Abstract
Pheromones mediate species-level communication in the search for mates, nesting, and feeding sites. Although the role of pheromones has long been discussed by various authors, their existence was not proven until the mid-twentieth century when the first sex pheromone was identified. From this finding, much has been speculated about whether this communication mechanism has acted as a regulatory agent in the process of speciation, competition, and sexual selection since it acts as an intraspecific barrier. Chrysomelidae is one of the major Phytophaga lineages, with approximately 40,000 species. Due to this immense diversity the internal relationships remain unstable when analyzed only with morphological data, consequently recent efforts have been directed to molecular analyses to establish clarity for the relationships and found their respective monophyly. Therefore, our goals are twofold 1) to synthesize the current literature on Chrysomelidae sex pheromones and 2) to test whether Chrysomelidae sex pheromones and their chemical structures could be used in phylogenetic analysis for the group. The results show that, although this is the first analysis in Chrysomelidae to use pheromones as a phylogenetic character, much can be observed in agreement with previous analyses, thus confirming that pheromones, when known in their entirety within lineages, can be used as characters in phylogenetic analyses, bringing elucidation to the relationships and evolution of organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Communication plays a key role in animal behavior. When animals communicate, they transfer information as encoded in a visual, auditive or olfactory signals (Endler 1993; Wyatt 2003). Insects are no different, but their diversity prompts the evolution of several mechanisms that encode information. However, olfactory communication is the most common among insects and it involves the use of chemical compounds to transfer information between individuals (Brezolin et al. 2018). Chemical communication is not something exclusive to insects. Darwin (1871) and many others, viz, Butler (1609) and Fabre (1879–1907) had already addressed that this mechanism is used by a range of organisms, including bacteria, humans, and many others, but it is certain that communication through chemical signals is a predominant feature in insects (Greenfield 2002; Reinhard 2004).
Insects are one of the most diverse animal groups, corresponding to more than half of all known living organisms (Cai et al. 2022; Sollai and Solari 2022; Stork 2018; Tihelka et al. 2021). Within Insecta, Coleoptera is the most biodiverse order of the animal kingdom with over 380.000 described species, and an estimated 1.5 million yet undescribed (Cai et al. 2022; Sollai and Solari 2022; Stork 2018). Chrysomelidae and six other beetle families, namely Vesperidae, Oxypeltidae, Disteniidae, Cerambycidae, Orsodacnidae and Megalopodidae, comprise the superfamily Chrysomeloidea. Along with Curculionoidea, these two superfamilies form the clade Phytophaga. This clade is the largest and most diverse lineage of phytophagous beetles, with approximately 125.000 described species. It is the second largest lineage of existing phytophagous animals, while Lepidoptera is the first one (Grimaldi and Engel 2005; Haddad and McKenna 2016; Marvaldi et al. 2009; McKenna et al. 2009, 2015; Robertson et al. 2015).
All chrysomelids are phytophagous and most adults feed on leaves, but adults of some species may feed on pollen. The feeding habits of larvae are more diverse in comparison to adults, being able to feed on roots or leaves, or inside stems and seeds. Many species are considered pests of cultivated plants and can cause direct damage to leaves, roots and stems, compromising the transport of nutrients in the plant. They can also cause indirect damage to plants by acting as vectors in the transmission of viruses (Goméz-Rodríguez et al. 2015; Leschen and Beutel 2014).
Some insects can sequester complex molecules from plants during feeding to produce their pheromones using simple biosynthetic steps such as, reduction, esterification, and epoxidation. There is evidence in the literature that diet has impact on the composition of both cuticle and volatile compounds involved in chemical communication in phytophagous beetles. For example, males of Hedypathes betulinus (Klug 1825) (Cerambycidae: Lamiinae) may sequester geranylacetone from green mate leaves during feeding (Andrade et al. 2019) and use it to produce the long-range sex pheromone fuscumol and fuscumol acetate through reduction and esterification steps (Fonseca and Zarbin 2009; Fonseca et al. 2010; Vidal et al. 2010). Impacts of the diet on the cuticular hydrocarbon composition have been reported in Chrysomelidae, females of Acanthoscelides obtectus (Say 1831) that have been fed with either beans or chickpeas in laboratory for 50 generations have shown differences in the levels of three cuticular hydrocarbon compounds (pentacosane, 9-methylheptacosane, and triacontane). Males of A. obtectus that have been reared on chickpeas for 50 generations in laboratory were able to discriminate between females based on these differences in the cuticle related to diet (Stojković et al. 2014). On the other hand, many other species use de novo biosynthesis to produce their pheromones such as using Acetyl-CoA for the biosynthesis of fatty acids or isoprenyl diphosphate for the biosynthesis of terpenes. These two biosynthetic pathways are very common in insects (Jurenka 2004). For example, several Lepidoptera species have pheromones derived from fatty acids (Ando et al. 2004) and several Coleoptera and Hemiptera species have pheromones derived from terpenoids (Jurenka 2004; Zou and Millar 2015).
The Chrysomelidae is the fourth largest family of Coleoptera, with approximately 40.000 described species (Zhang et al. 2022). This family has 12 subfamilies, namely Bruchinae, Cassidinae, Chrysomelinae, Criocerinae, Cryptocephalinae, Donaciinae, Eumolpinae, Galerucinae, Lamprosomatinae, Sagrinae, Spilopyrinae and Synetinae (Leschen and Beutel 2014; Haddad and McKenna 2016).
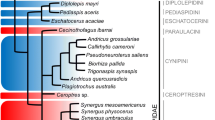
The 12 subfamilies of Chrysomelidae, were established by morphological analysis (Reid 1995, 2000). The phylogenetic relationships in the group have constantly changed until the advancement of molecular analyses, which provided some stability in the phylogeny of the following three main lineages: clade Chrysomelinae (including the subfamilies, Chrysomelinae and Galerucinae), clade Eumolpinae (including the subfamilies Cassidinae, Cryptocephalinae, Eumolpinae, Lamprosomatinae, Synetinae and Spilopyrinae) and clade Sagrinae (including the subfamilies Bruchinae, Criocerinae, Donaciinae and Sagrinae) (Gómez-Zurita et al. 2008). However, molecular techniques still diverge on establishing relationships between these clades (Fig. 1a, b, c, d) (Haddad and McKenna 2016; Nie et al. 2020b). The topology (clade Sagrinae (clade Chrysomelinae + clade Eumolpinae) is found in the most recent and extensive phylogeny studies (Douglas et al. 2023).
Phylogenetic relationships of the three main lineages of Chrysomelidae. Methods used to establish relationships between clades are specified in parentheses. a Gómez-Zurita et al. 2008 (multilocus ribosomal RNA), Zhang et al. 2018 (nuclear protein-coding), McKenna et al. 2019 (nuclear genes), Cai et al. 2022 (nuclear protein-coding), Douglas et al. 2023 (genome). b Nie et al. 2020b (mitogenome), Nie et al. 2020a, b (mitogenome), Zhang et al. 2022 (mitogenome). c Timmermans et al. 2015 (mitogenome), Goméz-Rodríguez et al. 2015 (mitogenome). d Phylogenetic relationships adapted from Douglas et al. 2023 (three major lineages indicated with numbers 1 clade Sagrinae, 2 clade Chrysomelinae, 3 clade Eumolpinae)
A character is defined as a heritable feature expressed as an independent variable, such as any characteristic of a taxon (Sereno 2007). Any character has phylogenetic potential. Individual characters contribute to evolutionary hypothesis that are expressed, for example, in a matrix. This matrix is used to analyze the phylogenetic relationships between the groups of interest. It is expected that pheromones have phylogenetic potential as a character since they have high specificity and could have evolved as any other trait. In this way, pheromones could be used as a phylogenetic character to solve problems in in the relationships between groups that morphological/molecular analyses were not able to. In addition, pheromones play a fundamental role in mediating insect communication and are widely used in insect pest control tactics (Casari and Ide 2012).
Therefore, our goals are twofold: 1) summarize and synthesize the current literature of pheromones involved in the location and recognition of mates in Chrysomelidae and, 2) analyze the use of Chrysomelidae sex-pheromones in phylogenetic analysis. Since Chrysomelidae is one of the largest beetle families and has a high degree of specificity with its host plants, describing how sex pheromones evolve is of extensive interest to both basic and applied research, such as in the elucidation of the internal relationships between groups and the improvement of current pest control tactics.
Material and Methods
Data Sampling
We performed a systematic search on Chrysomelidae pheromones using the Pherobase©, Web of Science©, and Google Scholar© online platforms. The Pherobase© was used as the main search database, while the Web of Science© and Google Scholar© were used as complementary databases. We reviewed all articles in the "Chrysomelidae" category, as well as in "Bruchidae", present in the Pherobase©. For the Web of Science search, the following parameters were used: ((all = (sex pheromone)) or (all = (Chrysomelidae)) and (all = (pheromone))). The Google Scholar search was performed using the following keywords: Chrysomelidae, pheromone and sex pheromone. A total of 262 articles were analyzed, published between the years of 1965–2023, and 206 taxa were reviewed in this work.
The taxa presented in this review are arranged in alphabetic order descending from family, subfamily, tribe, genus, until species. The subspecies level was included whenever possible. The following online platforms were used to check all species names and their current taxonomic status: The Encyclopedia of Life, The Catalogue of Life©, Inventaire National du Patrimoine Natural, Global Biodiversity Information Facility, Catálogo Taxonômico da Fauna do Brasil (Sistema de Informação sobre a Biodiversidade Brasileira—SiBBr), National Center for Biotechnology Information, and Integrated Taxonomic Information System. Species transferred from the Chrysomelidae to other families are not included in this work to avoid perpetuating taxonomic misunderstanding.
For taxa that share some chemical compounds or blends, additional comments about biology, biogeography, and feeding habits have been added to elucidate the interactions between these species.
Terminals, Characters, and Phylogenetic Analysis
The internal group is composed of thirty-nine recognized species of Chrysomelidae. In addition to Chrysomelidae, we included one species of Anthribidae, two species of Curculionidae, five species of Cerambycidae, and one species of Vesperidae in our phylogenetic analyses that represent the Phytophaga clade. One species of Silvanidae was selected as the outgroup representing the Cucujoidea superfamily (Supplementary Table 1).
The characters were constructed in accordance with the logical structure by following the criterion of character independence and mutually exclusive conditions of their states (Sereno 2007). Characters that could be coded as multi-state such as, biosynthesis pathway, functional groups, saturated compounds, and cyclic compounds, were categorized as present/absent for each possible state after establishing primary homology hypotheses (Tarasov and Solodovnikov 2011; Tarasov and Génier 2015). This kind of arrangement was used because of its efficiency in providing a direct answer to a hypothesis (the character) and an accurate separation of features into subsets of taxa (the present state) demonstrated in previous works (Tarasov and Solodovnikov 2011; Tarasov and Génier 2015). For these characters, this is justified by the difficulty of proposing homologies between such states since the routes required for the transformation of one molecule into another may be numerous and non-homologous as well as unknown. Added to this, we have the fact that each species can present more than one associated compound.
Unambiguous optimization makes it possible to visualize all characters, thus the characters will be presented in a list and will be followed by comments.
