Abstract
The interactions between Dacini male fruit flies and phytochemical male lures are unique. Lure response, fate and its effects after consumption on fruit fly mating behaviour are species- and lure-specific. Bactrocera frauenfeldi is known to respond to the phenylbutanoids raspberry ketone (RK) and cue lure (CL), anisyl acetone (AA), and zingerone (ZN), which are produced by some rainforest orchids. Here we compared the relative field responses of B. frauenfeldi males to these phenylbutanoids in two selected locations to determine the most attractive lure for this species. We also performed gas chromatographic-mass spectral analyses of male rectal pheromone glands to understand the fate of the ingested compounds. Results showed that B. frauenfeldi males were most responsive to CL, equally to RK and AA, while poorly to ZN in Cairns, a site with high population density. No significant difference was observed in Lockhart River which has a low population density of B. frauenfeldi. Chemical analyses showed that most of the ingested phenylbutanoids were sequestered into rectal glands, either unchanged or with minimal structural changes except for AA, which is converted to RK via a demethylation of the methoxy- to a hydroxy-moiety and reduced to 4-(4-methoxyphenyl)-2-butanol via the keto-moiety. This study provides both practical and ecological implications: it identified the most attractive lure, which is important for monitoring and management of B. frauenfeldi; and based on the relative responses, conversion and retention rates by B. frauenfeldi males, revealed the ecological significance of these phytochemical lures in plant-fruit fly co-evolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Males of many fruit fly species in the tribe Dacini (Diptera: Tephritidae: Dacinae) have long been known to be attracted to a particular group of plant secondary metabolites, i.e. phenylpropanoids and phenylbutonoids, hereafter referred to as male lures (Metcalf et al. 1983; IAEA 2003; Nishida and Tan 2016). Male fruit flies exhibit strong attraction to and actively forage for these lures in nature. This is followed by phagostimulant activities upon arrival at a source (Metcalf et al. 1983; Cunningham 1989). Such behaviour has been deliberately manipulated for pest management and control of fruit fly pests using these lures for population detection, surveillance, monitoring, suppression and eradication programmes (Tan et al. 2014; Vargas et al. 2014).
These male lures play an important role in the mating systems of fruit flies (see reviews by Shelly 2010; Tan and Nishida 2012; Segura et al. 2018). The consumed lures pass through the crop, and are either metabolized into analogues or sequestered unaltered and transported via the haemolymph to be stored in the male rectal (pheromone) gland prior to release as air-borne volatiles (Hee and Tan 2004). The incorporation of these analogues and sequestered compounds into the fruit fly pheromone blend enhances (a) the attractiveness of chemical sexual signals for conspecific female attraction (Shelly and Dewire 1994; Tan and Nishida 1996; Hee and Tan 1998; Wee et al. 2007, 2018b, c; Kumaran et al. 2014a; Rabiatul and Wee 2019); and/or (b) male behavioural sexual signalling (Shelly and Dewire 1994; Kumaran et al. 2014b; Rabiatul and Wee 2019), leading to increased male mating success. In some cases, F1 male offspring from females mated with lure-fed males was shown to have an enhanced lure-foraging ability than those sons from paternal males that were denied access to lures (Kumaran and Clarke 2014). With more research conducted, it becomes evident that male fruit flies’ sensitivity and attraction to male lures, fate and direct physiological effects of lures after ingestion, varies between fruit fly species and within species between lures. For example, zingerone (ZN) feeding increased male mating success of Bactrocera tryoni and Zeugodacus tau, with the enhancement of mating success effect last longer in the latter than the former, but no significant effect on Zeugodacus cucurbitae (Kumaran et al. 2014a; Shelly 2017; Wee et al. 2018a); male B. correcta is specifically and more attracted to β-caryophyllene than to methyl eugenol, a common male attractant used for population monitoring and control of this species and a few other Bactrocera pest species (Wee et al. 2018b).
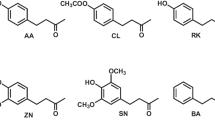
Bactrocera frauenfeldi (Schiner) is a polyphagous horticultural fruit pest infesting over 109 species of commercial and native fruits belonging to 37 families (Hancock et al. 2000; Leblanc et al. 2004, 2012). It is native to Papua New Guinea and its surrounding islands and has invaded several Pacific islands and north east Australia (Leblanc et al. 2012; Royer et al. 2016). The species has long been known to respond to two phenylbutanoids (Fig. 1), cue lure (CL) and raspberry ketone (RK) (Drew 1974). CL is readily hydrolysed to RK (Metcalf et al. 1979), which is found as a secondary metabolite in some plant families including Rosaceae, Asteraceae, Lamiaceae and Orchidaceae (e.g. Deifel 1989; Nishida and Tan 2016). CL is a commonly used male lure that attracts at least 286 fruit fly species belonging to the tribe Dacini (IAEA 2003, Doorenweerd et al. 2018). It was previously thought to exist only as a synthetic chemical but was recently detected in minute quantities in daciniphilous flowers (attracting only Dacini fruit flies) of Bulbophyllum hortorum J J Verm, O’Byrne and Lamb (Katte et al. 2020).
