Abstract
Floral chemical components are important cues used by plants to attract pollinators. One outstanding case is “fruit fly orchids” in the genus of Bulbophyllum to attract their pollinators by releasing characteristic fragrances. Dacini fruit flies are main or exclusive pollinators which are strongly attracted to certain natural chemicals, either methyl eugenol (ME: a phenylpropanoid) or raspberry ketone (RK: a phenylbutanoid). Furthermore, zingerone (ZN: a phenylbutanoid) has been characterized as the attractant for both ME- and RK-sensitive fruit fly species. In the present study, we examined chemical profiles of two closely related Bulbophyllum orchids—B. hortorum, and B. macranthoides subsp. tollenoniferum—distributed in Papua New Guinea and the Southeast Asian countries, respectively. We first observed that RK-sensitive flies were attracted to these orchids by ex situ cultivation in Penang, Malaysia. These Bulbophyllum orchids contained RK and/or ZN as their main floral components. Other than these attractants, multiple phenylbutanoids including potential attractants for RK-sensitive species were identified from these orchids. Therefore, we examined attractiveness of potential phenylbutanoid attractants to an RK-sensitive melon fly, Zeugodacus cucurbitae, using laboratory-reared flies. Furthermore, we analyzed molecular phylogenetic relationships among phenylpropanoid- or phenylbutanoid-producing orchids to see a relation between chemical profiles and phylogenetic classification in the related species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floral fragrance is one of several key factors used by insect pollinators to visit specific flowers. A group of “fruit fly orchids” in the genus Bulbophyllum produces specific volatiles as floral fragrance to attract potential pollinators, exclusively the tephritid fruit fly species, which include destructive horticultural pests in both tropical and temperate regions. Males of many such species belonging to two genera–Bactrocera and Zeugodacus—of the Tribe Dacini (consisting four genera including Dacus) under the subfamily Dacinae (Tephritidae: Diptera)—are strongly attracted to certain natural chemicals, methyl eugenol (ME: a phenylpropanoid) or raspberry ketone (RK: a phenylbutanoid). This leads to voracious ingestion of these compounds by the male flies, and their subsequent utilization as sex pheromones or sex pheromone precursors (Tan et al. 2014). Furthermore, zingerone (ZN: a phenylbutanoid) has been characterized as the attractant for both ME- and RK-sensitive fruit fly species likely owing to its hybrid chemical structure between ME and RK. Notably, an analogous synthetic acetyl derivative, cue-lure (CL), has been developed as a highly potent attractant, and used for pest management of RK-sensitive fruit fly species, particularly the melon fly, Z. cucurbitae (Coquillett) (Beroza et al. 1960).
Bulbophyllum is the largest genus in the family Orchidaceae, and it comprises more than 2000 species (Frodin 2004). Two closely related species—Bulbophyllum hortorum J.J. Verm., P. O'Byrne and A.L. Lamb and B. macranthoides Kraenzl. subspecies tollenoniferum (J.J. Sm.) J.J. Verm.—are epiphytic orchids mainly distributed in the Papua New Guinea and Southeast Asia regions, respectively. B. hortorum is related to B. patens that is allopatrically distributed in Thailand, Peninsular Malaysia, Sumatra and Borneo (Vermeulen 2008). Various tephritid fruit flies—including ME- and RK-sensitive species—are attracted to flowers of B. patens and B. baileyi, because these species contain ZN as a major component in the floral fragrance (Tan and Nishida 2000, 2007). However, there is limited information about tephritid fruit flies that are attracted to flowers of B. hortorum and B. macranthoides subsp. tollenoniferum. In the present study, we report that flowers of B. hortorum and B. macranthoides subsp. tollenoniferum selectively attract mainly RK-sensitive fruit flies. We also characterized floral volatile components that are attractive to the dacine fruit flies. Since multiple phenylbutanoids have been detected, including potential attractants for the dacine fruit flies from these floral fragrances, we also observed their attractiveness to an RK-sensitive species, Z. cucurbitae, using laboratory bioassays. Furthermore, we analyzed molecular phylogenetic relationships between the two Bulbophyllum species, and their related fruit fly-attracting (daciniphilous) Bulbophyllum species, using DNA sequences of a non-coding plastid region and a nuclear ribosomal internal transcribed spacer (ITS) region.
Materials and methods
Plants and flowers
Original plants of B. hortorum and B. macranthoides subsp. tollenoniferum from Papua New Guinea and Indonesia, respectively, for ex situ cultivation in Penang were given as research materials from P. T. Ong of Forest Research Institute Malaysia, Kepong, Selangor, Malaysia.