We excluded the cuticle compounds of two species of Chrysochus and Phaedon cochleariae from our analyses due to the high number of compounds identified for cuticle of these species (i.e., 27 compounds identified from the cuticle of Chrysochus spp. and 67 compounds identified in the cuticle of Phaedon cochleariae) and the lack of data that provides behavioral responses to any of these compounds as a short-range sex- pheromone of these species. We do not encode the functional groups and biosynthesis pathways for Zabrotes and Decellebruchus due to the absence of data in the literature for these species. Therefore, we only encode the four following characters for Zabrotes and Decellebruchus: the emitting sex (female), aggregation pheromone (absent), long-range sex-pheromone (present), and short-range sex-pheromone (present) (also known as contact sex-pheromone).
The matrix was build using the NEXUS program version 0.5.0 (Page 2001). For better visualization, the states were coded with a dash (-) when inapplicable and with a question mark (?) when not observed (missing data). However, when such states are analyzed in programs, they end up being interpreted under all possibilities of states present in the character in question (e.g., 0/1).
A supertree was assembled using phylogenetic hypotheses from different taxonomic levels (Phytophaga: (Cai et al. 2022), Chrysomeloidea: (Nie et al. 2020a, b), Chrysomelidae: (Douglas et al. 2023), Bruchinae: (Kergoat et al. 2015), Acallymma + Diabrotica: (Derunkov et al. 2015), Leptinotarsa + Zygogramma: (Gómez-Zurita et al. 2008), Aphthona: (Konstantinov 1998), Callosobruchus: (Tuda et al. 2006), Diabrotica: (Eben et al. 2013) and Galerucella (Borghuis et al. 2009) to produce a reference topology. We optimized the proposed characters of this reference topology using only the unambiguous changes to describe their evolutionary pathways. Visualization and handling of the resulting cladograms was done in ASADO ver. 1.61 TNT-MRBAYES SLAVER (vl 5.30) (Nixon 2022) and was exported for editing in a specific vectorization program.
For the Bayesian inference, the pheromone dataset was partitioned according to the homoplasy criterion (Rosa et al. 2019). In this criterion, the characters are partitioned according to their homoplasy values, therefore, the characters are organized in partitions corresponding to their values. We measured the homoplasy values using TNT (Goloboff 2008) through an implicit weighing analysis, with the default concavity parameter (k = 3). The values are normalized between 0 and 1, with the lowest value corresponding to no homoplasy, and no values are assigned to non-informative characters (Supplementary 2). In addition, we included the non-informative characters in the partition that corresponds to no homoplasy (homoplasy value = 0.00), as suggested by Gonçalves et al. (2022). The morphological data were modeled according to suggestions by Rosa et al. (2019), as follows: ascertainment bias as variable, branch lengths linked among-partition rate variation, equal rates among-character rate variation, and the branch length prior equal to 10.
The Bayesian inference was carried out in MrBayes 3.2.7 (Ronquist et al. 2012) through the CIPRES Science Gateway portal (Miller et al. 2010). We use two independent runs with four chains each, 2 × 106 generations and 25% of burn-in. Convergence of the chains was checked in MrBayes 3.2.7 and Tracer 1.6 (Rambaut et al. 2013) using as reference ESS (effective sample size) > 200, ASDSF (average standard deviation of split frequencies) ≤ 1 and PSRF (potential scale reduction factor) ~ 1.0. Convergence for topologies was checked through the visualization of the trace plots generated by the function analyze.rwty in the package RWTY (Warren et al. 2017) in the R environment, and according to the analytical references suggested by Wilgenbusch et al. (2004), Nylander et al. (2008), Rambaut et al. (2013) and Warren et al. (2017). The trace plots results are shown in the supplementary 3. For the trees we applied the majority rule consensus with all-compatible groups added (Contype = allcompat), and the posterior probability was used as branch support.
Results and Discussion
Our literature review found sex pheromone were reported in ten subfamilies belonging to the Chrysomelidae. Six of these subfamilies, namely Bruchinae, Chrysomelinae, Criocerinae, Cryptocephalinae, Eumolpinae and Galerucinae, have either the long- or short-range sex pheromone, or the aggregation pheromone described in the literature (Supplementary Table 1 and Fig. 6). Three subfamilies, namely Cassidinae, Donaciinae and Synetinae, only have reports of interactions mediated by chemical signals or initial investigation on cuticle and tarsal profile (Geiselhardt et al. 2011; Valkama et al. 1997), and for the last subfamilies, Lamprosomatinae, Sagrinae, and Spilopyrinae, any semiochemical-mediated interaction has been found. Currently, these three taxa are comprised by 250, 40 and 39 species, respectively, included species with economic importance and potential use for biological control (Caxambú and Almeida 1999; Chamorro 2014; Jolivet et al. 2014; Lawrence and Lawrence 2014).
Additionally, all the semiochemicals reported for Chrysomelidae, pheromones (1–66) and kairomones (67–75), were presented in Figs. 2 and 3.
Chemical structures of compounds identified as pheromones in Chrysomelidae from Acanthoscelides obtectus: 1–6; Bruchus rufimanus: 7; Callosobruchus analis: 8–10; C. chinensis: 9, 11–13; C. maculatus: 8, 11, 14–18; C. rhodesianus: 19–22; C. subinnotatus: 8, 14; Caryedon serratus: 23, 24; Gastrophysa atrocyanea: 25–31; Leptinotarsa decemlineata: 32; Oulema melanopus: 33; Labidostomis lusitanica: 34; Costalimaita ferruginea: 35, 36; Altica litigate: 37, 38; Aphthona czwalinae, A. cyparissiae, and A. flava: 37–44; Epitrix fuscula: 37, 38, 43–46; E. hirtipennis: 45–50; Phyllotreta cruciferae: 37–42; P. pusilla: 42, 51–55; P. striolata: 37–42, 56–58; P. vittula: 37, 38, 40–42, 56; Diabrotica. balteata: 59; D. cristata: 60; D. undecimpunctata howardi: 61; D. virgifera virgifera: 62; Diorhabda elongata: 63, 64; Galerucella calmariensis and G. pusilla: 65; Acalymma vittatum: 66. Blue: fatty acid/polyketide-derived from acetate pathway pheromones, red: terpenes, green: nitrogenous metabolites and black: biosynthesis not proposed
Overview of Pheromones in Chrysomelidae
Subfamily Bruchinae Latreille, 1802
With approximately 1.650 described species (Morse 2014), individuals allocated to this subfamily are easily recognized by the habit of living inside seeds during their larval stage. Although, there is 36 host plant families recorded as hosts (Johnson 1981, 1989; Johnson et al. 2001; Santos and Ribeiro-Costa 2019), Bruchinae shows a marked preference for Fabaceae (Johnson 1981). The subfamily is distributed throughout the world, except for Antarctica and New Zealand (Kingsolver 2002). Most species are endemic to the Americas and their distribution generally corresponds to the distribution of their host plants. Currently, the subfamily has 69 genera, allocated in six tribes, Amblycerini Bridwell, 1932, Bruchini Latreille, 1802, Eubaptini Bridwell, 1932, Kytorhinini Bridwell, 1932, Pachymerini Bridwell, 1929, and Rhaebini Blanchard, 1845 (Morse 2014; Viana 2016). Bruchini concentrates about 80% of all species in the subfamily, followed by Amblycerini (10%), Pachymerini (9%), and the remaining 1% distributed among the other tribes (Johnson and Romero-Nápoles 2004).
It is worth noting that in this review we are treating Bruchinae as a subfamily belonging to Chrysomelidae (Bocak et al. 2014; Bouchard et al. 2011; Duckett et al. 2004; Farrell 1998; Farrell and Sequeira 2004; Gómez-Zurita et al. 2008; Reid 1995; Reid 2000). Still dealing with the Bruchinae and the names mentioned in Pherobase, we came across in the database with the name Bruchidius atrolineatus (Pic 1921), a species that in 2016, was transferred to the genus Decellebruchus, and in this review will be treated as Decellebruchus atrolineatus (Pic, 1921), following the work of Napóles (2016).
Tribe Amblycerini Bridwell, 1932
Zabrotes Horn, 1885
The Zabrotes subfasciatus (Boheman 1833) is a major pest of Phaseolus vulgaris L. (Fabaceae), the common bean. Although the genus being native to the Tropical Region, Z. subfasciatus has a wide geographic distribution, as it was introduced over the years in Europe, becoming a major pest of Leguminosae (Sari et al. 2003). In relation to the semiochemicals, Pimbert and Pouzat (1988) analyzed, via electroantennogram, the response of males of Z. subfasciatus in relation to compounds emitted by females and noted a strong attractiveness, indicating that females emit sexual pheromone that is strongly attractive to males.
Tribe Bruchini Latreille, 1802
Acanthoscelides Schilsky, 1905
Acanthoscelides is the largest genus of Bruchini, with about 340 valid names and more than 200 to be described (Johnson 1990). This genus is distributed in the new world and has several species of economic importance, as occurs in all Bruchinae groups. However, what stands out is the proportion of the number of known taxa and the amount of work describing pheromones, which is almost non-existent, only one representative, A. obtectus (Say, 1831), cosmopolitan species, popularly known as bean weevil and is found infesting Phaseolus vulgaris L.
Hope et al. (1967) was the first to suggest the presence of a compound released by males, with a role as a stimulus for copulation. Three year later, Horler (1970) indicate a candidate to sex pheromone in A. obtectus, according to the author, the male, few days after emergence produce a substance that attracted the female, being identified as (E)-methyl tetradeca-2,4,5-trienoate (1). Landor et al. (1971), Kocienski et al. (1977), and Pirkle and Boeder (1978) carried out the synthesis of the pheromone and only in 1981, Mori et al. (1981) synthesized both enantiomers of the dry bean beetle pheromone in high optical purity, confirming the (R)-1 as a natural pheromone.
Vuts et al. (2015a) carried out a series of studies to identify the roles played by the pheromone 1 in the sexual behavior of A. obtectus and found that males chose virgin females over other males, and preferred virgin over mated females. They also found that males transfer the pheromone to the females during mating, resulting in mated females being avoided by courting males. Ultimately the authors found, using gas chromatography of hexane extracts, the presence of two male-specific compounds, in addition to pheromone 1, the octadecanal (2), these, in turn, were absent from the behaviorally active female samples, this last compound was mentioned for the first time by Vuts et al. (2015a).
Almost 50 years later Hope's work, the pheromone bouquet of A. obtectus is finally published, the (E,R)-methyl tetradeca-2,4,5-trienoate (1), octadecanal (2), (2E,4Z,7Z)-methyl deca-2,4,7-trienoate (3), (2E,4Z)-methyl deca-2,4-dienoate (4), and the sesquiterpenes (3Z,6E)- and (3E,6E)-α-farnesene (5 and 6) was identified male-specific volatile component of A. obtectus (Vuts et al. 2015b). In the tests realized by the authors, the virgin females were weakly attracted when pure methyl (E,R)-methyl tetradeca-2,4,5-trienoate (1) was tested alone, meanwhile when a mixture of the six identified compounds was released, they proved to be as active as headspace odors collected from live males (Horler 1970; Vuts et al. 2015b).
Bruchus Linnaeus, 1767
Bruchus rufimanus Boheman, 1833, has been considered a pest in Africa, Asia, North America, and Europe (Bruce et al. 2011). In this species, three classes of semiochemicals regulate the interaction between B. rufimanus, its host plant, and the sexual partner (Segers et al. 2021). Kairomones released by Vicia faba flowers (Linnaeus 1753) allows adults to find the food source (Bruce et al. 2011), males then produce sex pheromones for attraction and copulation with females (Bruce et al. 2011), and finally, kairomones are released by the pods allowing females to find sites for oviposition (Frérot et al. 2015; Frérot and Leppik 2016).