Recently, in separate preliminary field tests conducted in Australia (Royer 2015) and Papua New Guinea (Nishida and Tan 2016), anisyl acetone (AA) and zingerone (ZN) (Fig. 1) baited traps also attracted males of B. frauenfeldi. AA, an aromatic ketone, was amongst the first few synthetic chemicals reported as potential fruit fly baits to the melon fly, Zeugodacus cucurbitae (Barthel et al. 1957). AA was recently detected in Bulbophyllum orchid flowers in Papua New Guinea and South-East Asia regions, and it attracts many CL/RK-responsive Dacini fruit fly species, including B. frauenfeldi (Katte et al. 2020; Royer et al. 2020). ZN, in contrast, is a unique phenylbutanoid compound that has the ability to attract male fruit flies from CL/RK-responsive as well as methyl eugenol (a phenylpropanoid; ME)-responsive groups of Dacini fruit flies (Tan and Nishida 2000, 2007; Nakahira et al. 2018; Leblanc et al. 2018a, b, 2019). Furthermore, it is also highly specific to certain species that are non-responsive to either CL/RK or ME (Fay 2012; Leblanc et al. 2018a, b, 2019; Royer 2015; Royer et al. 2018, 2019).
Bactrocera frauenfeldi is one of numerous CL/RK-responsive Dacini fruit fly species that are invasive across the Australasian region. However, little is understood of its interactions with phenylbutanoid lures in comparison to other pest species, such as melon fly (Nishida et al. 1993). Therefore, a detailed study to identify the most attractive lure for this species and the fate of lure after consumption by males is warranted to fill a key gap in the understanding of male lures for the species.
In this paper, we investigated the relative field attractancy of ZN, RK, AA and CL to male B. frauenfeldi in north Queensland, Australia to determine the most attractive lure for the species. Two locations were chosen: Lockhart River and Cairns with known low and high B. frauenfeldi population densities, respectively. We also conducted chemical analyses to understand the fate of different phenylbutanoids after ingestion by B. frauenfeldi, on a comparative, quantitative, and temporal basis.
Materials and methods
Insects
Bactrocera frauenfeldi laboratory strain pupae were obtained from the Department of Agriculture and Fisheries (DAF), Cairns, Queensland. Emerged flies were held in screen cages (35 × 35 × 35 cm) provided with water and a mixture of protein hydrolysate-sugar (ratio 1:3) ad libitum. Flies were sex-segregated within three days of adult emergence and maintained at 27 °C and 70% RH in an insectary illuminated with natural light in addition to fluorescent lighting in a 8:16 h light: dark regime. Sexually mature virgin flies (14–17 day-old) were used for all studies unless otherwise stated.
Chemicals
Figure 1 shows the various phenylbutanoids used in this study. Anisyl acetone (AA; 4-(4-methoxyphenyl)-2-butanone; CAS 104-20-1) (purity ≥ 98%); Cue lure (CL; 4-(p-acetoxyphenyl)-2-butanone, CAS 3572-06-3) (99.4% purity); raspberry ketone (RK; 4-(4-hydroxyphenyl)-2-butanone; CAS 5471-51-2) (purity > 98%); and zingerone (ZN; 4-(4-hydroxy-3-methoxyphenyl)-2-butanone; CAS 122-48-5) (purity > 96%) were obtained from Sigma-Aldrich. We also synthesized 4-(4-methoxyphenyl)-2-butanol (AL; 4-(4-methoxyphenyl)-2-butanol; CAS 67952-38-9) and zingerol (ZL; 4-(4-hydroxy-3-methoxyphenyl)-2-butanol; CAS 39728-80-8) from commercial AA (TCI, Japan) and ZN, respectively, using sodium borohydride (Wako, Japan). The compound identification for AL was confirmed by mass spectra fragmentation pattern and reference to NIST 11.0 library: GCMS: 180 (M + , 24%), 162 (22%), 147 (74%), 122 (18%), 121 (100%), 91 (32%), 78 (13%), 77 (21%), 45 (9%).
Field trapping
Lures were dosed at 0.5 ml (0.5 g for crystalline lures) to 0.08 ml Hy-Mal® (containing a.i. 1150 g/L maldison) dispensed onto a 3.8 cm long dental wick (Livingstone Int., Rosebury, New South Wales). CL and AA were in liquid form. ZN and RK, which are crystalline, were liquefied in absolute ethanol in a ratio of 3:1. All lures were applied with a graduated pipette to the dental wicks separately. Lures were placed in Steiner traps made of 1 L clear cylinder hung horizontally with 2.5 cm wide ingress tubes on the flat ends.
Traps were placed at two locations in north Queensland, Australia, Lockhart River and Cairns, with low and high populations of B. frauenfeldi, respectively (12.1 versus 220.8 flies/trap/week using CL-baited traps dosed at 3 mL lure to 1 mL malathion [500 g/L] in 2012–2014; Royer, unpublished data). Lockhart River is on Cape York Peninsula (Lat. − 12.79, Lon. 143.30) at an altitude of 17 m, with mean annual rainfall of 2047 mm and mean annual temperature 25.9 °C (min. 22.0 °C, max. 29.8 °C). Cairns is in far north Queensland (Lat. − 16.87, Lon. 145.75) at an altitude 2 m, with a mean annual rainfall of 1987 mm and mean annual temperature 25.0 °C (min. 20.8 °C, max. 29.1 °C).