Insects
We used a laboratory-reared colony of Z. cucurbitae maintained by the Okinawa Prefectural Plant Protection Center in Japan. The strain originating from Taiwan in 2008 was kept at 25 °C and 60–70% relative humidity under a photoperiod of 14 h light/10 h dark, with dawn and dusk twilights. The adult flies were provided with water and a diet comprising four parts sucrose and one part dry yeast AY-65 (Asahi Food & HealthCare, Ltd., Tokyo, Japan).
Observation of fruit flies attracted to orchid flowers
Preliminary observations showed that feral RK-sensitive Dacini fruit flies were attracted to flowers between 08:00 and 11:00 h after which there was normally no attraction. Therefore, observations were made for five flowers of each species from 07:30 to 12:00 h (dawn breaks at ca 07:00 h) on the day the flowers freshly bloomed. It should be noted that the flowers of the two species did not bloom simultaneously on the same day.
Chemicals
We synthesized 4-(4-hydroxy-3,5-dimethoxyphenyl)-2-butanone from syringaldehyde (Wako Pure Chemical Co., Osaka, Japan). We tentatively named this compound as “syringerone (SN)” to avoid a possible misuse and confusion with “syringyl acetone [1-(4-hydroxy-3,5-dimethoxyphenyl)-2-propanone]”. Briefly, the aldol condensation of syringaldehyde with acetone yielded “dehydrosyringerone [1-(4-hydroxy-3,5-dimethoxyphenyl)-3-buten-2-one]”, as described previously (Kuo et al. 2005). The product was then reduced to SN using palladium-activated carbon (Wako Pure Chemical Co., Osaka, Japan). We also synthesized 4-(4-methoxyphenyl)-2-butanol (AL), rhododendrol (RL) and zingerol (ZL) from commercial anisyl acetone (AA) (Tokyo Chemical Industry Co., LTD., Tokyo, Japan), raspberry ketone (RK) (Tokyo Chemical Industry Co., LTD., Tokyo, Japan) and zingerone (ZN) (Sigma-Aldrich, St. Louis, MO, USA), respectively, using sodium borohydride (Wako Pure Chemical Co., Osaka, Japan).
Extraction of floral fragrance
Extraction of floral fragrance was performed as described in the previous papers by Tan and Nishida (2000) and Nakahira et al. (2018) with slight modifications. Briefly, a whole flower or each floral part was weighed and then immersed in sufficient ethanol in a glass vial. An aliquot of each extract was concentrated in vacuo; and extracted with a mixture of 10% ethyl acetate in hexane and saturated NaCl solution. The organic layer was dried over anhydrous Na2SO4, and carefully concentrated under a N2 stream for gas chromatography (GC) quantification and coupled gas chromatography/mass spectrometry (GC–MS) analyses.
Quantification of phenylbutanoids
A portion of the floral fragrance samples prepared as described above was used for quantification. The sample solution (1 μl) with an internal standard (1-pentadecanol) was subjected to GC quantification by comparing the flame ionization signal intensities with that of the respective authentic standards. Concentrations of phenylbutanoids (ppm) were calculated from contents per fresh weight of whole flower or each floral part.
Instrument
GC–MS analyses were performed on an Agilent 5975/6890 N GC–MS instrument equipped with an Agilent HP-5MS 5% phenyl methyl siloxane-coated capillary column (30 m × 0.25 mm, 0.25 μm film thickness). The temperature of the GC oven was programmed from 60 (2 min holding) to 290 °C at a rate of 10 °C/min. The GC was equipped with a total ion monitor. GC quantification was performed on an HP-6850 gas chromatograph equipped with an Agilent HP-5MS 5% phenyl methyl siloxane-coated capillary column (15 m × 0.25 mm, 0.25 μm film thickness) with a flame ionization detector using the same program of the GC–MS analyses for the GC oven.
