Nishida, R. A review on natural phenylbutanoid attractants: Occurrence, distribution, and role in nature, especially in relation to Dacini fruit fly behavior and pollination. J Chem Ecol (2024). https://doi.org/10.1007/s10886-024-01499-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10886-024-01499-6