For each location, five sites were chosen and served as blocks. Each block consisted of four traps baited with each of the lures and arranged in a randomised complete block design. Traps within blocks were placed 30 m apart, and blocks were at a minimum of 300 m apart. Traps were hung approximately 1.5 m from the ground in or near shaded host trees, such as tropical almonds (Terminalia catappa L.) and mangoes (Mangifera indica L.). Traps were rotated each week to avoid any positional effect. Trap catches were cleared weekly for eight consecutive weeks during summers of 2017–2018 and 2019 (Lockhart River from 13 December 2017 to 5 February 2018 and Cairns from 14 January 2019 to 11 March 2019). There were 40 replications for each lure at each location, and trap capture was expressed as mean flies/trap/week (FTW). Trapped flies were identified to species under a stereomicroscope using taxonomic keys by Drew (1989).
Male lure feeding and rectal gland excision
Male lure feeding was conducted in the morning between 08:30 and 13:00 h. A sexually mature and virgin male was allowed to feed on either RK, ZN or AA. RK, AA and ZN were chosen for chemical analysis, because they were known to exist as natural floral attractants in many plant species when the study was conducted in 2016, while CL was not known to exist as a natural compound then (Katte et al. 2020). 20 µg of a known male lure was dispensed using a 10 µL pre-calibrated microcapillary glass pipette (Drummond®) on a piece of filter paper (4 cm dia.) placed on a disposable Petri dish. Feeding period was limited to 30 min. Subsequently, fed-flies were removed and kept in a 500 mL ventilated plastic container provided with artificial adult diet (see “Materials and Methods”—Insects) and water ad libitum until required for further analysis.
After 6 h, 1-, 3- and 5-day post treatment (DPT), a treated male was lightly cold-immobilised at − 20 °C, decapitated and then, the rectal pheromone gland was carefully excised. Each extracted gland was soaked in a glass insert (250 or 300 µl; Sigma-Aldrich®) with a known amount of absolute ethanol, and 50 ng of 1-dodecanol as an internal standard was added. For male lure feeding and each post-feeding treatment, six replications were conducted. The rectal glands extracted from the respective lure-deprived males were similarly processed as controls.
Chemical analysis of excised male rectal glands
Chemical analysis and quantification were conducted using a Shimadzu 8040 Triple Quadrupole Gas Chromatography-Mass Spectrometer (electron impact at 70 eV), equipped with a HP-5 column (non-polar; 30 m × 0.25 mm × 0.25 µm, GS-Tek (USA), fused with silica column coated with cross-linked bonded dimethylpolysiloxane). The carrier gas was helium, and the oven temperature was programmed from 60 °C (1 min holding) to 240 °C (hold 1 min) at a rate of 10 °C/min.
Prior to chemical analysis, the soaked gland within the glass insert was homogenised using a custom-made mini glass rod. The macerated tissue in ethanol was then vortexed and centrifuged in a fixed angle rotor at 1000 rpm for 3 min. The supernatant was then transferred to a new glass insert placed inside an auto-sampler vial for GC injection. One-µl aliquot was injected into the GCMS for analysis. For quantification, separate calibration curves were plotted for RK, ZN, ZL and AL using various dosages with co-injection of the internal standard in absolute ethanol. Based on these calibration curves, peak areas of the respective chemicals in each rectal gland, relative to the internal standard, were quantified.
Statistical analysis
For field trapping data, a cross-sites meta-analysis was conducted using generalised linear mixed models (GLMM) in GenStat (2016), adopting restricted maximum likelihood (REML). The over-dispersed Poisson distribution with the log link function was adopted with the fixed effects of lure, site, week of sampling, and the ‘site by lure’ interaction. The ‘site by week’ interaction was initially included in the model, but omitted as it was not significant. The random effects were the weekly trap services nested within the blocks, with the temporal correlations between these successive trap services accounted for under an autoregressive (of order one) error structure. The variance components were restricted to not permit negative estimates. The standard errors for each mean were formed on the link (natural logarithm) scale and back-transformed to counts forming asymmetrical ranges. These were averaged to provide an approximate standard error for each mean. Pairwise testing between the lures was conducted using protected t-tests.
For rectal gland analysis, statistical analysis was performed using Sigma Plot 12.0, and α was set at P = 0.05 for all comparisons. Data were subjected normality and variance homogeneity tests prior to one-way analysis of variance. For normally distributed data, means were separated by Holm-Sidak method. When normality or homogeneity data was not met, Kruskal–Wallis on Ranks was used, and means were separated by Dunn’s Method.
Results
Field trapping
In Cairns, trap catches of B. frauenfeldi males ranked in decreasing order were CL > AA = RK > ZN, whereas in Lockhart River, trap catches were CL = AA = RK > ZN. The main effect for lures was significant (F = 10.4, df = 3,308; P < 0.001), and Table 1 shows the lure means separately for each site.
Mean weekly trap catches over time at each location are shown in Fig. 2. The two locations had notably different overall fly densities, although the relative trap catches using the different lures were similar. At Lockhart River (with low population density of B. frauenfeldi), mean FTW was less than 3 for all the lures. There was no significant difference in the mean FTW between AA, CL and RK (Table 1), while no B. frauenfeldi was captured in ZN-baited traps throughout the eight-week trapping trials. In Cairns, which has a high population density during summer, CL-baited traps captured the highest number of B. frauenfeldi males amongst the lures tested (ca. 130 FTW). This was followed by RK and AA-baited traps, which captured an average of 80–90 FTW, while ZN trapped very low number of males throughout the eight-week trapping trials (Table 1). Bactrocera frauenfeldi populations gradually declined over the trapping periods in both sites. From previous trapping in Cairns and Lockhart River, populations generally peak around December to February (Royer JE, unpublished data).