Laboratory bioassays
Virgin male flies were selected and separately caged at 7 days after adult eclosion. Ten males were transferred into a plastic container (75 mm × 75 mm × 45 mm) with a lid, having a hole (35 mm diameter) that was covered with a plastic mesh (Fig. S1a), for acclimatization 1 day prior to commencement of a bioassay. The males were allowed access to water and food ad libitum. Prior to the experiment, we impregnated pieces of filter paper (21 mm diameter, Kiriyama No. 5B, Tokyo, Japan) with 100 µg of a test compound (test compounds: AA, AL, CL, RK, RL, SN, ZN, and ZL) dissolved in 25 μL of ethanol, and dried at room temperature. Each filter paper was then placed at the center of the plastic mesh on the lid of container (Fig. S1b). Filter paper treated with ethanol alone was used as experimental controls. The number of males located on the filter paper was counted as attracted flies every 5 min for 30 min (i.e., six observations per experimental replication). Three replicates were conducted for each compound. Furthermore, (1) to test whether numbers of attracted flies depend on time after the treatment of a test compound, the average numbers of attracted flies at each interval were analyzed using Friedman test for each compound; and (2) to compare attractiveness, the average numbers of attracted flies at every 5 min interval were calculated for each compound tested.
DNA extraction, PCR, sequencing and phylogenetic analysis
Total genomic DNA was prepared from individual flowers using DNeasy Plant Mini Kit (Quiagen, Crawley, UK). Along with B. hortorum and B. macranthoides, total genomic DNAs of phenylpropanoid- (namely, B. cheiri and B. vinaceum) or phenylbutanoid-producing orchids (namely, B. ecornutum, B. macranthum, B. patens, and B. praetervisum) were prepared for phylogenetic analysis. The DNA sample information including GenBank accession numbers is shown in Table S1. Five non-coding regions of chloroplast genome (cp), including the rps16 intron, trnG intron, petB intron, trnL intron, and trnL-trnF spacer region, and the nuclear ITS region (small subunit ribosomal RNA, internal transcribed spacer 1, 5.8S ribosomal RNA, and internal transcribed spacer 2) were amplified and sequenced using the primers and procedures previously described by Azuma et al. (2010) with slight modifications. PCR for cp DNA regions was carried out using LA-Taq DNA polymerase (Takara, Bio Inc., Tokyo, Japan). KOD DNA polymerase (Toyobo, Osaka, Japan) was used for PCR amplification of ITS region. For rps16, trnG, trnL, and trnL-trnF, the reaction condition was 94 °C for 1 min followed by 35 cycles of 94 °C for 20 s, 58 °C for 10 s, 68 °C for 2 min, with a final extension at 72 °C for 10 min. Same conditions were applied for petB, excepting that the annealing and extension of cycles were performed at 50 °C for 1 min and 30 s, 72 °C for 2 min. For ITS, the reaction conditions were 94 °C for 1 min followed by 35 cycles of 94 °C for 20 s, 56 °C for 30 s, 68 °C for 2 min, with a final extension at 68 °C for 10 min. The primers used for PCR are listed in Table S2. The PCR products were enzymatically cleaned with Calf intestine Alkaline Phosphatase (Toyobo, Osaka, Japan) and Exonuclease I (Takara, Bio Inc., Tokyo, Japan), and directly sequenced with ABI 3130xl Genetic Analyzer (Applied Biosystems and Hitachi, Ltd. Tokyo, Japan) using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) according to the conditions in the manufacturer’s protocol. The DNA sequence data have been deposited in the DDBJ/EMBL-Bank/GenBank as listed in Table S1. Sequence alignment was performed using the software Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic tree was generated by aligning the 3478–3545 bp nucleotide sequences of amplified fragments by the PCR. The phylogenetic tree was constructed using maximum likelihood (ML) from 1000 replicate bootstrap sampling under the GTRGAMMA model using the software RAxML ver. 8 (Stamatakis 2014).
Statistical analysis
Statistical analyses were conducted using the R software (http://www.r-project.org). Quantitative differences of numbers of attracted Z. cucurbitae to the different phenylbutanoids tested were analyzed using the Tukey’s HSD test.
Results
Fruit fly species attracted to orchid flowers
The fruit flies attracted to flowers of both the Bulbophyllum species were RK-sensitive species (Fig. 1), except Ba. dorsalis and its hybrid that are ME-sensitive. Table 1 shows that more Dacini fruit fly species were attracted to B. hortorum, than to B. macranthoides subsp. tollenoniferum. It is interesting to note that (1) two individuals of Dacus maculipterus attracted to B. hortorum are not the common Dacus species previously recorded in Peninsular Malaysia; and (2) one feral ME-sensitive species, Ba. dorsalis, was attracted to flowers of the two Bulbophyllum species. Apparently, more Z. cucurbitae males were attracted to B. macranthoides subsp. tollenoniferum than to B. hortorum. However, it should be noted that two of the B. hortorum flowers were observed while the weather, with intermittent drizzle/rain, was not conducive to fly attraction. We have observed B. hortorum pollinia removal by an attracted Z. cucurbitae that needed to be pulled open the spring-lip consistently in a closed position; but to date have not seen pollinia been removed by an attracted fly for B. macranthoides subsp. tollenoniferum.