Bruce et al. (2011), identified nine organic compounds released by flowers and identified that the combination of (R)-linalool (67), cinnamyl alcohol (68), cinnamaldehyde (69) are attractive to B. rufimanus. In the same study, it was shown that only males release the undecene (7), pheromone, which, despite being effective in attracting females under laboratory conditions, when tested in the field, attracted only when together with the kairomones emitted by flowers of V. faba (Bruce et al. 2011; Frérot et al. 2015). After copulation in flowers, females lay their eggs in pods, and this behavior is mediated by a combination of (Z)-hex-3-en-1-yl acetate (71), and more five smaller compounds similar to those found in flowers by Bruce et al. (2011), linalool (67), ocimene (71), α-humulene (72), β-cariofilene (73) e limonene (74) (Frérot and Leppik 2016).
Callosobruchus Pic, 1902
It is the best-studied genus of this tribe, with five species with some type of chemical compound described in Pherobase. This number is justified by the fact that several species are currently considered of economic importance, causing severe damage to stored grains, especially legumes intended for human consumption. Another peculiar feature is observed in Callosobruchus, where two types of pheromones are involved in mating. The first is a sex pheromone released by females, attracting males even at a certain distance, and a second, also a sex pheromone, acting through contact between individuals, that is, at a short distance, and exerting an arousal response only in males, with exposure of the male genital organ, followed by copulation (Shimomura et al. 2010a; Tanaka et al. 1981).
Callosobruchus analis (Fabricius 1781)
Preliminary evidence for a female sex attractant in C. analis, was reported by Cork et al. (1991) as being (Z)-3-methylhept-2-enoic acid (8). According to Tanaka et al. (1981), in this species, two types of pheromones are found during mating, which are subsequently emitted by the female, the first is a sex pheromone that attracts the male at a given distance, and the second is a sex pheromone of contact, released at a short distance and which causes the male to expose his genital organ for copulation.
Using filter paper collection and further analysis using Gas Chromatography-Mass Spectrometry (GC–MS), Shimomura et al. (2010a), were able to identify the following compounds, (E)-3,7-dimethyloct-2-enedioic acid (9) and 2,6-dimethyloctanedioic acid (10), the callosobruchusic acid, which had been previously identified as a contact sex pheromone for C. maculatus (Fabricius 1775) and C. chinensis (Linnaeus 1758), respectively. Although, unlike the latter two, the two compounds in C. analis are stereochemically pure. According to Shimomura et al. (2010a), C. analis has highly specific mating behavior, as does C. rhodesianus (Pic, 1902), when compared to two other species that live in sympatry, C. chinensis and C. maculatus. In addition, Yajima et al. (2007) identified (R)- and (S)-9 in the natural product at 3:1 ratio and (2R,6S)-, (2S,6R)-, (2S,6S)- and (2R,6R)-10 at 43:38:18: trace for C. chinensis.
Callosobruchus chinensis Linnaeus, 1758
A first indication that in Callosobruchus females are responsible for releasing the sex pheromone was published by Honda and Yamamoto (1976), when studying C. chinensis. Later Tanaka et al. (1981; 1982) indicated that Erectin, (2E,6E)-2,6-dimethylocta-2,6-dienedioic acid (11), was produced by both sexes, but only played an excitatory role in males, with exposure of the genitalia and subsequent copulation attempt. In 1990, Ueno et al. identified the kairomone ( +)-catechin (75) as a stimulant for oviposition, and more than thirty years after the first work, attraction compounds released by females are described by Shimomura et al. (2008), suggesting the following molecules (2Z,6E)- and (2E,6E)-7-ethyl-3,11-dimethyldodeca-2,6,10-trienal (12 and 13), with the synthesis and field testing of these compounds being later performed by Chiluwal et al. (2017), proving their attractiveness. Shimomura et al. (2016) also indicated that (E)-3,7-dimethyloct-2-enedioic acid (9) act as contact pheromone (Shimomura et al. 2016; Tanaka et al. 1981).
Callosobruchus maculatus (Fabricius, 1775)
The cowpea weevil, C. maculatus, was studied for the first time by Rup and Sharma (1978), where the authors proposed that the female would be responsible for the release of the sex pheromone. In 1986, Rup developed some bioassays and observed that prior to copulation, the female called the male and directed him to the mating site of interest. Phillips et al. (1996) suggested that five carboxylic acids, released by females, had a synergistic effect on the behavior of males of C. maculatus, these are (Z)- and (E)-3-methylhept-2-enoic acid (8 and 14), (Z)- and (E)-3-methylhept-3-enoic acid (15 and 16) and 3-methylhept-2-enoic acid (17). Following the author’s, tests with single-vial and wind-tunnel demonstrated that all five acids are biologically active for male, but the 15 and 16 are highest attractive, besides stimulating, in addition to flying that was stimulated by the other acids, this two stimulating the approach and contact (Phillips et al. 1996). In addition to the long distance sex pheromone, females of C. maculatus also produce a contact sex pheromone, the 2,6-dimethyloctane-1,8-dioc acid (11) and nonanedioic acid (18) following Nojima et al. (2007).
Callosobruchus rhodesianus (Pic, 1902)
The female sex pheromone of the C. rhodesianus was first suggested by Shimomura et al. (2010b), when the authors hypothesized that the 7-ethyl-3,11-dimethyl-6,10-dodecadienal (19) would be attractive to males, being the absolute configuration of the natural compound confirmed as (3S,6E)-19. Bioassays using Y-tube olfactometer showed that only the (S)-19 is attractive to males, while the (R)-19 and the racemate are not attractive to the males. In the same year, Shimomura et al. (2010c) suggested another female compound as a pheromone candidate, the (E)-6-ethyl-2,10-dimethylundeca-5,9-dienal (20). The authors indicated that this compound would possibly work as a minor sex attractant pheromone. The 6,10,14-trimethylpentadecan-2-one (21) and nonadecan-2-one (22) were identified as the sex contact pheromone, produced by the females (Shimomura et al. 2016).
Callosobruchus subinnotatus (Pic, 1914)
The last species of the genus addressed in this review is C. subinnotatus, with only one study related to this species. Shu et al. (1999) proposed that the female produces sex pheromone, attracting males to the emitting source. The authors analyzed the compounds using the Gas Chromatographic and Mass Spectrometric and suggested that the sex pheromone consisted of two short-chain fatty acids, the (Z)- and (E)-3-methylhept-2-enoic acid (8 and 14), being the composition of these molecules confirmed by electrophysiological and behavioral bioassays, where they were used synthetic compounds.
Despite the C. rhodesianus and C. maculatus have overlapping host plant, Vigna unguiculata (Linnaeus) Walp., the natural distribution (prior to anthropogenic dispersal) and pheromones are distinct (Tuda et al. 2006), resulting in the hindrance of cross copulation in these two species. For the other Callosobruchus species, namely C. analis, C. chinensis, and C. subinnotatus, as much as they share one compound, the other compounds that form the blend in these species are distinct (Table I), as are their respective host plants.
Decellebruchus Borowiec, 1987
Sex pheromones were first reported by Nammour et al. (1988), where females of D. atrolineatus (Pic, 1921) were identified as responsible for the release of the sex pheromone. Later experiments confirmed the attractiveness of males to compounds released by females (Pouzat and Nammour 1989) and the location of possible glands involved in the release of this pheromone (Biemont et al. 1992). It is worth noting that none of the works mentioned proposed the structure of the sex pheromone.
Tribe Pachymerini Bridwell, 1929
Caryedon Schoenherr, 1823
The genus has only one species represented, Caryedon serratus (Olivier 1790). Following the pattern of other species, C. serratus is also considered of economic importance, since it attacks Arachis hypogaea (Linnaeus, 1753) (Fabaceae). Furthermore, after a series of investigations of attractiveness, using five hexadecanoic acid ester derivatives, the authors found that, the males are significantly attracted to benzoyl hexadecanoate. By investigating other platforms and repositories, it was possible to locate other works addressing the use of chemical communication in C. serratus.
Chaibou et al. (1993) indicated the presence of pheromone released by females during scotophase, this being attractive to males, but no structure was suggested. Panday et al. (2011) in order to establish an attractiveness for males and females, as a counterpoint to the use of insecticides, performed an attractiveness test with extracts of pods and kernels in different solvents, being possible to observe a greater attractiveness of both sexes for the methanol extract of shelled groundnut. Based on this result, the authors analyzed it in the Gas Chromatograph-Mass Spectrometry and found the presence of octadeca-9,12-dienoic acid (23) and palmitic acid (24), as major constituents, may be responsible for the attractiveness. Some years later, Jyothi et al. (2014), attested to the presence of a sex pheromone released by females and an aggregation pheromone, the latter released by males. For this, the authors used a Y-tube olfactometer for the bioassays and investigated the responses of males and females in relation to their conspecific headspace extracts and groundnut seed extracts. Through the results obtained, the authors hypothesized that females would be responsible for the release of sex pheromones, and males for the aggregation pheromone. Beyond that, both sexes responded considerably to groundnut seed extracts, with females having a superior response to males, 76.1% to 67.6%, respectively, these results agree with those obtained previously by Panday et al. (2011) and Chaibou et al. (1993).
Subfamily Cassidinae Gyllenhal, 1813
The Cassidinae is the second largest subfamily of Chrysomelidae (Fukumori et al. 2022), with approximately 6.000 species described with worldwide distribution, nevertheless most species of this subfamily are found in the Neotropical region (Chaboo 2007). Despite the diversity of this group, there is only one record of cuticle chemical compounds identified in this subfamily and one study of female contact sex pheromone (Geiselhardt et al. 2011; Kawazu et al. 2011).
Tribe Cassidini Gyllenhal, 1813
Cassida Linnaeus, 1758
Geiselhardt et al. (2011) investigated the chemical composition of tarsi and elytra SPME samples of Cassida viridis Linnaeus, 1758 and 34 other Coleoptera species, that have convergently developed widened tarsi for substrate adhesion. Their results indicate that there are no substantial differences in the chemical composition of tarsal and elytral samples in all species studied. All chemical components identified in this study are characteristic for insect cuticular lipids, such as saturated, unsaturated, and methyl-branched hydrocarbons (Geiselhardt et al. 2011). Therefore, no pheromones have been described to date in this subfamily.
Tribe Cryptonychini Chapuis, 1875
Brontispa Sharp, 1904
The Brontispa longissima (Gestro 1885), popularly known as coconut hispine beetle, is a serious pest of Cocos nucifera L., and other several ornamental palms (Liebregts and Chapman 2004, Nakamura, Konishi and Takasu 2006). In 2011, Kawazu et al. investigated the existence of the females contact sex pheromone. The results indicated the presence of a sex pheromone in B. longissima and suggested that these compounds consist of one or more less-polar compounds, like saturated hydrocarbons.
Subfamily Chrysomelinae Latreille, 1802
This subfamily is the fifth largest in Chrysomelidae, with about 3.000 species (Reid 2006), allocated in 132 genera and 2 tribes, Chrysomelini and Timarchini (Riley et al. 2002). However, on the Pherobase platform, more than 50 articles are found for the subfamily, a higher amount compared to Galerucinae. This number is also justified by the pest status of some taxa, especially regarding commodities, such as Solanum tuberosum (Linnaeus, 1753).