Captures (mean flies/trap/week) of B. frauenfeldi males using different lure-baited traps in Lockhart River (13 December 2017–5 February 2018) and Cairns (14 January–11 March 2019; bottom). Anisyl acetone (AA), raspberry ketone (RK) and zingerone (ZN). ZN captured zero flies at Lockhart River and very low numbers of flies at Cairns (data not shown)
Rectal gland analyses
Chemical analyses consistently showed the presence of two major endogenous spiroacetal compounds in the rectal gland of B. frauenfeldi males recently reported by Noushini et al. (2020), with (E,E)-2-ethyl-8-methyl-1,7-dioxaspiro[5,5]undecane being most abundant followed by (E,E)-2,8-dimethyl-1,7-dioxaspiro[5,5]undecane (Fig. 3). The consumed RK was sequestered into the rectal glands unchanged, while AA was converted into RK and AL. The consumed ZN was largely sequestered in the rectal gland unchanged, with a small portion converted into ZL (Fig. 3).
Gas chromatograms of rectal gland extracts of Bactrocera frauenfeldi males after consuming selected phenylpropanoid attractants: control, anisyl acetone (AA)-fed, raspberry ketone (RK)-fed, and zingerone (ZN)-fed males. Compound 1 = (E,E)-2,8-dimethyl-1,7-dioxaspiro[5,5]undecane (Rt = 7.12 min); compound 2 = (E,E)-2-ethyl-8-methyl-1,7-dioxaspiro[5,5]undecane (Rt = 8.20 min); IS = internal standard, 1-dodecanol (Rt = 11.673 min); AL = 4-(4-methoxyphenyl)-2-butanol (Rt = 12.16 min); RK = raspberry ketone (Rt = 12.64 min); ZN = zingerone (Rt = 13.78 min); ZL = zingerol (Rt = 14.08 min)
Table 2 shows the quantities of phenylbutanoid analogues present in the male rectal gland at 6 h, 1-, 3- and 5-day after an initial feeding on each of the respective phenylbutanoid attractants. Following consumption of RK, significant quantities were accumulated on a temporal basis in the rectal glands of B. frauenfeldi males (F = 5.709, df = 3, 20; P = 0.005). From the initial amount of 20 µg RK offered, approximately 35% was sequestered at 6 h post-feeding (Table 2). Quantities of RK detected had increased significantly to ca. 70% from the initial 20 µg RK offered (13.3–13.9 µg per gland) at 1- and 3-DPT, respectively (0.013 ≤ P ≤ 0.008; Holm-Sidak method), before decreasing to ca. 12 µg per gland (~ 60%) at 5-DPT (Table 2).
After consumption, AA was converted to RK and AL (Table 2). In the rectal gland, the accumulation of RK increased significantly with time post-feeding (H = 9.298, df = 3; P = 0.026, Kruskal–Wallis), while the amount of AL remained at or below 1.5 µg per gland (F = 0.154, df = 3, 18; P > 0.05). At 6 h post-feeding, the amount of RK detected in the male rectal gland was ca. 0.8 µg. Thereon, the accumulated RK increased significantly with time, from ca. 1.8 µg to ca. 3.7 µg per gland at 1-day and 3-day, respectively (P < 0.05, Dunn’s Method). At 5-DPT, the amount of RK varied greatly between individual glands, with an average of 3.81 ± 1.43 µg per gland (Table 2). Interestingly, the average amount of pooled RK and AL present in the rectal gland was not more than 25% of the AA offered to the male B. frauenfeldi at any given time.
Approximately 65% of the ingested ZN was sequestered as early as 6 h with a small fraction being converted to ZL (Table 2). The quantities of ZN and ZL accumulated in the male rectal glands, ranging from 13–17 µg per gland and 55–99 ng per gland (65–85% from the initial 20 µg ZN offered), respectively, remained high and not significantly different over time (F(ZN) = 0.95; F(ZL) = 1.161, df = 3, 20; P > 0.05).
Discussion
The current results suggest that CL serves as the most attractive lure for B. frauenfeldi, followed by AA and RK, as indicated by the highest FTW in Cairns, which has a high fly population density. This is the first account of AA being an equally effective lure as RK in trapping B. frauenfeldi males. AA has previously trapped B. frauenfeldi in Australia (Royer 2015) and low numbers of other CL/RK-responsive species in Australia and New Caledonia (Royer 2015; Royer et al. 2019, 2020). Although ZN was the least effective phenylbutanoid lure in attracting B. frauenfeldi males in the field, laboratory observations showed that the males readily feed on ZN at close range (Wee SL, personal observation).
Fruit fly-lure response is a species-specific and lure-specific phenomenon. Males of a fruit fly species may show responses to more than one lure; and when they do, there is usually a particular lure that it would be most attracted to (Barthel et al. 1957; Metcalf et al. 1979, 1983; Royer 2015; Royer et al. 2018, 2019; Wee et al. 2018a, b), as also demonstrated in this study. According to Metcalf et al. (1979, 1983), tephritid male fruit flies’ response to lures implicated an intimate plant-fruit fly co-evolution relationship with positive reinforcement towards mutualistic benefits. The Dacini fruit flies seemingly had developed specific olfactory sensillae preadapted for responding to these male lures at a very low olfactory threshold, often at nanogram or submicrogram levels (Wee et al. 2002, 2018a, b).