Chemical profiles of whole flowers
Typical gas chromatograms of the whole floral extracts of B. hortorum and B. macranthoides subsp. tollenoniferum are shown in Fig. 2. We identified the floral components by comparing their GC-retention times and mass spectra with those of the authentic samples, as shown in Table 2. We detected the phenylbutanoid attractants—AA, RK and ZN—for Bactrocera and/or Zeugodacus species in both the Bulbophyllum species. We also found the corresponding alcohols —4-(4-methoxyphenyl)-2-butanol (AL), rhododendrol (RL) and zingerol (ZL)—as minor components in both the species. Notably and surprisingly, we also detected a trace amount of CL in three of the samples (namely, BH2, BH8 and BH10) of B. hortorum (Fig. 2 and Fig. S2). Furthermore, we identified 4-(4-hydroxy-3,5-dimethoxyphenyl)-2-butanone in B. macranthoides subsp. tollenoniferum, but not in B. hortorum. We propose herewith a name of this compound as “syringerone (SN)” as an analog of zingerone (ZN) to avoid confusion with syringyl acetone. The amounts of the major components, i.e., AA, RK and ZN, in B. hortorum and B. macranthoides subsp. tollenoniferum were highly variable amongst individual floral specimens (Fig. S3). For B. hortorum flowers, RK was the most prominent component and ZN was the second largest component in most samples (10 of 14 samples) (Fig. S3a). The amount of AA was highly variable between individual flowers. Nevertheless, it was less than 10% of the total amounts of phenylbutanoids in several samples (BH1, 8, 9, 10 and 14). AA was a major component in BH2, and the second largest component in BH3, BH5, BH6 and BH7. Notably, we did not detect AA and RK, but only a small amount of ZN in BH10. RK and ZN were also major components in B. macranthoides subsp. tollenoniferum, but their amounts and concentrations varied greatly between individual flowers (Fig. S3b). RK was not detected in one sample BM4. We also detected AA and SN as relatively minor components in all B. macranthoides subsp. tollenoniferum samples.
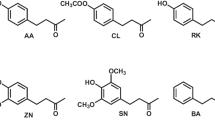
Total ion chromatogram of floral extracts of the fruit fly orchids, Bulbophyllum hortorum and B. macranthoides subsp. tollenoniferum. A partial chromatogram (in the frame) showing CL detected from B. hortorum floral specimen BH2 (intensity magnified). Structures of the phenylbutanoids are shown in the bottom. AA: anisyl acetone, AL: 4-(4-methoxyphenyl)-2-butanol, RK: raspberry ketone, RL: rhododendrol, ZN: zingerone, ZL: zingerol, SN: syringerone, CL: cue-lure
Distribution of phenylbutanoids in the floral organs
The floral morphology and fragrance are important for navigation of pollinators and subsequent pollination, as shown in the field observation. Therefore, we examined distribution of the major phenylbutanoids—AA, RK, ZN and SN—in each part of the flowers. The amounts and concentrations of phenylbutanoids in the individual floral parts of B. hortorum and B. macranthoides subsp. tollenoniferum are shown in Fig. 3. Although the lateral sepals contained the largest amounts of phenylbutanoids, the concentrations of those compounds were the highest in the lip. The reason is because the lip is lightest in weight among the floral parts. The compositions of phenylbutanoids in each floral part were different between B. hortorum and B. macranthoides subsp. tollenoniferum. For B. hortorum, the concentration of each phenylbutanoid in the lip was approximately twice or more than that detected in other flower parts (Fig. 3a). Similar amounts and concentrations of AA, RK and ZN were found in the lips, while RK and ZN were major components in the lateral sepals. Contrastingly, the lip of B. macranthoides subsp. tollenoniferum contained ZN as a major component with AA and SN as minor components (Fig. 3b); and notably RK was not present. The medial and lateral sepals and petals possessed RK and ZN as major components with AA and SN as minor components. The column has very low content and concentration of ZN with a minute amount of AA; while RK and SN were not detected.