The advantage of this interest, which is too focused on some groups, as is the case of Chrysomelinae, is the possibility of discovering a range of interactions mediated by semiochemicals, as is the case of secretions for the protection of immature forms described for Linaeidea and Chrysophtharta (Moore 1967; Sugawara et al. 1979), sequestration and use of cantharidin in a mimetic way, through predation of individuals of the Meloidae family (Islami and Nikbakhtzadeh 2009), identification of glands involved in the release of chemical compounds in Leptinotarsa (Daloze et al. 1986), Chrysolina (Laurent et al. 2003) and Chrysomela (Wallace and Blum 1969), defensive compounds, of the monoterpene type, used as markers of choice by the predator, in the plant-herbivore-predator interaction (Sears et al. 2001), tarsal and elytral cuticular compounds, the latter being involved in sex-specific recognition in Gastrophysa (Sugeno et al. 2006; Geiselhardt et al. 2011) and Phaedon (Geiselhardt et al. 2009).
The largest number of interactions described, however, was due to the sequestration of chemical compounds from the host plants, present in Oreina (Ehmke et al. 1999; Pasteels et al. 1988), Platyphora (Pasteels et al. 2001) and Phratora (Pasteels et al. 1983), Kairomones in Gastrolina (Matsuda 1978), Gastrophysa (Matsuda and Matsumoto 1975; Matsuda 1976), Leptinotarsa (Visser et al. 1979), Phaedon (Nielsen 1978; Tanton 1965) and Phratora (Peacock et al. 2001), and defensive compounds released by larvae and adults of Calligrapha (Timmermans et al. 1992), Chrysolina (Pasteels et al. 1979), Chrysomela (Blum et al. 1972; Hollande 1909; Matsuda and Sugawara 1980; Schütz et al. 1997; Termonia and Pasteels 1999), Gastrophysa (Pauls et al. 2016; Sugawara et al. 1979), Gonioctena (Dettner and Schwinger 1987), Plagiodera (Meinwald et al. 1977) and Phratora (Pauls et al. 2016).
Tribe Chrysomelini Latreille, 1802
Gastrophysa Chevrolat, 1836
Popularly known as the japanese green duck leaf beetle, the Gastrophysa atrocyanea Motschulsky, 1860, uses a blend of contact pheromone encountered in the female body as the attraction to copula, 9-methylheptacosane (25), 11-methylheptacosane (26), 13-methylheptacosane (27), 9-methylnonacosane (28), 11-methylnonacosane (29), 13-methylnonacosane (30), 15-methylnonacosane (31) (Sugeno et al. 2006).
Leptinotarsa Chevrolat, 1837
The Leptinotarsa decemlineata Say, 1824, is considered a pest in Solanaceae plantations, such as eggplant, tomato, pepper, tobacco, but mainly potato (Kuhar et al. 2006), has description in the literature. According to Oliver et al. (2002), the aggregation pheromone (S)-3,7-dimetil-2-oxo-6-octene-1,3-diol (32), is released only by males, but recognized by both sexes (Dickens et al. 2002). Cuticular hydrocarbons are also known to L. decemlineata, being the profile of males and females different from each other, being able to play a role of recognition between the sexes (Dubis et al. 1987), in addition to a series of studies that prove insect-plant interaction mediated by kairomones (Bolter et al. 1997; DeWilde et al. 1969; McIndoo 1926; Dickens 1999, 2000; Schanz 1953; Schütz et al. 1997).
Phaedon Dahl, 1828
The Phaedon cochleariae (Fabricius 1792), commonly called mustard beetle, also use the contact pheromone, being 65 compounds identified by Geiselhardt et al. (2009), for both females and males, and this was used for the male to discriminate and chose the female for copulation, since the compounds differ in quantity between the sexes.
Tribe Doryphorini Motschulsky, 1860
Zygogramma Chevrolat, 1836
The Zygogramma bicolorata (Pallister 1953) is mainly used as a control agent for Parthenium, a plant considered toxic to animals, including humans. Qadir et al. (2021) analyzed the cuticular profile of females and males and reported the presence of the chain-length which ranged from C14 to C36. In the study, no sex-specific differences were observed in the CHC profiles, but they did show a difference quantitatively. The authors also observed that the abdominal extracts of the females attracted the males, resulting in their aggregation behavior on the paper disks. The hypothesis raised in the paper then, is that females release short-range sex pheromones to attract males (Qadir et al. 2021).
Subfamily Criocerinae Latreille, 1807
This subfamily has 1500 living species allocated in 3 tribes and 20 genus (Bukejs and Schmitt 2016). Despite it has a cosmopolitan distribution, the vast majority of the species are found in the Subtropical and Tropical regions. Of the three tribes, only Lemiini is represented, which is the largest tribe with eleven genera, including here the two largest genera, Lema, sensu lato, which comprises about 60% of all known species in the subfamily, and Oulema, with 128 species (Vencl and Leschen 2014). Although most species of the subfamily, both in the larval and adult stages, attack the leaf surface, some species may attack ovules or other parts of flowers, and as stem-borers and gall-forming. Some species have become pests of commercial products, such as asparagus, lilies, potatoes, rice, and other grains (Vencl and Leschen 2014).
Tribe Lemiini Heinze, 1962
Oulema Des Gozis, 1886
This genus has a single description of aggregation pheromone, and for a species that is considered an important pest of wheat, barley, and oats, Oulema melanopus (Linnaeus, 1758). The pheromone, (E)-8-hydroxy-6-methyloct-6-en-3-one (33), was first suggested by Cossé et al. (2002), and according to the authors it is released by males but is attractive to both sexes. The following year, Rao et al. (2003), confirmed the pheromone and attested to its attractiveness in the field.
Subfamily Cryptocephalinae Gyllenhal, 1813
With 5.300 species distributed worldwide, Cryptocephalinae has its richness concentrated in the tropical and subtropical regions. Two tribes, Chlamisini Gressitt, 1946 and Clytrini Lacordaire, 1848, have cuticular profiles described each with a genus, Neochlamisus Karren, 1972 and Lachnaea, respectively (Geiselhardt et al. 2011; Rutledge et al. 2014). In addition to these two, the genus Labidostomis, belonging to the Clytrini, is represented by a single known aggregation pheromone (López et al. 2022).
Tribe Clytrini Lacordaire, 1848
Labidostomis lusitanica (Germar, 1824)
The L. lusitanica is a polyphagous species, consuming a variety of plant genera, such as Populus, Polygonum, Quercus, Rumex, and Salix, and is considered a pest of species of economic importance pistachio nuts, Pistacia vera (Anacardiaceae), feeding voraciously on pistachio crops, being capable of defoliate the youngest plants within hours (Gómez et al. 2021). Due to this economic appeal, López et al. (2022) initiated studies to understand the interactions of this species mediated by chemical compounds. It was possible to observe that in the field, males and females aggregate on pistachio trees, and in the laboratory, it was proven that the male emits a compound that causes this behavior, the 2-isobutyl-3-methoxypyrazine (34). In olfactometer tests, both sexes responded to the pheromone (López et al. 2022).
Subfamily Eumolpinae Thomson, 1859
With a cosmopolitan distribution, the subfamily has about 500 genera and more than 7.000 species, with the distribution of these individuals, concentrated mainly in the tropics The adults feed on the outer part of the leaves, mostly dicotyledon, some of which are toxic, while the larvae feed on underground roots, but there are some species that feed on monocotyledon and gymnosperms (Jolivet et al. 2014).
Tribe Eumolpini Hope, 1840
Chrysochus Chevrolat, 1836
The Chrysochus cobaltinus LeConte, 1857 utilizes cuticular hydrocarbon profiles in its close-range sex communication, here the cuticular compounds found in the female’s body, are responsible for stimulating the males to copulate (Peterson et al. 2007). In addition to discovering that the CHCs compounds mediate communication between males and females of the species, the authors tried to understand whether these hydrocarbon profiles would be efficient, to the point of preventing two closely related species that share the same biogeographic region from mating (Supplementary Table 1).
Therefore, they carried out a series of experiments with the two species that form a hybrid zone in Washington State (WA) (U.S.A.), Chrysochus cobaltinus and Chrysochus auratus (Fabricius, 1775), where conspecific dead females have been offered to males with their cuticles intact, removed and painted with hexane, and with male and female cuticular extract of both species (conspecific and heterospecific). Based on the results, where males responded positively to the unmanipulated conspecific female cadavers to the detriment of all other treatments, it was possible to demonstrate that the hydrocarbon profiles playing an important role with respect to the evolution of reproductive isolation (Howard et al. 2003; Peterson et al. 2007). In the bioassays conducted years earlier, it was possible to observe that males from the hybrid zone tend to be more selective than males from outside these zones, just as females are more distinguishable (Peterson et al. 2005), suggesting that this set of reproductive traits are continuously reinforcing sexual isolation through natural selection, thus preventing hybridization (Dobzhansky 1940).
Despite being in a hybrid zone, these two Chrysochus species have different feeding habits, Chrysochus auratus has a restrictive diet, feeding exclusively on Apocynum cannabinum and A. androsaemifolium (Apocynaceae) (Dobler and Farrell 1999; Dussourd and Eisner 1987; Williams 1992), while C. cobaltinus has a broader diet, feeding on Asclepias speciosa and A. eriocarpa (Asclepiadaceae) in addition of the two species of Apocynum (Dickinson 1995; Dobler and Farrell 1999). Therefore, the feeding habit together with species- and sex-specific recognition through CHCs profile, contribute to the reproductive isolation of these two species that are in a hybrid zone.
Costalimaita Bechyně, 1954
Data on Costalimaita ferruginea (Fabricius 1801) were found in two unpublished theses, (R Molinário de Souza, PhD Thesis, Federal University of Viçosa, 2013) and (ME Vieira Xavier, PhD thesis, Federal University of Alagoas, 2019). Both report aggregation behavior in freshly fed Eucalyptus leaves. Despite this, the authors found different results regarding other compounds related to the species, so for the analyses only the data from Xavier (2019) was considered, because it is a more complete work, in our point of view. The compound heptadecane was specific in samples from C. ferruginea females. The compounds tetradecene (35) and (Z)-9-octadecenoic acid (36), were specific to males.
Subfamily Galerucinae Latreille, 1802
The 14.500 currently known species are allocated in 1.100 genus and two tribes, distributed worldwide (Nadein and Bezděk 2014; Nie et al. 2018; Douglas et al. 2023). Adults consume the outer part of leaves, pollen and nectar from female flowers, and the larvae are fruit and leaf miners, and may also feed on the outer part of roots, buboes, and leaves (Nadein and Bezděk 2014).
Tribe Alticini Newman, 1835
This tribe contains approximately 10.000 species allocated in 601 genera (Douglas et al. 2023), and the biodiversity of this taxon is concentrated mainly in the tropics of the southern hemisphere (Nadein and Bezděk 2014). Eleven genera allocated to Alticini have some type of chemical compound described in the literature, ranging from flavonoids (Matsuda 1978), emission of volatiles associated with host plants (Kumano-Nomura and Yamaoka 2009), sesquiterpenes released by males (Bartelt et al. 2001), defensive squirts (Evans et al. 2000), alkaloid sequestration (Dobler et al. 2000), even aggregation pheromones (Beran et al. 2011, 2016; Zilkowski et al. 2006).
Altica Geoffroy, 1762
Altica fragariae (Nakane, 1955) and Altica viridicyanea (Baly, 1874)
In order to understand the roles of the CHC’s profiles in two sympatric species, Xue et al. (2016) have developed some mating bioassays, with females and males of the A. fragariae and A. viridicyanea. The result showed that both males preferred a conspecific female instead of the female of the sister species, and they preferred the females with the CHCs intact, indicating that the CHCs play a crucial role in the process of the male detecting the conspecific female for mating, discrimination between related species, age and to distinguish the sexual mature female from immature one (Xue et al. 2016, 2018).