According to Metcalf and Metcalf (1992), the 2-butanone side chain, is a primary chemical moiety for fruit fly attractiveness. All tested phenylbutanoid lures possess the 2-butanone side chain which is responsible for attracting the CL/RK-responsive fruit flies. For ZN, apart from the butanone side chain that attracts the CL/RK-responsive fruit flies, it also possesses a methoxy-moiety attached to the benzene ring that enables attraction of ME-responsive fruit flies (Tan and Nishida 2000, 2007; Nishida and Tan 2016). However, in the case of B. frauenfeldi, this additional methoxy-moiety in ZN may have affected the chemical molecular conformation in olfactory perception of male lures thus rendering the low to zero responsiveness in B. frauenfeldi towards ZN in the field. In general, vapour pressure directly affects the volatility of a chemical, but in this case, low attraction of B. frauenfeldi to ZN was unlikely to be caused by this, as the vapour pressure of ZN is at least six times higher than RK. By comparison, the vapour pressure for CL and AA are five and 349 times that of RK, respectively (Hanssen 2015; Park et al. 2016). The low attractiveness of ZN appears to strengthen our previous finding that chemical molecular conformation is more important than vapour pressure in fruit fly olfactory perception of phytochemical lures (Wee et al. 2018a).
Our study also shows that different fruit fly population density could potentially affect the outcome and interpretation of a comparative lure-response field trial. At the low population density site (Lockhart River), there was no difference in the trap captures of B. frauenfeldi between CL, RK and AA. However, when the same trial was repeated in Cairns, a site with a high population of B. frauenfeldi, a significant difference was found in the FTW between these lures. This may be due to the population density of B. frauenfeldi at Lockhart River being too low to enable the relative attractiveness of lures to be compared statistically and in a meaningful manner. Moreover, low numbers of B. frauenfeldi males were also trapped at ZN-baited traps in Cairns, while none were captured in the low fly populated with at Lockhart River. ZN had previously captured low numbers of B. frauenfeldi in similar sites in Cairns (Royer 2015). Interestingly, this species was not attracted and trapped by ZN in Papua New Guinea, even though the FTW there was three times higher than that of Cairns (Royer et al. 2018). One possible explanation for this discrepancy is the environmental conditions in Cairns differed from that of Papua New Guinea, with the former received higher mean annual rainfall (1992 mm) than the latter (1017 mm). According to Wee and Shelly (2013), differences in environmental condition, e.g. a wetter versus a drier local climate, may affect fruit fly response to male lures.
The decline in B. frauenfeldi over the trapping period is thought to be due to declining populations rather than the lure becoming depleted. From previous trapping, B. frauenfeldi population peaks vary from year to year, but generally in Lockhart River the peak is December to January and in Cairns, January to February (Royer JE unpublished data). This corresponds with the declines seen here from mid-January in Lockhart River and February in Cairns. Lure loss due to fly consumption before they were knocked down by the insecticide is possible but unlikely to have a great impact on lure depletion. Moreover, 500 mg of lure was used in this study. CL has been shown to have a release rate of 0.38 mg/day at 24 °C and 0.62 mg/day at 27 °C and RK a release rate of 0.02 mg/day at 24 °C (Metcalf and Metcalf 1992). Even at CL’s highest release rate, only 35 mg would be released over the eight-week trapping period. Therefore, the use of 500 mg is ample for the eight-week trapping period.
Chemical analyses showed that the consumed RK was sequestered unaltered into the male rectal gland of B. frauenfeldi. A similar fate is also reported for RK with other RK/CL-responsive Dacini males, namely Z. cucurbitae (Coquillett), Z. tau (Walker) and B. tryoni (Froggatt) (Nishida et al. 1993; Tan and Nishida 1995, 2000; Nakahira et al. 2018). Although chemical analysis on CL-fed B. frauenfeldi males was not included in this study, the aforementioned chemical analyses for Z. cucurbitae, Z. tau and B. tryoni have consistently shown that CL was invariably converted into RK (references as cited above). Park et al. (2016) showed that CL remained intact in the atmosphere up to the point of contacting a fly’s chemoreceptors, where CL is subsequently hydrolysed to form RK.
This is the first report of chemical analysis on the fate of AA, a lesser known natural phenylbutanoid, after consumption by a Dacini male. The consumed AA was biotransformed into RK and AL and stored as major and minor components, respectively, in the rectal glands of B. frauenfeldi males. The biotranformation of AA to AL involves a reduction of the keto- to a hydroxy-moiety. A similar reduction scheme also occurs in other fruit fly species. For instance, consumed ZN was reduced to ZL in B. dorsalis (Tan and Nishida 2000), Z. cucurbitae and Z. tau (Nakahira et al. 2018), as well as B. frauenfeldi in this study; CL/RK was reduced to rhododendrol in Z. cucurbitae and Z. tau (Nakahira et al. 2018) and B. tryoni (Kumaran et al. 2014a, b). All of these reduced chemical forms are usually present in minute/trace amount or may only be occasionally detected in male rectal glands. However, the demethylation of the methoxy-moiety of AA to a hydroxy-moiety to form RK is rather unusual and an interesting biochemical phenomenon that warrants in-depth biochemical investigations.