Distribution of the major phenylbutanoids—AA, RK, ZN and SN—in the floral parts of two Bulbophyllum species. Amounts (left) and concentrations (right) of the volatiles in each floral part are shown in the graphs (mean ± SE). aB. hortorum (n = 4–5), bB. macranthoides subsp. tollenoniferum (n = 3). AA: anisyl acetone, RK: raspberry ketone, ZN: zingerone, SN: syringerone
Attraction of Z. cucurbitae to phenylbutanoids
Because we found that RK-sensitive fruit flies were selectively attracted to the flowers of B. hortorum and B. macranthoides subsp. tollenoniferum, we examined the attraction of male Z. cucurbitae to filter papers treated with 100 μg of each authentic phenylbutanoid found in the Bulbophyllum flowers during a 30 min test period (Fig. S1C). We confirmed that CL attracted almost all males as the most potent attractant during the test period (Fig. 4). Whereas RK attracted most of males as a major floral attractant of the fruit fly orchids, the number of attracted males to its corresponding alcohol RL was significantly fewer than that to RK (Fig. S4). We found that the number of attracted males was significantly affected by the time after exposure to RL probably owing to its sustained release property, but not to the other compounds (Friedman test, p < 0.05). We also found that ZN moderately attracted males, but the number of attracted males to the corresponding alcohol, ZL, was significantly fewer. Both AA and its corresponding alcohol AL similarly attracted males to some extent. Contrary to the attraction of males to the di- and tri-substituted phenylbutanoids, the tetra-substituted phenylbutanoid–SN did not attract any male fly. We further tested whether the male fly was attracted to the filter papers treated with 1000 µg of SN for 30 min, but no attraction was observed even at higher concentrations.
Phylogenetic analysis
We constructed a phylogenetic tree of daciniphilous orchids by aligning the 3478–3545 bp nucleotide sequences of amplified fragments of non-coding plastid region and nuclear ribosomal ITS region by PCR (Fig. 5). In addition to B. hortorum and B. macranthoides subsp. tollenoniferum, we selected the phenylbutanoid-producing orchids—namely, B. patens, B. macranthum, B. praetervisum, and B. ecornutum (Nakahira et al. 2018; Tan and Nishida 2000, 2005)—and also several phenylpropanoid (e.g., ME-)-producing orchids—such as B. cheiri, B. vinaceum and a variety of B. macranthum from the Philippines (Nakahira et al. 2018; Tan et al. 2002, 2006)—for the analysis. We found that B. hortorum and B. macranthoides subsp. tollenoniferum were distinctly separated from each other, but formed a lineage in the phylogenetic trees. This lineage was relatively closer to that consisting of two sibling species—B. macranthum and B. praetervisum. The lineage of B. patens was relatively apart from those consisting of B. macranthoides subsp. tollenoniferum, B. hortorum, B. macranthum, and B. praetervisum. The lineages of ME-producing orchids, B. cheiri and B. macranthum (Philippines variety), were clearly differentiated from those of other orchids producing phenylpropanoids. Contrastingly, the lineages of RK-producing B. ecornutum and ME-producing B. vinaceum are clearly separated from those of other Bulbophyllum orchid species analyzed in this study.
Discussion
We found that a series of phenylbutanoids commonly shared in both B. hortorum and B. macranthoides subsp. tollenoniferum as floral volatiles, in which RK and ZN were major components with varied contents and concentrations among individual flowers even within a species. Because each flower was derived from a single plant cluster (or clone), physiological factors such as floral age and actual timing of flower picking for extraction would affect the differences of the composition. Although collecting multiple plants from different locations may be extremely difficult owing to the rarity of both B. hortorum and B. macranthoides subsp. tollenoniferum in their natural habitats, possible variation of chemical components among different plant clusters should be analyzed. While RK has been well characterized as a powerful attractant for RK-sensitive fruit fly species, ZN attracts both ME- and RK-sensitive fruit fly species including Ba. dorsalis and Ba. albistrigata possibly owing to its structural resemblance to both ME and RK (Tan and Nishida 2000). We also detected AA in the flowers of B. hortorum and B. macranthoides subsp. tollenoniferum. Notably, AA has been reported as an attractant for RK-sensitive Z. cucurbitae (Barthel et al. 1957). AA was a minor component in most flowers of B. hortorum and B. macranthoides subsp. tollenoniferum, but detected as a highly prominent component in one of the B. hortorum flowers. The results suggest that some individual flowers of B. hortorum would utilize AA as an additional factor to attract RK-sensitive fruit flies as their pollinators.