Altica litigata Fall, 1910
Known to cause damage to plants in the Lythraceae and Onagraceae families, Altica litigata, has had its pheromones investigated (Carruthers et al. 2011) and two molecules have been proposed, (6R7S)-himachala-9,11-diene (37) and (6R,7S)-2,2,6-trimethyl-10-methylene-bicyclo[5.4.0]-undec-1,11-ene (38), both of which are emitted by males of this species.
Aphtona Chevrolat in Dejean, 1836
Bartelt et al. (2001), investigated the pheromones of some species of Aphthona Chevrolat, 1836, namely, A. czwalinae Weise 1888, A. cyparissiae (Koch 1803), and A. flava Guillebeau, 1894. These species are known to consume leafy spurge, Euphorbia esula Linnaeus, 1753, species belonging to the Euphorbiaceae family. The results showed that the possible pheromones of the studied species of Aphthona were a himachalane-type terpenes, being two new enantiomers hydrocarbons (6R,7S)-himachala-9,11-diene (37) and (6R,(6R,7S)-2,2,6-trimethyl-10-methylene-bicyclo[5.4.0]-undec-1,11-ene (38), trans-α-himachalene (39), γ-cadinene (40) and (R)-ar-himachalene (41), one norsesquiterpene ketone (1S,2R)-2,6,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (42) and two alcohols, (3R,9R,9aS)- and (3S,9R,9aS)-3,5,5,9-tetramethyl-2,3,5,6,7,8,9,9a-octahydro-1H-benzo[7]annulen-3-ol (43 and 44), found in the three species studed (Bartelt et al. 2001).
According to Gassmann et al. (1996) these species have an overlapping distribution in Eastern Europe and share the same host plants, however there are two distinct groupings. A. cyparissiae and A. flava are brownish species and are preferentially distributed in open and dry areas, while A. czwalinae are black and are found preferentially in closed and humid areas. In his taxonomic revision, Konstatinov (1998), establishes taxonomic groups that reflect these ecological differences, in addition to other morphological ones, being the cyparissiae group for the species A. cyparissiae and A. flava and the hammarstroemi group for A. czwalinae. Such conditions together, and possibly behavioral aspects, with the differences found by Bartelt et al. 2001 for the blends of each species prevent cross-attraction between them.
Epitrix Foudras in Mulsant, 1859
Epitrix fuscula Crotch, 1873, had its compounds elucidated by Zilkowski et al. (2006). They proposed six specific male compounds, the two most abundant were (2E,4E,6Z)- and (2E,4E,6E)-nona-2,4,6-trienal (45 and 46). The other four minor compounds are himachalane-type sesquiterpenes, two of them are hydrocarbons, (6R,7S)-himachala-9,11-diene (37) and (6R,7S)-2,2,6-trimethyl-10-methylene-bicyclo[5.4.0]-undec-1,11-ene (38), and the other two are alcohols, (3R,9R,9aS)- and (3S,9R,9aS)-3,5,5,9-tetramethyl-2,3,5,6,7,8,9,9a-octahydro-1H-benzo[7]annulen-3-ol (43 and 44). Two years late, Zilkowski et al. (2008) indicated that Epitrix hirtipennis (Melsheimer 1847) also emits a blend of 2,4,6-nonatrienals, namely (2E,4E,6Z)-, (2E,4E,6E)-, (2E,4Z,6Z)-, (2Z,4E,6E)-, (2Z,4E,6Z)- and (2E,4Z,6E)- nona-2,4,6-trienall (45–50).
Longitarsus Berthold, 1827
The Longitarsus jacobaeae (Waterhouse 1858) was studied by Zhang and McEvoy (1994). It was possible to observe in the laboratory and field bioassays that male individuals were attracted when filter paper or tansy ragwort leaves, Senecio jacobaea Linnaeus, 1753, were offered right after the females were exposed, indicating that these would be responsible for the attraction of the opposite sex. No pheromone or structure was suggested by the authors, only the indication that the female would be responsible for the emission and that this would be a possible aggregation pheromone.
Phyllotreta Chevrolat in Dejean, 1836
Phyllotreta cruciferae (Goeze, 1777)
The first works indicating that the male crucifer flea beetle, P. cruciferae, released aggregation pheromones, this being capable to attracts both sexes when the males are feeding on host plant, were carried out by Peng and Weiss (1992) and Peng et al. (1999), through bioassays carried out in the laboratory and in the field. Only two year later, Bartelt et al. (2001) proposed six male-specific sesquiterpenes as candidates for aggregation pheromone of P. cruciferae.
Posteriorly the syntheses of some himachalene-type sesquiterpenes are proposed by the Muto et al. (2004) and Mori (2005). Soroka et al. (2005) performed a series of field experiments with males and female of P. cruciferae. They observed that individuals were attracted by the pheromone compound and allyl isothiocyanate, the latter coming from Brassica napus Linnaeus, the host plant of the beetles. The authors noticed a considerable increase in attractiveness when they offered the pheromone mixture and allyl isothiocyanate together.
Tóth et al. (2011) realized similar field test, using pure enantiomers of P. cruciferae pheromone components (6R,7S)-himachala-9,11-diene (37), (6R,7S)-2,2,6-trimethyl-10-methylene-bicyclo[5.4.0]-undec-1,11-ene (38), ( +)-α-himachalene (39), γ-cadinene (40) and (R)-ar-himachalene (41), (1S,2R)-2,6,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (42), in combination with isothiocyanate, and obtained results similar to those found by Soroka et al. (2005). The authors observed that the compound (6R,7S)-himachala-9,11-diene (37) alone was as attractive as the mixture, indicating that this is the main compound of the pheromone of P. cruciferae (Tóth et al. 2005).
Phyllotreta pusilla Horn, 1889
The P. pusilla, another crop pest, has the male aggregation pheromone described, when Bartelt et al. (2001) proposed six new sesquiterpenes for the Phyllotreta genus, being five for the P. pusilla, (1S,2R)-2,6,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (42), (3S,9R,9aS)- and (3R,9R,9aS)-5,5,9-trimethyl-2,3,5,6,7,8,9,9a-octahydro-1H-benzo[7]annulene-3-carbaldehyde (51 and 52), (4aS,5R,8aS)-5-methyl-8a-(prop-1- en-2-yl)-3,4,4a,5,6,7,8,8a-octahydronaphthalene-2-carbaldehyde (53), (9R,9aS)-5,5,9-trimethyl-5,6,7,8,9,9a-hexahydro-1H-benzo[7]annulen-3-yl]methanol (54) and (9R,9aS)-5,5,9-trimethyl-5,6,7,8,9,9a-hexahydro-1H-benzo[7]annulene-3-carbaldehyde (55).
Phyllotreta striolata (Fabricius, 1803)
This species is considered an important pest of Brassicaceae in North America, as well in Southeast Asia. Beran et al. (2011), using the Taiwanese P. striolata population, found that the males emitted volatiles by feeding and these volatiles can attract males and females in the field. Therefore, the authors analyzed the compounds and found that six sesquiterpenes were emitted by feeding males, (6R,7S)-himachala-9,11-diene (37), ( +)-α-himachalene (39), γ-cadinene (40) (R)-ar-himachalene (41), trans-α-himachalene (56), and β-himachalene (57), being the sesquiterpene 37 was active male-specific compound. Field tests were developed for verified the of the compound of both host plants and males. Based on the results, where the synthetic pheromone required the concomitant presence of the host plant's volatile, allyl isothiocyanate, to be attractive, the authors hypothesized that the adults respond to the pheromone only when the specific host volatiles are present (Beran et al. 2011).
In this same year, Bartelt et al. (2001) studied the American P. striolata population, and founded more three specific-males compounds, an sesquiterpenoid ((3S,9R,9aS)-3-hydroxy-3,5,5,9-tetramethyl-5,6,7,8,9,9ahexahydro-1H-benzo[7]annulen-2(3H)-one (58), being the major compound, (6R,7S)-2,2,6-trimethyl-10-methylene-bicyclo[5.4.0]-undec-1,11-ene (38) and (1S,2R)-2,6,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (42), both detected as minor compound. Following these results, Beran et al. (2016) reanalyze the compounds emitted by the Taiwanese population of P. striolata and indicate that the blends of male sesquiterpenoids are qualitatively different between the Taiwanese and American populations.
Beran et al. (2016) identified an evolutionarily new family of terpene synthesis genes in P. striolata, one of which is directly involved in the biosynthesis of the male aggregation pheromone. Still in this work (Beran et al. 2016), the authors, through a phylogenetic analysis of the genes, suggest that the trans isoprenyl diphosphate synthase gene family was present in the ancestor of Galerucinae, having this functionally diversified.
Phyllotreta vittula (Redtenbacher, 1849)
Tóth et al. (2011), indicated that P. vittula and P. cruciferae (European population) had similarity regarding pheromones, as P. vittula responded to the majority compound emitted from the male of P. criciferae, the (6R,7S)-2,2,6-trimethyl-10-methylene-bicyclo[5.4.0]-undec-1,11-ene (38). It is worth mentioning that, in the test field, P. cruciferae prefers the major compound, plus the 3-butenyl isothiocyanate, the host plant compounds, whereas P. vittula prefers the major compound in conjunction with allyl isothiocyanate, also the host plant compounds. The another’s compounds found in the P. vittula extracts are (6R,7S)-himachala-9,11-diene (37), γ-cadinene (40) (R)-ar-himachalene (41), (1S,2R)-2,6,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (42) and trans-α-himachalene (56), being the bouquet an aggregation pheromone.
Tribe Galerucini Latreille, 1802
This tribe counts with 4.500 species allocated in 499 genus (Douglas et al. 2023). There are a diversity of chemical interactions described for Galerucini, elytral cuticular hydrocarbons and tarsal secretions (Geiselhardt et al. 2011), anthraquinones deposited together with eggs to prevent predation, which is carried out mainly by ants (Hilker and Schulz 1991; Hilker et al. 1992) and floral volatiles to attract pollinators (Andrews et al. 2007), and aggregation pheromone in Diorhabda elongata (Brullé 1832), Galerucella calmariensis (Linnaeus, 1767) and Galerucella pusilla (Duftschmid 1825) (Bartelt et al. 2006; Cossé et al. 2005).
Acalymma Barber, 1947
Popularly known as striped cucumber beetle, the Acalymma vittatum (Fabricius, 1775) is the cause of great economic losses in cucumber and melon cultivars. Given its economic appeal, Morris et al. (2005) investigated the pheromone of this species, and an ꞵ-lactone, vittatalactone, was suggested as a compound emitted by the male, activating aggregation behavior in females. The structure was determined to be vittatalactone, (3R,4R)-3-methyl-4-(1,3,5,7-tetramethylloctyl)oxetan-2-one (59), using GC–MS combined with microderivatization techniques, and NMR (Morris et al. 2005).
Diabrotica Chevrolat, 1836
Among all the genera allocated in Galerucinae, Diabrotica draws attention for the number of studies, more than 30. The justification is given by the number of species that are considered of economic importance, as is the case of Diabrotica virgifera LeConte, 1868, which in its larval stage is considered the main pest of corn in the USA (Eben 2022). If we compare with the current number of species allocated to Diabrotica, around 400 (Derunkov and Konstantinov 2013; Eben 2022), the number of taxa with pest status becomes negligible, but the fact is that the genus now has greater attention for preying on items that are considered commodities, especially in countries that are large producers, such as the USA, the main corn exporter with a record crop valued at US$ 18.7 billion in 2021 (U.S. Department of Agriculture n.d).