The attraction of B. frauenfeldi males to CL, AA, RK and ZN and their consumption, bio-conversion and accumulation in the male pheromone glands are interesting behavioural and physiological phenomena. The trend of RK accumulation shows that the consumed RK was detected in the rectal gland as soon as 6 h, peaked at 1- to 3 -DPT and began to decrease at 5-DPT onwards, possibly being released as pheromonal components during courtship, as seen in the other mentioned CL/RK-responsive species (Nishida et al. 1990, 1993; Tan and Nishida 1995, 2000; Nakahira et al. 2018). Although the accumulation of RK and AL following AA consumption showed a significant increase with time, the pooled amount of RK and AL appeared to be low compared to other male lures, representing about 25% of the total AA offered. Observations during fly feeding showed that B. frauenfeldi males would more readily feed on ZN than AA at close range, i.e. once they located the lure source, the males spent a longer time feeding on ZN than AA. This may explain the lower accumulation rate of lure metabolites for AA-fed males. The reason behind this behaviour is not known and needs further investigation.
Conversely, following the consumption of ZN, over half the amount consumed was detected in the rectal gland at 6 h but most ZN was retained in the rectal gland for up to 5-DPT without any indication of decrease. Although the functional importance of ZN in B. frauenfeldi is yet to be clarified, the results may indicate ZN has a relatively small role in the chemical ecology of B. frauenfeldi. This may explain why B. frauenfeldi males are not strongly seeking for ZN in the field although they readily feed on ZN at close range. Nevertheless, the discoveries of AA, RK and ZN in flowers of certain daciniphilous Bulbophyllum species (Tan and Nishida 2000, 2005, 2007; Nishida and Tan 2016; Nakahira et al. 2018; Katte et al. 2020) and the Dacini assemblage attracted to these flowers in tropical rainforests may have demonstrated the relative ecological and physiological significance of these phenylbutanoids in the mutualistic interactions between Dacini fruit fly fauna and Bulbophyllum orchid flora via floral synomone (Tan and Nishida 2015).
The attraction, consumption, bio-conversion and dynamics of the lure metabolite accumulation may offer a glimpse of the ecological importance and utilisation of phenylbutanoids by the Dacini fruit flies, so as to improve mate attraction and male mating competitiveness. Our study indicates that: (a) CL is the most attractive lure while AA is equally attractive as RK for B. frauenfeldi males; (b) the conversion of ingested AA into RK and AL by B. frauenfeldi males involves a demethylation of the methoxy- to a hydroxy-moiety, and a reduction of the keto-moiety, respectively; (c) the relative responses, conversion and retention rates of different lures by B. frauenfeldi males might be of ecological and plant-fruit fly co-evolutionary significances.
References
Barthel WF, Green N, Keiser I, Steiner LF (1957) Anisylacetone, synthetic attractant for male melon fly. Science 126:3275
Cunningham RT (1989) Biology and physiology: parapheromones. In: Robinson AS, Hooper G (eds) Fruit flies: their biology, natural enemies and control, world crop pests 3(A). Elsevier, Amsterdam, pp 221–230
Deifel A (1989) 4-(4-Hydroxyphenyl)-2-butanone (raspberry ketone). Review of natural occurrence and biogenesis. (in German) Z. Lebensm Unters Forsch 188:330–332
Doorenweerd C, Leblanc L, Norrbom AL, San Jose M, Rubinoff D (2018) A global checklist of the 932 fruit fly species in the tribe Dacini (Diptera: Tephritidae), Zookeys (730): 19–56.Drew RAI (1974) The response of fruit fly species (Diptera: Tephritidae) in the South Pacific area to male attractants. J Aust Entomol Soc 13:267–270
Drew RAI (1989) The tropical fruit flies (Diptera: Tephritidae: Dacinae) of the Australasian and Oceanian Regions. Mem Qld Mus 26:1–521
Fay HAC (2012) A highly effective and selective male lure for Bactrocera jarvisi (Tryon) (Diptera: Tephritidae). Aust J Entomol 51:189–197
GenStat (2016) GenStat for Windows, Release 16.1. VSN International Ltd., Oxford
Hancock DL, Hamacek EL, Lloyd AC, Elson-Harris MM (2000) The distribution and host plants of fruit flies (Diptera: Tephritidae) in Australia. Queensland Department of Primary Industries Information Series Q199067, Brisbane
Hanssen BL (2015) Synthesis and analysis of zingerone analogues as fruit fly attractants. MSc. Thesis, Department of Chemistry and Biomolecular Sciences, Macquarie University, Sydney, Australia
Hee AKW, Tan KH (1998) Attraction of female and male Bactrocera papayae to conspecific males fed with methyl eugenol and attraction of females to male sex pheromone components. J Chem Ecol 24:753–764
Hee AKW, Tan KH (2004) Male sex pheromonal components derived from methyl eugenol in the hemolymph of the fruit fly Bactrocera papayae. J Chem Ecol 30:2127–2138
IAEA (International Atomic Energy Agency) (2003) Trapping guidelines for area-wide fruit fly programmes. Joint FAO/IAEA Division, Vienna, p 47