Consistent with the chemical profiles of fruit fly attractants including ZN, RK and AA, we observed that RK-sensitive fruit fly species were mainly attracted to both B. hortorum and B. macranthoides subsp. tollenoniferum. Interestingly, these phenylbutanoid attractants were accompanied by their corresponding reduced forms as minor components in a pairwise manner, i.e., AA/AL, RK/RL and ZN/ZL. This pairwise formation of phenylbutanoids has also been detected in other related Bulbophyllum species, namely B. patens, B. macranthum and B. praetervisum (Nakahira et al. 2018; Tan and Nishida 2000). This also suggests that the reduction reactions of phenylbutanoid attractants similarly occur in these fruit fly orchids. Although the amounts of these alcohols—AL, RL and ZL—were extremely low, Z. cucurbitae males were attracted to these compounds in the laboratory bioassays. The results further suggest that the respective alcohols may effectively play some roles in attracting certain specific pollinator fruit fly species in the natural habitat.
We also noted that the presence of SN was found in flowers of B. macranthoides subsp. tollenoniferum. This is the first report for the identification of SN as a floral component, although SN has been reported as a constituent of commercially processed dry ginger (Jolad et al. 2005). However, SN did not attract Z. cucurbitae in the laboratory bioassays. Nevertheless, it is interesting to determine whether this compound could attract any other fruit fly species in their natural habitat as an additive attractant, because the chemical structure is similar to that of ZN, i.e., the only difference is the presence of another methoxy moiety at the 5-position on the aromatic ring of ZN.
Interestingly, we detected CL as a minor component in three of B. hortorum floral specimens (Fig. 2 and Fig. S2). Synthetic CL was first developed as an effective male attractant for pest management (Beroza et al. 1960). The presence of CL in B. hortorum flowers seems to involve a novel enzyme, such as acetyltransferase to biosynthesize CL from RK. Since CL is a more effective fruit fly attractant than RK because of its higher volatility, the acquirement of an enzyme, to convert RK to CL could add an evolutionary advantage for B. hortorum to attract its pollinators, even though its quantity in the floral tissues was found to be very small. The phenylbutanoid with an acetyl group in its aromatic ring is the first reported case, we first reported it as ‘unpublished data’ in a review by Nishida and Tan (2016), of its natural existence in an organism—B. hortorum.
Bulbophyllum hortorum and B. macranthoides subsp. tollenoniferum contained multiple phenylbutanoids as floral components. It is worth noting that most flowers of both species contained RK and ZN as the major components. In contrast, some Bulbophyllum species generally produce a limited number of fruit fly attractants as floral components. For example, ME is contained as the major component in the flower of B. cheiri which selectively attracts several ME-sensitive fruit fly species including Ba. dorsalis and Ba. umbrosa (Tan et al. 2002). Similarly, RK and its corresponding alcohol RL are major and minor floral components, respectively, of B. ecornutum (formerly published as B. apertum) to attract several RK-sensitive fruit fly species (Tan and Nishida 2005). Considering the structural difference between RK and ZN, it is possible that B. hortorum and B. macranthoides subsp. tollenoniferum acquired biosynthetic enzymes for ZN production to attract both ME- and RK-sensitive species (Tan and Nishida 2000). The biosynthetic pathway of RK has been well characterized. Initially, 4-hydroxybenzalactone is synthesized from p-coumaroyl-CoA and malonyl-CoA by a polyketide synthase, benzalacetone synthase (BAS) (Abe et al. 2001). It is then converted into RK by raspberry ketone/zingerone synthase 1 (RZS1) that catalyzed NADPH-dependent reduction of α,β-unsaturated double bond of the side chain of the intermediate (Koeduka et al. 2011). As ZN is produced from 3-methoxy-4-hydroxybenzalactone by RZS1 in the same reaction for the production of RK, feruloyl-CoA in which the methoxy moiety is present at the 3-position on the aromatic ring of p-coumaroyl-CoA could be a precursor as the first step in the ZN biosynthesis. Therefore, ZN-producing orchid species have probably acquired enzymes to biosynthesize 3-methoxy-4-hydroxybenzalactone from feruloyl-CoA and malonyl-CoA by a mutation of benzalacetone synthase. Indeed, a mutation of a small number of amino acids in an active-site pocket to bind a substrate drastically changes substrate specificity of the benzalacetone synthase (Morita et al. 2010).