Diabrotica balteata LeConte, 1865
In 1987, the sex pheromone released by females of D. balteata, was identified as 6,12-dimethylpentadecan-2-one (60) (Chuman et al. 1987). Field trials with traps were performed by McLaughlin et al. (1991), and the results indicated that the bioactive form of this sex pheromone is (R,R)- 6,12-dimethylpentadecan-2-one.
Diabrotica cristata (Harris, 1836)
The synthetic pheromone compound from D. virgifera virgifera proved to be attractive to D. virgifera zeae Krysan & Smith, 1980 and D. porracea Harold, 1875, both belonging to the virgifera species group, with stereoisomerism of this compound causing the specific response among the taxa in the cluster (Guss et al. 1983a, 1984). D. cristata, also belonging to this group, is an exception, with males being attracted to the compound (2S,8R)-8-methyldecan-2-yl acetate (61) (Guss et al. 1983a).
Diabrotica undecimpunctata howardi Barber, 1947
Sex pheromones released by females were described for D. undecimpunctata howardi Barber, 1947, and the synthetic compound 10-methyltridecan-2-one (62) is attractive to males of D. undecimpunctata undecimpunctata Mannerheim, 1843 and D. undecimpunctata duodecimnotata (Guss et al. 1983b).
In addition to sex pheromone-mediated interactions, Diabrotica spp. also has kairomones as mediators (Ventura et al. 2000; Marques et al. 2009). Metcalf et al. (1980) demonstrated that some bitter substances, characteristic of the Cucurbitaceae, act as kairomones in relation to some species of Diabrotica. These toxic substances, which emerged as repellents against herbivory, became phagostimulants, indicating a coevolutionary association between Cucurbitaceae and Diabrotica, which, according to the authors, has been regulated by a single group of terpenoids, the cucurbitacins, acting on host selection, and by a series of volatile phenylpropanoids that stimulate pollen collection, thus fertilizing cucurbits (Metcalf et al. 1980; Metcalf 1994).
Diabrotica virgifera virgifera LeConte, 1868
Despite having been indicated by Ball and Chaudhury (1973) and Guss (1976), the first sex pheromone for Diabrotica was only described in 1982, with the female of D. virgifera virgifera, responsible for the release of the propionate compound of 8-methyldecan-2-yl propanoate (63) (1982).
Diorhabda Weise, 1883
The mediterranean tamarisk beetle Diorhabda elongata, is used as biological control the saltcedar, Tamarix spp., species that is exotic and invasive in the US, causing annual losses of about $285,000,000 (Zavaleta 2000). Cossé et al. (2005) investigated the possible pheromones through the analysis of the volatiles collected from the males and females. The authors founded that the males produced two compounds, (2E,4Z)-hepta-2,4-dienal (64) and (2E,4Z)-hepta-2,4-dien-1-ol (65), that elicited antennae of males and females. In field tests, the compound 65 was more attractive than the mixture of both compounds, in a 1:1 ratio, with males and females attracted in a similar proportion, confirming the authors' hypothesis that these would be aggregation pheromone (Cossé et al. 2005).
Galerucella Crotch, 1873
Galerucella calmariensis (Linnaeus, 1767), G. pusilla (Duftschmidt 1825) and G. tenella (Linnaeus, 1971)
Galerucella calmariensis, Galerucella pusilla and Galerucella tenella occur throughout Europe (except the very north) and their geographic distribution seem to follow their exclusive/primary host plants (Blossey 1995; Fors et al. 2015; Manguin et al. 1993). Galerucella calmariensis and G. pusilla are monophagous species that feed exclusively on Lythrum salicaria Linnaeus, 1753, (Lythraceae) while G. tenella is a polyphagous species that primarily feeds on Filipendula ulmaria and other Rosaceae species (Borghuis et al. 2009; Fors et al. 2015). The occurrence of G. calmariensis and G. pusilla species overlap in location, ecological niche, and time of year in the native Europe (Manguin et al. 1993). It is not clear in the literature how much overlap in geographical distribution exists between G. calmariensis/G. pusilla and G. tenella in their native range, but these three species seem to have been collected in areas of Sweden where their primary/exclusive host-plant species are present within close distance (Fors et al. 2015). Galerucella calmariensis and G. tenella were introduced into the United States in the early 1990`s as biological control agents for the invasive L. salicaria (Hight et al. 1995).
There are reports in the literature that G. calmariensis, G. pusilla and G. tenella produce the same compound as aggregation pheromone. Bartelt et al. (2006) studied volatiles compounds of Galerucella calmariensis and Galerucella pusilla, and found out that males of these two species produce the dimethylfuran lactone, 12,13-dimethyl-5,14-dioxabicyclo[9.2.1]-tetradeca-1(13),11-dien-4-one (66), as aggregation pheromone. This compound from natural and synthetic sources elicited responses on females and males of the two species in both laboratory and field settings (Bartelt et al. 2006, 2008). On a later study, Fors et al. (2015) investigated the production of and response to the dimethylfuran lactone in five species of Galerucella, including G. tenella, G. calmariensis and G. pussila. They also detected the lactone 66 in volatile collections of male G. tenella. The behavioural attraction of male and female G. tenella to the synthetic lactone 65 were confirmed with olfactometer assays in the laboratory (Fors et al. 2015). Although these three species produce and respond to the same pheromone compound and have similar geographical distributions, cross-attraction between G. tenella and G. calmariensis/G. pusilla is unlikely due to differences in ecological niche. In fact, the attraction of G. pusilla and G. tenella to the lactone 65 is synergized by their respective host-plant odours in laboratory settings (i.e., there is a synergistic effect between the lactone 65 and L. salicaria odour in the attraction of G. pusilla and F. ulmaria odour in the attraction of G. tenella) (Fors et al. 2015). The reproductive isolation between G. calmariensis and G. tenella may be at least partially maintained by differences in several morphological characters between these two species, such as colour, size, and male genitalia (Manguin et al. 1993).
Pheromone production in G. calmariensis seems to be related to feeding on L. salicaria. Bartelt et al. (2008) investigated this relationship between pheromone production in G. calmariensis and host plant feeding by transferring pheromone producing males feeding on L. salicaria to roses and willows as an alternative food source in the laboratory. They observed that pheromone emission ceased when beetles were transferred to the alternative hosts. In addition, beetle survival rate was lower when feeding on the alternative hosts in comparison to L. salicaria. Pheromone emission returned to typical levels a week after survivor beetles feeding on rose and willow were transferred back to L. salicaria (Bartelt et al. 2008). This is an indication that pheromone production in G. calmariensis, G. pusilla and G. tenella could be related to the sequestration and metabolization of compounds from the primary/exclusive host plant since these three species produce and respond to the same pheromone compound and they have similar molecular characteristics (Borghuis et al. 2009; Hambäck et al. 2013).
Pyrrhalta Joannis 1865
Pyrrhalta aenescens Fairmaire, 1878 and Pyrrhalta maculicollis (Motschulsky, 1853)
Widely distributed in eastern Asia, both species are considered a serious pest of elm trees. Although they are very similar with respect to morphology, genitalia, and molecular studies have been able to distinguish them (Nie et al. 2012). Zhang et al (2014) investigated mate recognition via cuticular hydrocarbons in order to understand how the isolation of these two sympatric species occurs. Mating experiments revealed that is a strong sexual isolation between them, where males preferred the conspecific females with intact CHCs and conspecific CHCs reapplied after the process of removal, and heterospecific females when they have been treated with the CHCs of the conspecific females. Furthermore, chemical analyses have shown that the hydrocarbon profiles vary between the two species, P. aenescens profile consisted of mono-methyl-branched alkanes between C22 and C29, while P. maculicollis dimethyl-branched alkanes between C29 e C35 (Zhang et al. 2014).
The Neglected Taxa
The following subfamilies have some kind of investigation regarding chemical compounds but not a description of pheromones. Some of these neglected taxa make up a large majority of Chrysomelidae species, such as Cassidinae, so future research regarding pheromones of these lineages is necessary for understanding the evolution of compounds within the family.
The Cassidinae is the second largest subfamily of Chrysomelidae (Fukumori et al. 2022), with approximately 6.000 species described with worldwide distribution, nevertheless most species of this subfamily are found in the Neotropical region (Chaboo 2007). Despite the diversity of this group, there is only one record of cuticle chemical compounds identified in this subfamily. Geiselhardt et al. (2011) investigated the chemical composition of tarsi and elytra SPME samples of Cassida viridis Linnaeus, 1758 and 34 other Coleoptera species, that have convergently developed widened tarsi for substrate adhesion. Their results indicate that there are no substantial differences in the chemical composition of tarsal and elytral samples in all species studied. All chemical components identified in this study are characteristic for insect cuticular lipids, such as saturated, unsaturated, and methyl-branched hydrocarbons (Geiselhardt et al. 2011). Therefore, no pheromones have been described to date in this subfamily.
The Criocerinae has two genera in which interactions mediated by chemical cues have been described, namely Lema and Oulema. Plant–insect chemical mediated interactions involving the single species of the genus Lema, namely L. unicolor Clark, 1866, have been suggested by Pedersen et al. (2013) as it was observed visiting flowers of the orchid Luisia curtissi Seidenf, 1997 in northern Thailand. However, no pheromones have been identified for L. unicolor. In the other hand, Oulema has the aggregation pheromone produced by male Oulema melanopus (L.) identified by Cossé et al. (2002), and its activity has been confirmed in both laboratory and field settings (Cossé et al. 2002; Rao et al. 2003).
The Donacia brevicornis Ahrens, 1810 and D. marginata Hoppe, 1795, both species pertaining to the subfamily Donaciinae Kirby, 1837, are studied by Geiselhardt et al. (2011). As a result, there is a chemical congruence between elytral and tarsal cuticular lipids for a whole grouping of species that have enlarged tarsi and specialized attachment structures. However, this pattern did not hold in a phylogenetic context suggesting that this congruence is not conserved within Donaciini. A similar result was obtained by Martin and Drijfhout (2009), when investigating the similarity of cuticular hydrocarbons in Formicidae, in the light of a hypothetical phylogenetic relationships.
The Synetinae, has only two genera, Syneta Dejean, 1835, and Thricolema Crotch, 1874, the first with eleven species and the second with only one. Syneta has a Holarctic distribution, while Thricolema occurs in California and Oregon (Silfverberg 2010). About chemical communication, the only mention to the subfamily is by Valkama et al. (1997), which describe the attractiveness of the Ips typographus (Curculionidae, Scolytinae) pheromone trap, Ipslure® (2-methyl-3-buten-2-ol, cis-verbenol and ipsdienol). As a result, 40. 884 individuals belonging to 53 species and 18 families were captured, including Syneta betulae (Fabricius, 1792), with five individuals captured.
Pheromones Through the Lineages of Chrysomelidae
Based on the knowledge we have so far about Chrysomelidae, it is possible to affirm that both females and males are responsible for the emission of pheromones (Supplementary Table 1), but when we analyze them in relation to their respective functions, the aggregation pheromones are mostly related to males, with a single exception Longitarsus jacobeae (Alticini), while the contact and sexual pheromones are mostly found in females, except for a single lineage of Bruchinae composed of Acanthoscelides obtectus and Bruchus rufimanus (Bruchinae), both emit sexual pheromones (Supplementary Table 1 and Fig. 6). In the outgroup this pattern is only repeated in Hedypathes betulinus (Cerambycidae: Lamiinae).