Katte Y, Tan KH, Su ZH, Hajime O, Nishida R (2020) Floral fragrances in two closely related fruit fly orchids, Bulbophyllum hortorum and B. macranthoides (Orchidaceae): assortments of phenylbutanoids to attract tephritid fruit fly males. Appl Entomol Zool 55:55–64
Kumaran N, Clarke AR (2014) Indirect effects of phytochemicals on offspring performance of Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). J Appl Entomol 138:361–367
Kumaran N, Hayes RA, Clarke AR (2014a) Cuelure but not zingerone make the sex pheromone of male Bactrocera tryoni (Tephritidae: Diptera) more attractive to females. J Insect Physiol 68:36–43
Kumaran N, Prentis PJ, Mangalam KP, Schutze MK, Clarke AR (2014b) Sexual selection in true fruit flies (Diptera: Tephritidae): transcriptome and experimental evidences for phytochemicals increasing male competitive ability. Mol Ecol 23:4645–4657
Leblanc L, William J, Allwood AJ (2004) Host fruit of mango fly (Bactrocera frauenfeldi (Schiner)) (Diptera: Tephritidae) in the Federated States of Micronesia. Micronesica 37:21–31
Leblanc L, Vueti ET, Drew RAI, Allwood AJ (2012) Host plant records for fruit flies (Diptera: Tephritidae: Dacini) in the Pacific islands. Proc Hawaii Entomol Soc 44:11–53
Leblanc L, Doorenweerd C, Jose MS, Sirisena UGAI, Hemachandra KS, Rubinoff D (2018a) Description of a new species of Dacus from Sri Lanka, and new country distribution records (Diptera, Tephritidae, Dacinae). ZooKeys 795:105–114
Leblanc L, Doorenweerd C, Jose MS, Pham HT, Rubinoff D (2018b) Descriptions of four new species of Bactrocera and new country records highlight the high biodiversity of fruit flies in Vietnam (Diptera, Tephritidae, Dacinae). ZooKeys 797:87–115
Leblanc L, Hossain MA, Doorenweerd C, Khan SA, Momen M, San Jose M, Rubinoff D (2019) Six years of fruit fly surveys in Bangladesh: a new species, 33 new country records and discovery of the highly invasive Bactrocera carambolae (Diptera, Tephritidae). ZooKeys 876:87–109
Metcalf RL, Metcalf ER (1992) Plant kairomones in insect ecology and control. Chapman and Hall, New York
Metcalf RL, Metcalf ER, Mitchell WC, Lee LWY (1979) Evolution of olfactory receptor in oriental fruit fly Dacus dorsalis. Proc Natl Acad Sci USA 76:1561–1565
Metcalf RL, Mitchell WC, Metcalf ER (1983) Olfactory receptors in the melon fly Dacus cucurbitae and the oriental fruit fly Dacus dorsalis. Proc Natl Acad Sci USA 80:3143–3147
Nakahira M, Ono H, Wee SL, Tan KH, Nishida R (2018) Floral synomone diversification of Bulbophyllum sibling species (Orchidaceae) in attracting fruit fly pollinators. Biochem Syst Ecol 81:86–95
Nishida R, Tan KH (2016) Search for new fruit fly attractants from plants: A review. In: Sabater-Munoz B, Vera T, Pereira R, Orankanok W (eds) Proceedings of the 9th international symposium on fruit flies of economic importance, 12–16 May 2014, Bangkok, Thailand, pp 249–262
Nishida R, Tan KH, Takahashi S, Fukami H (1990) Volatile components of male rectal glands of the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). Appl Entomol Zool 25:105–112
Nishida R, Iwahashi I, Tan KH (1993) Accumulation of Dendrobium (Orchidaceae) flower fragrance in the rectal glands by males of the melon fly, Dacus cucurbitae (Tephritidae). J Chem Ecol 19:713–722
Noushini S, Perez J, Park SJ, Holgate D, Alvarez VM, Jamie I, Jamie J, Taylor P (2020) Attraction and electrophysiological response to identified rectal gland volatiles in Bactrocera frauenfeldi (Schiner). Molecules 25:1275
Park SJ, Morelli R, Hanssen BL, Jamie JF, Jamie IM, Siderhurst MS, Taylor PW (2016) Raspberry ketone analogs: vapour pressure measurements and attractiveness to Queensland Fruit Fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). PLoS ONE 11:1–16
Rabiatul AS, Wee SL (2019) Zingerone improves mating performance of Zeugodacus tau (Diptera: Tephritidae) through enhancement of male courtship activity and sexual signaling. J Insect Physiol 119:103949. https://doi.org/10.1016/j.jinsphys.2019.103949
Royer JE (2015) Responses of fruit flies (Tephritidae: Dacinae) to novel male attractants in north Queensland, Australia, and improved lures for some pest species. Austral Entomol 54:411–426
Royer JE, Wright CL, Hancock DL (2016) Bactrocera frauenfeldi (Diptera: Tephritidae), an invasive fruit fly in Australia that may have reached the extent of its spread due to environmental variables. Aust Entomol 55:100–111
Royer JE, Agovaua S, Bokosou J, Kurika K, Mararuai A, Mayer DG, Niangu B (2018) Responses of fruit flies (Diptera: Tephritidae) to new attractants in Papua New Guinea. Aust Entomol 57:40–49
Royer JE, Mille C, Cazeres S, Brinon J, Mayer DG (2019) Isoeugenol, a more attractive male lure for the cue-lure-responsive pest fruit fly Bactrocera curvipennis (Diptera: Tephritidae: Dacinae), and new records of species responding to zingerone in New Caledonia. J Econ Entomol 112:1502–1507
Royer JE, Tan KH, Mayer DG (2020) Comparative trap catches of male Bactrocera, Dacus and Zeugodacus fruit flies (Diptera: Tephritidae) with four floral phenylbutanoid lures (anisyl acetone, cue-lure, raspberry ketone and zingerone) in Queensland, Australia. Environ Entomol nvaa056