The distribution of attractants in the floral tissues plays an important role in leading a fruit fly bearing pollinia to a precise position so as to complete pollination by depositing pollinia on to the stigma. In the case of B. ecornutum, RK is present only in the floral lip, thereby, explains why an attracted fly would eventually be led to feed on the lip surface (Tan and Nishida 2005). For B. hortorum and B. macranthoides subsp. tollenoniferum, the attractant chemicals were found in all floral parts including sepals and petals. However, the concentration of the attractants was significantly higher particularly in B. hortorum, which may effectively entice a fruit fly pollinator, of the right size/weight to open the closed spring-loaded lip, to the right position on the adaxial (upper) surface of the lip for either removal or deposition of pollinia during pollination (Fig. 1). The complete process of pollination (i.e., from pollinia removal to deposition) of B. stockeri (actually is B. hortorum) has been reported by Ong (2013).
The phylogenetic analysis based on chloroplast DNA and nuclear ITS sequences revealed the separation of ME-producing daciniphilous orchids—namely, B. cheiri and B. macranthum (Philippines variety), from RK/ZN-daciniphilous orchids—namely, B. patens, B. macranthum, B. praetervisum, B. hortorum, and B. macranthoides—within a clade. However, B. vinaceum and B. ecornutum are ME- and RK-producing daciniphilous orchids, respectively; and yet placed together in another clade which probably reflects possession of a common feature, a stiff hamulus (Tan and Nishida 2005; Tan et al. 2006), but not found in the other Bulbophyllum species, that supports the pollinia in their respective pollinaria. Thus, molecular types of the attractants contained in the daciniphilous orchids show no obvious association with the phylogenetic relationship, suggesting that a change of biosynthesis of attractants in daciniphilous orchids might have easily occurred during a co-evolutionary process. It may be important to understand how fruit fly pollinators affect the change of biosynthesis of attractants in daciniphilous orchids. Characterization of pollinators of daciniphilous orchids in the natural habitats will provide insights into mechanisms underlying formation of relationships between daciniphilous orchids and fruit flies via floral attractants.
References
Abe I, Takahashi Y, Morita H, Noguchi H (2001) Benzalacetone synthase: a novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum. Eur J Biochem 268:3354–3359. https://doi.org/10.1046/j.1432-1327.2001.02255.x
Azuma H, Harrison RD, Nakamura K, Su ZH (2010) Molecular phylogenies of figs and fig-pollinating wasps in the Ryukyu and Bonin (Ogasawara) Islands, Japan. Genes Genet Syst 85:177–192. https://doi.org/10.1266/ggs.85.177
Barthel W, Green N, Keiser N, Steiner LF (1957) Anisylacetone, synthetic attractant for male melon fly. Science 126:654. https://doi.org/10.1126/science.126.3275.654
Beroza M, Alexander BH, Steiner LF, Mitchell WC, Miyashita DH (1960) New synthetic lures for the male melon fly. Science 131:1044–1045. https://doi.org/10.1126/science.131.3406.1044
Frodin DG (2004) History and concepts of big plant genera. Taxon 53:753–776. https://doi.org/10.2307/4135449
Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN (2005) Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE 2 production. Phytochemistry 66:1614–1635. https://doi.org/10.1016/j.phytochem.2005.05.007
Koeduka T, Watanabe B, Suzuki S, Hiratake J, Mano J, Yazaki K (2011) Characterization of raspberry ketone/zingerone synthase, catalyzing the alpha, beta-hydrogenation of phenylbutenones in raspberry fruits. Biochem Biophys Res Commun 412:104–108. https://doi.org/10.1016/j.bbrc.2011.07.052
Kuo P, Damu AG, Cherng CY, Jeng JF, Teng CM, Lee EJ, Wu TS (2005) Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch Pharm Res 28:518–528. https://doi.org/10.1007/BF02977752
Morita H, Shimokawa Y, Tanio M, Kato R, Noguchi H, Sugio S, Kohno T, Abe I (2010) A structure-based mechanism for benzalacetone synthase from Rheum palmatum. Proc Natl Acad Sci USA 107:669–673. https://doi.org/10.1073/pnas.0909982107
Nakahira M, Ono H, Wee SL, Tan KH, Nishida R (2018) Floral synomone diversification of Bulbophyllum sibling species (Orchidaceae) in attracting fruit fly pollinators. Biochem Syst Ecol 81:86–95. https://doi.org/10.1016/j.bse.2018.10.002