Exploring the chemical structures of the pheromones, we found that alcohol and ester are exclusive to males. The most representative functional group in the pheromones of Chrysomelidae were the carboxylic acids with ten compounds (8–11, 14–18, 23, 24) and three of them were a diacids (9–11), used by Callosobruchus analis, C. chinensis, C. maculatus, C. subinnotatus and Caryedon serratus. These unusual compounds as pheromones are polar with lower volatility than similar compounds containing other functional groups like alcohols, esters, and aldehydes. These pheromones are probably left on the trail to direct individuals of the opposite sex to the meet. Fatty acid/polyketide-derived from acetate pathway and terpenes were also present in the pheromones including an allene (1) from Acanthoscelides obtectus, homosesquiterpene, (2E)- and (2Z)-homofarnesals (11 and 12), from Callosobruchus rhodesianus and a norsesquiterpenes, (1S,2R)-2,6,6-trimethylbicyclo[5.4.0]undec-7-en-9-one (42), from Aphthona cyparissiae, A. czwalinae, A. flava, Phyllotreta cruciferae, P. pusilla, P. striolata and P. vittula.
Galerucini is the second major group, after Bruchini, where the females are responsible for the production of sexual pheromones, with four species allocated only in Diabrotica (Supplementary Table 1). Interestingly, all the four species of Diabrotica presented a high similarity in the pheromones, being two methyl-branched ketones (59 and 61) in D. balteata and D. undecimpunctata howardi, and two esters from the reduction of the methyl-branched ketones to secondary alcohols (60 and 62) in D. cristata and D. elongata. This distinction in functional groups corroborates two distinct lineages present in the genus, poliphagous species from fucata group with D. balteata and D. undecimpuctata and oligophagous from virgifera group with D. cristata and D. virgifera virgifera (Eben and Espinosa de Los Monteros 2013).
In the Alticini we noticed the opposite, males are the sex predominantly responsible for the emission, with nine species allocated in the Aphthona, Epitrix and Phyllotreta. Interestingly, the male-specific pheromones reported for the species were cyclic terpenes, himachalane-type sesquiterpenes and γ-cadinene (37–44, 51–58) present in E. fuscula, P. striolata, P. cruciferae, A. czwalinae, A. cyparissiae, and A. flava. These compounds are different in the unsaturation position and the presence of oxygenated groups but come from the same biosynthetic pathway.
Terminals, Characters, and Phylogenetic Analysis
Beyond the study of pheromones for pest application, we use these compounds as characters in a phylogenetic hypothesis, to try to understand how the evolution of these groups occurs. Most commonly found in the literature are phylogenetic trees using morphology, molecular data, or a combination of both to elucidate internal relationships and understand the evolution of taxons. Recently the use of pheromones in phylogenetic analysis has been raised, especially in Lepidoptera, where the study of semiochemicals is more advanced, but for the Chrysomelidae, this is the first investigation.
A phylogenetic tree is a diagram-like representation that deals with the relationships of evolutionary descent among populations, species, or higher taxa (Wiley and Lieberman 2011). In this representation, the lines represent species over time and the joining of these lines, nodes, the moments of speciation, i.e., the moment when a parent species splits into two or more daughter species, or cladogenesis from a common ancestor (Wiley and Lieberman 2011). Other elements that can be added to this representation are the characters, which can be of different origins (morphological, molecular, ecological), and their conditions in each taxon indicating how the descent of such characteristics occurs (Hinchliff et al. 2015). Thus, a tree, a phylogenetic hypothesis, can then be proposed through analysis with the purpose of knowing the evolutionary history of a taxon and thus produce classifications that reflect such relationships within this group (Huson and Bryant 2006). Another result to be analyzed may be its predictive potential, since it is possible to assume the condition of a taxon according to the evolution of the group in which it is found or is evolutionarily related (Bush 2001). However, for this, one should aim for a greater representativeness of the taxa in question.
A complete table of the species and characteristics related to pheromones is provided in the supplementary material (Supplementary 2). The comparison of the taxa conditions enabled the construction of a matrix (Supplementary 3) with 68 characters that follow. The characters are discussed according to the optimizations made in the reference topology (Fig. 5).
We emphasize that the investigation of morphological characters related to the production of pheromones such as the positioning of the glands was not possible. The presence of these characters would substantially modify the coding structure since they would make some characters dependent on them while others would appear as inapplicable.
List of Characters
-
1.
Male pheromone emitter: (0) absent; (1) present. The data examined indicate that males may or may not produce pheromones while females seem to obligately produce sexual contact pheromones (see character 5). Thus, the presence of pheromone production by males does not exclude pheromone production by the female (next character). Such a character has 12 steps, that is, 12 changes throughout evolution, being the highest number of steps among all characters.
-
2.
Female pheromone emitter: (0) absent or unchecked; (1) present.
-
3.
Aggregation pheromone: (0) absent or unchecked; (1) present. Males mostly produce aggregation pheromones, the exceptions here being only Bruchus and Acanthoscelides from Bruchinae as well as Hedypathes (Cerambycidae) in the outgroup.
-
4.
Sexual pheromone: (0) absent or unchecked; (1) present. Females mostly produce sex pheromones, the exceptions here being only Longitarsus de Alticini.
-
5.
Pheromone, sexual, contact: (0) absent or unchecked; (1) present. It is noteworthy that the species of Callosobruchus and Myllocerinus (Sun et al. 2017) also produce distance sex pheromone (character 6). Which makes us hypothesize that possibly all females produce at least sexual contact pheromones. A significant portion of the species present questions for this character since there are no tests for this purpose.
-
6.
Pheromone, sexual, distance: (0) absent or unchecked; (1) present. For males, sex pheromones are presented exclusively as distance pheromones, unlike females who produce distance and/or contact sex pheromones according to their molecular weight. However, investigations in this regard deserve to be carried out.
-
7.
Pheromone, biosynthesis, acetate: (0) absent; (1) present. The three distinct biosynthesis found, acetate, terpene (character 21) and nitrogenous metabolites (character 28) are not mutually exclusive since each taxon can produce compounds from different routes.
-
8.
Pheromone, biosynthesis, acetate, type: (0) acyclic; (1) cyclic. Although there is the possibility of more than one type occurring in the set of compounds of a species, we did not find such a condition.
-
9.
Pheromone, biosynthesis, acetate, methyl-branched: (0) absent; (1) present.
-
10.
Pheromone, biosynthesis, acetate, starter unit, acetyl: (0) absent; (1) present.
-
11.
Pheromone, biosynthesis, acetate, starter unit, propionyl: (0) absent; (1) present.
-
12.
Pheromone, biosynthesis, acetate, extender units, 2: (0) absent; (1) present. Produced only by male Diorhabda of Galerucini.
-
13.
Pheromone, biosynthesis, acetate, extender units, 3: (0) absent; (1) present.
-
14.
Pheromone, biosynthesis, acetate, extender units, 4: (0) absent; (1) present. Produced only by male Acallymma of Galerucini and Acanthoscelides of Bruchinae.
-
15.
Pheromone, biosynthesis, acetate, extender units, 5: (0) absent; (1) present. Produced only by male Bruchus of Bruchinae as well as Oryzaephilus (Silvanidae) of the outgroup (Pierce et al. 1985).
-
16.
Pheromone, biosynthesis, acetate, extender units, 6: (0) absent; (1) present. Produced only by male Diorhabda of Galerucini, Epitrix of Alticini and Bruchus of Bruchinae.
-
17.
Pheromone, biosynthesis, acetate, extender units, 7: (0) absent; (1) present. Produced only by male Caryedon of Bruchinae as well as Myllocerinus (Curculionidae) in the outgroup.
-
18.
Pheromone, biosynthesis, acetate, extender units, 8: (0) absent; (1) present.
-
19.
Pheromone, biosynthesis, acetate, extender units, 9: (0) absent; (1) present. Produced only by female Gastrophysa of Chrysomelinae and Callosobruchus rhodesianus of Bruchinae.
-
20.
Pheromone, biosynthesis, acetate, extender units, 13: (0) absent; (1) present. Produced only by female Tetropium (Cerambycidae) in the outgroup (Silk et al. 2007; Silk et al. 2011).
-
21.
Pheromone, biosynthesis, terpene: (0) absent; (1) present.
-
22.
Pheromone, biosynthesis, terpene, type: (0) acyclic; (1) cyclic.
-
23.
Pheromone, biosynthesis, terpene, monoterpene: (0) absent; (1) present. Produced only by male Leptinotarsa of Chrysomelinae.
-
24.
Pheromone, biosynthesis, terpene, triterpene: (0) absent; (1) present. Produced only by male Araecerus (Anthribidae) in the outgroup (Yang et al. 2017).
-
25.
Pheromone, biosynthesis, terpene, sesquiterpene: (0) absent; (1) present.
-
26.
Pheromone, biosynthesis, terpene, norsesquiterpene: (0) absent; (1) present. Produced only by male Aphtona and Phyllotreta of Alticini as well as Tetropium and Hedypathes (Cerambycidae) in the outgroup.
-
27.
Pheromone, biosynthesis, terpene, homosesquiterpene: (0) absent; (1) present. Produced only by female Callosobruchus chinensis and C. rhodesianus of Bruchinae.
-
28.
Pheromone, biosynthesis, nitrogenous metabolites: (0) absent; (1) present.
-
29.
Pheromone, functional group, alcohol: (0) absent; (1) present. The alcohol functional group was verified only in males.
-
30.
Pheromone, functional group, alcohol, primary: (0) absent; (1) present.
-
31.
Pheromone, functional group, alcohol, secondary: (0) absent; (1) present.
-
32.
Pheromone, functional group, alcohol, tertiary: (0) absent; (1) present.
-
33.
Pheromone, functional group, alcohol, allylic: (0) absent; (1) present.
-
34.
Pheromone, functional group, aldehyde: (0) absent; (1) present.
-
35.
Pheromone, functional group, amide: (0) absent; (1) present. The amide functional group was verified only in females and until now only in Migdolus (Vesperidae) in the outgroup (Leal et al. 1994).
-
36.
Pheromone, functional group, amine: (0) absent; (1) present. The amine functional group was verified only in males and until now only in Cryptocephalinae.
-
37.
Pheromone, functional group, carboxylic acid: (0) absent; (1) present.
-
38.
Pheromone, functional group, ester: (0) absent; (1) present.
-
39.
Pheromone, functional group, ester, primary: (0) absent; (1) present.
-
40.
Pheromone, functional group, ester, secondary: (0) absent; (1) present.
-
41.
Pheromone, functional group, ester, methyl-ester: (0) absent; (1) present.
-
42.
Pheromone, functional group, eter: (0) absent; (1) present. The eter functional group was verified only in males and until now only in Cryptocephalinae.
-
43.
Pheromone, functional group, hydrocarbon: (0) absent; (1) present.
-
44.
Pheromone, functional group, ketone: (0) absent; (1) present.
-
45.
Pheromone, saturated: (0)absent; (1) present.
-
46.
Pheromone, unsaturated: (0) absent; (1) present.
-
47.
Pheromone, unsaturated, monoene: (0) absent; (1) present.
-
48.
Pheromone, unsaturated, diene: (0) absent; (1) present.
-
49.
Pheromone, unsaturated, triene: (0) absent; (1) present.
-
50.
Pheromone, unsaturated, tetraene: (0) absent; (1) present.
-
51.