Segura DF, Belliard SZ, Vera MT, Bachmann GE, Ruiz MJ, Jofre-Barud F, Fernández PC, López ML, Shelly TE (2018) Plant chemicals and the sexual behavior of male Tephritid fruit flies. Ann Entomol Soc Am 111:239–264
Shelly TE (2010) Effects of methyl eugenol and raspberry ketone/cue lure on the sexual behavior of Bactrocera species (Diptera: Tephritidae). Appl Entomol Zool 45:349–361
Shelly TE (2017) Zingerone and the mating success and field attraction of male melon flies (Diptera: Tephritidae). J Asia-Pac Entomol 20:175–178
Shelly TE, Dewire AM (1994) Chemically mediated mating success in male oriental fruit flies (Diptera: Tephritidae). Ann Entomol Soc Am 87:375–382
Tan KH, Nishida R (1995) Incorporation of raspberry ketone in the male rectal glands of the Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Appl Entomol Zool 30:494–497
Tan KH, Nishida R (1996) Sex pheromone and mating competition after methyl eugenol consumption in the Bactrocera dorsalis complex. In: McPheron BA, Steck GJ (eds) Fruit fly pests—a world assessment of their biology and management. St.Lucie Press, Florida, pp 147–153
Tan KH, Nishida R (1998) Ecological significance of male attractant in the defence and mating strategies of the fruit fly pest, Bactrocera papayae. Entomol Exp Appl 89:155–158
Tan KH, Nishida R (2000) Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J Chem Ecol 26:533–546
Tan KH, Nishida R (2005) Synomone or kairomone? Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J Chem Ecol 31:497–507
Tan KH, Nishida R (2007) Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochem Syst Ecol 31:334–341
Tan KH, Nishida R (2012) Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci 12:56
Tan KH, Nishida R (2015) Pollination of bactrocerophilous Bulbophyllum orchids. In: Elliott J, Kuraweil HF, O’Byrne P, Tan KW, van Schans AS, Wong SM, Yam TW (eds) Proceedings of the 20th world orchid conference, Singapore, pp 273–279
Tan KH, Nishida R, Jang EB, Shelly TE (2014) Pheromones, male lures, and trapping of tephritid fruit flies. In: Shelly TE, Epsky N, Jang EB, Reyes-Flores J, Vargas RD (eds) Trapping and the detection, control, and regulation of tephritid fruit flies. Springer, Dordrecht, pp 15–74
Vargas RI, Leblanc L, Pinero JC, Hoffman KM (2014) Male annihilation, past, present, and future. In: Shelly TE, Epsky N, Jang EB, Reyes-Flores J, Vargas RD (eds) Trapping and the detection, control, and regulation of tephritid fruit flies. Springer, Dordrecht, pp 493–511
Wee SL, Shelly TE (2013) Capture of Bactrocera fruit flies in traps baited with liquid versus solid formulations of male lures in Malaysia. J Asia-Pac Entomol 16:37–42
Wee SL, Hee AKW, Tan KH (2002) Comparative sensitivity to and consumption of methyl eugenol in three Bactrocera dorsalis (Diptera: Tephritidae) complex sibling species. Chemoecology 12:193–197
Wee SL, Tan KH, Nishida R (2007) Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J Chem Ecol 33:1272–1282
Wee SL, Abdul Munir MZ, Hee AKW (2018a) Attraction and consumption of methyl eugenol by male Bactrocera umbrosa Fabricius (Diptera: Tephritidae) promotes conspecific sexual communication and mating performance. Bull Entomol Res 108:116–124
Wee SL, Chinvinijkul S, Tan KH, Nishida R (2018b) A new and highly selective male lure for the guava fruit fly Bactrocera correcta. J Pest Sci 91:691–698
Wee SL, Peek T, Clarke AR (2018c) The responsiveness of Bactrocera jarvisi (Diptera: Tephritidae) to two naturally occurring phenylbutaonids, zingerone and raspberry ketone. J Insect Physiol 109:41–46
Acknowledgements
The authors would like to thank Sybilla Oczkowicz (DAF, Cairns) for pupae supply, Stacey Carseldine and John Pritchard (contracted by DAF) for assistance with field trials. The critical review of this manuscript by R. Nishida (Kyoto University) and two anonymous reviewers are much appreciated. The Central Analytical Research Facility operated and provided by the Institute for Future Environments, Queensland University of Technology, Australia was also greatly appreciated. The work is partly supported by International Atomic Energy Agency Research Contract 20443 (@ST-2016-004) and the Australian Endeavour Research Fellowship 2016 (5596-2016) awarded to S. L. Wee.
Author information
Authors and Affiliations
Contributions
SLW, KHT and JR conceived and designed the experiments. SLW, JR and JH performed the experiments. SLW and DM analysed the data. SLW, KHT and JR wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants or animals (vertebrates) performed by any of the authors.
Additional information
Communicated by Günther Raspotnig.
Rights and permissions
About this article
Cite this article
Wee, S.L., Royer, J.E., Herring, J. et al. Relative response of male Bactrocera frauenfeldi (Diptera: Tephritidae) to phenylbutanoid phytochemicals: implications for fruit fly control and plant–insect interactions. Chemoecology 30, 305–314 (2020). https://doi.org/10.1007/s00049-020-00320-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00320-6