Nishida R, Tan KH (2016) Search for new fruit fly attractants from plants: A review. In: Sabater-Munoz B, Vera T, Pereira R, Orankanok W (eds) Proceedings of the 9th International Symposium on Fruit Flies of Economic Importance, Bangkok, Thailand, pp 249–262
Ong PT (2013) The pollination of two Bulbophyllum species. Orchid Review 2013 September Issue:152–155
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Tan KH, Nishida R (2000) Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J Chem Ecol 26:533–546. https://doi.org/10.1023/A:1005477926244
Tan KH, Nishida R (2005) Synomone or kairomone? – Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J Chem Ecol 31:497–507. https://doi.org/10.1007/s10886-005-2023-8
Tan KH, Nishida R (2007) Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochem Syst Ecol 35:334–341. https://doi.org/10.1016/j.bse.2007.01.013
Tan KH, Nishida R, Toong Y (2002) Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. J Chem Ecol 28:1161–1172. https://doi.org/10.1023/A:1016277500007
Tan KH, Tan LT, Nishida R (2006) Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination. J Chem Ecol 32:2429–2441. https://doi.org/10.1007/s10886-006-9154-4
Tan KH, Nishida R, Jang EB, Shelly TE (2014) Pheromones, male lures, and trapping of tephritid fruit flies. In: Shelly T, Epsky N, Jang EB, Reyes-Flores, Vargas R (eds) Trapping and the detection, control, and regulation of tephritid fruit flies. Springer, Dordrecht, pp 15–74. https://doi.org/10.1007/978-94-017-9193-9
Vermeulen JJ (2008) New species of Bulbophyllum from eastern Malesia (Orchidaceae). Nord J Bot 26:129–195. https://doi.org/10.1111/j.1756-1051.2008.00220.x
Acknowledgements
We thank Jaap J. Vermeulen for confirmation of the species and subspecies of the Bulbophyllum species; and Jane Royer for the Dacus species identification. We thank Atsushi Honma and Yasutsune Sadoyama of Okinawa Prefectural Plant Protection Center for providing Z. cucurbitae. We also thank Ayako Sasaki for technical assistance. R. Nishida was partly supported by the Grant-in-Aid for Scientific Research from JSPS (Nos. 19310142 and 23380035) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. H. Ono was partly supported by JSPS KAKENHI Grant Number 26450466.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13355_2019_653_MOESM1_ESM.tif
Supplementary material 1: Fig. S1 Laboratory bioassay to evaluate the attractiveness of the phenylbutanoids. a The plastic container (75 mm × 75 mm × 45 mm) with a lid having a hole (35 mm diameter) bored and covered with a plastic mesh was used for the laboratory bioassays. b Flies were supplied with water filled in a 2.0-ml microtube and food. To test each compound, a filter paper (21 mm diameter) treated with 100 µg of a test compound was placed on the plastic mesh of the lid of the container. c Several males were attracted to the plastic mesh just at bottom of the treated filter paper (arrow), and voraciously fed on an active compound applied to the filter paper. (TIFF 4351 kb)
13355_2019_653_MOESM2_ESM.tif
Supplementary material 2: Fig. S2 A trace amount of cue-lure was detected from two floral specimens (BH2, BH8 and BH10) of the fruit fly orchids, Bulbophyllum hortorum. ZN: zingerone; CL: cue-lure. (TIFF 100 kb)
13355_2019_653_MOESM3_ESM.tif
Supplementary material 3: Fig. S3 Amounts of the major phenylbutanoids—AA, RK, ZN and SN—in the whole flowers of the fruit fly orchids, Bulbophyllum hortorum (a) and B. macranthoides subsp. tollenoniferum (b). AA: anisyl acetone; RK: raspberry ketone; ZN: zingerone; SN: syringerone. (TIFF 612 kb)
13355_2019_653_MOESM4_ESM.tif
Supplementary material 4: Fig. S4 Average numbers of attracted flies at 5 min intervals to each attractant—calculated from data as shown in Fig. 4. Different alphabets indicate significant differences between compounds (p < 0.05 by Tukey’s HSD test). (TIFF 516 kb)
Rights and permissions
About this article
Cite this article
Katte, T., Tan, K.H., Su, ZH. et al. Floral fragrances in two closely related fruit fly orchids, Bulbophyllum hortorum and B. macranthoides (Orchidaceae): assortments of phenylbutanoids to attract tephritid fruit fly males. Appl Entomol Zool 55, 55–64 (2020). https://doi.org/10.1007/s13355-019-00653-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00653-x