Pheromone, unsaturated, hexaene: (0) absent; (1) present.
-
52.
Pheromone 8: (0) absent; (1) present.
-
53.
Pheromone 9: (0) absent; (1) present.
-
54.
Pheromone 11: (0) absent; (1) present.
-
55.
Pheromone 14: (0) absent; (1) present.
-
56.
Pheromone 37: (0) absent; (1) present.
-
57.
Pheromone 38: (0) absent; (1) present.
-
58.
Pheromone 39: (0) absent; (1) present.
-
59.
Pheromone 40: (0) absent; (1) present.
-
60.
Pheromone 41: (0) absent; (1) present.
-
61.
Pheromone 42: (0) absent; (1) present.
-
62.
Pheromone 43: (0) absent; (1) present.
-
63.
Pheromone 44: (0) absent; (1) present.
-
64.
Pheromone 45: (0) absent; (1) present.
-
65.
Pheromone 46: (0) absent; (1) present.
-
66.
Pheromone 56: (0) absent; (1) present.
-
67.
Pheromone 66: (0) absent; (1) present.
-
68.
Pheromone 77: (0) absent; (1) present.
Implication of Pheromones in Phylogenetic Hypotheses
The Bayesian inference analyses with the pheromone dataset did not present difficulties in the mixing of the chains and convergence for the topologies. The two runs were successful in exploring the sample space, presenting ASDSF values below 0.001, ESS values greater than 1000 and PSRF equal to 1.0 for all parameters, without exceptions (Supplementary 3). The partitioning of the morphological data under the homoplasy criterion resulted in a scheme of nine partitions, with homoplasy values varying between 0 and 0.70, plus non-informative characters (Supplementary 2). 25% (17) of the characters had zero homoplasy and approximately 14.7% (10) of the characters were non-informative.
The Fig. 4 shows a majority rule tree resulting from the Bayesian analysis using the pheromone dataset, with posterior probability as branch support. Although the recovered topology does not reflect widely known phylogenetic relationships (Nie et al. 2020a, b; Douglas et al. 2023), analyzes of the pheromone dataset shed new light on our understanding of clade recognition in Chrysomelidae. First, the topology does not support the monophyly of Chrysomelidae and also for the known arrangement of the subfamilies (Fig. 4). For genera with more than one species, Galerucella (Galerucinae, Galerucini), Aphthona and Epitrix (Galerucinae, Alticini) and Chrysochus (Eumolpinae) were recovered as monophyletic. Phyllotreta, are paraphyletic in relation to Aphthona while all other genera are polyphyletic.
Phylogenetic relationship among Chrysomelidae based in the pheromone dataset. Tree resulting from a Bayesian analysis with 68 characters split into eight partitions under homoplasy criterion. Consensus tree with all compatible groups added and posterior probability as support branch. Classification proposed by Bouchard et al. (2011) on the right
Such a result may also be related to factors such as the low sampling in relation to the richness and diversity of the family's eating habits, since the information is concentrated on groups of economic interest. Added to this, such works, for the most part, are incomplete in relation to the complexity that the chemical communication of the taxon in question may become. The revisitation and completeness of this information for the taxa reviewed here is of paramount importance even before the inclusion of new taxa. In this way, we would have a matrix with a smaller number of missing data, making this information base more robust. As an example of this data incompleteness, two of the main stored grain pests, Bruchus pisorum and Decellebruchus atrolineatus (= Bruchidius atrolineatus) (Rees 2007), do not have their chemical compounds identified.
Despite the divergences still present in current phylogenetic hypothesis (Zhang et al. 2022), we assume for the purposes of discussion the clade Sagrinae as a sister lineage to the others (clade Sagrinae (clade Chrysomelinae + clade Eumolpinae)). When analyzing proposed characters on the pre-established topology for the family, considering the positioning of lineages that have no associated pheromones (Chrysomelinae-Timarchini, Donaciinae, Lamprosomatinae, Sagrinae, Spilopyrinae and Synetinae), we obtain a cladogram with 263 steps, consistency index of 25 and retention index of 53 (Fig. 5). In this analysis Chrysomelidae does not show any homoplasy or synapomorphy due to pheromone characters. However, this feature should be better investigated in a comparative way with other families of Phytophaga such as the positioning of the producing glands and derived characters such as sexual behavior.
Phylogenetic relationship among Chrysomelidae based in a supertree (see material and methods for details). Unambiguous state changes under parsimony criteria are represented by filled circles for reversible or non-reversible synapomorphic transformations and by empty circles for homoplastic transformations. Classification proposed by Bouchard et al. (2011) on the right. numbers in parentheses correspond to the pheromone numbers in table 1 and PI: Present and not identified
At the level of the three main clades, we have two homoplasies that supports the clade Eumolpinae and Chrysomelinae (absence of sexual pheromone of distance, 6:0; presence of methyl-branched in acetate biosynthesis, 9:1). First condition with reversal in Diabrotica. In Sagrinae clade also presented on Bruchus rufimanus and Acanthoscelides obtectus as well as Malodon, Tetropium and Hedypathes (Cerambycidae) in the outgroup. Second condition with reversal in Diorhabda elongata and Epitrix. In Sagrinae clade also presented on Callosobruchus subnnotatus, C. maculatus and C. analis as well as Araecerus (Anthribidae) in the outgroup.
Synapomorphies are observed at some levels such as the tribe Alticini (pheromone 37, 56:1; pheromone 38, 57:1 both with reversion in Phyllotreta pusilla and Epitrix hirtipennis and inapplicability in Longitarsus jacobaeae), genera group as Epitrix, Longitarsus and Aphtona (tertiary alcohol, 32:1 with inapplicability in Epitrix hirtipennis and Longitarsus jacobaeae) and the genera Epitrix (pheromone 45, 64:1; pheromone 46, 65:1) and Galerucella (pheromone 66, 67:1) (Fig. 5). In de outgroup the pheromone 77, 68:1 is a synapomorphy that supports the two subfamilies (Spondylidinae and Lamiinae) of Cerambycidae (Fig. 5).
Considering this trend of clusters when analyzing only the pheromone characters, we can deduce that such relationships have the potential to be better clarified if these characters are used to complement morphological, molecular, and ecological characters (Fig. 6).
Conclusion
Chrysomelidae, as well as other phytophagous groups, has been shown to be an excellent model for studying insect-plant interactions. Regarding pheromones, it is evident that the majority described so far in the literature are aggregation pheromones. Nevertheless, the knowledge is very low, regarding pheromones in Chrysomelidae when compared to the nearly 40,000 species described for this family, we credit this fact to the little knowledge regarding natural history for a considerable portion of individuals, small size of individuals compared to other groups belonging to Cerambycidae and Curculionidae, for example, making it difficult to collect volatiles and carry out the experiments, and finally, the interest directed towards the groups that cause damage to grains intended for human consumption, given the economic appeal, massive investment and the expressive number of individuals found in the field, thus facilitating the development of experiments.
Pheromones have proven to be strong candidates as characters for use in phylogenetic analysis, being used most successfully in Lepidoptera, a group that has been and is extensively studied and has a vast knowledge regarding pheromones and other types of communication mediated by chemical molecules. The opposite is observed in Chrysomelidae, where we found a derisory number of papers dealing with pheromones in several subfamilies, such as Cassidinae, Criocerinae and Eumolpinae, and in other subfamilies such as Synetinae, Spilopyrinae, Donaciinae and Sagrinae there is no information, resulting in a low sampling of taxa for analysis. Another problem that was observed is the lack of uniformity in the investigations, where one species has data on cuticle, aerations, bioassays, and others only an arena test, resulting in an analysis with missing data for several important taxa, as is the case of Zabrotes subfasciatus.
Therefore, we strongly suggest that other research groups start working with other lineages that are outside our biogeographic occurrence, such as Palophaginae and Zeugophorinae (subfamilies of Megalopodidae), Philinae and Vesperinae (subfamilies of Vesperidae), Orsodacnidae, Dorcasominae and Spondylidinae (subfamilies of Cerambycidae), Cassidinae, Criocerinae, Donaciinae, Eumolpinae, Sagrinae, Spilopyrinae, and Synetinae (subfamilies of Chrysomelidae), and finally Disteniidae and Oxypeltidae, both belonging to Phytophaga clade. These future studies should be as exploratory as possible by investigating the volatile and cuticular compounds in a transparent way, highlighting possible absences as informative conditions for character coding. This will contribute to future and more robust analyses, not only in Chrysomelidae, but in Phytophaga as a whole.
Although this is the first analysis in Chrysomelidae to use pheromones as a phylogenetic character, much can be observed in agreement with previous phylogenetic analyses (morphological and molecular), thus confirming that pheromones can be used as characters in phylogenetic analyses in conjunction with morphological, ecological, and molecular data, bringing elucidation to the relationships and evolution of organisms in all taxonomic levels. It is certain that, as more pheromones are described, especially from the two scarcer lineages, we will be able to use the chemical characters with more congruence since we will have a larger and more robust elucidative sample.
References
Ando T, Inomata S-i, Yamamoto M (2004) Lepidopteran sex pheromones. In: Schulz S (ed) The chemistry of pheromones and other semiochemicals I. Topics in current chemistry. Springer, Berlin, Heidelberg, pp 51–96. https://doi.org/10.1007/b95449
Andrade SMM, Szczerbowski D, Vidal DM, Allison JD, Zarbin PHG (2019) Mate recognition by the green mate borer, Hedypathes betulinus (Coleoptera: Cerambycidae): the role of cuticular compounds. J Insect Behav 32:120–133. https://doi.org/10.1007/s10905-019-09719-8
Andrews ES, Theis N, Adler LS (2007) Pollinator and herbivore attraction to Cucurbita floral volatiles. J Chem Ecol 33:1682–1691. https://doi.org/10.1007/s10886-007-9337-7
Ball HJ, Chaudhury MFB (1973) A sex attractant of the western corn rootworm. J Econ Entomol 66:1051–1053
Bartelt RJ, Cossé AA, Zilkowski BW, Weisleder D, Momany FA (2001) Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles. J Chem Ecol 27(12):2397–2423. https://doi.org/10.1023/a:1013667229345
Bartelt RJ, Cossé AA, Zilkowski BW, Weisleder D, Grode SH, Wiedenmann RN, Post SL (2006) Dimethylfuran-lactone pheromone from males of Galerucella calmariensis and Galerucella pusilla. J Chem Ecol 32:693–712. https://doi.org/10.1007/s10886-005-9026-3
Bartelt RJ, Cossé AA, Zilkowski BW, Wiedenmann RN, Raghu S (2008) Early-summer pheromone biology of Galerucella calmariensis and relationship to dispersal and colonization. Biol Control 46(3):409–416. https://doi.org/10.1016/j.biocontrol.2008.05.010
Beran F, Mewis I, Srinivasan R, Svoboda J, Vial C, Mosimann H, Boland W, Buttner C, Ulrichs C, Hansson BS, Reinecke A (2011) Male Phyllotreta striolata (F.) produce an aggregation pheromone: identification of male-specific compounds and interaction with host plant volatiles. J Chem Ecol 37:85–97. https://doi.org/10.1007/s10886-010-9899-7
Beran F, Jiménez-Alemán GH, Lin M-Y, Hsu Y-C, Mewis I, Srinivasan R, Ulrichs C, Boland W, Hansson BS, Reinecke A (2016) The aggregation pheromone of Phyllotreta striolata (Coleoptera: Chrysomelidae) revisited. J Chem Ecol 42:748–755. https://doi.org/10.1007/s10886-016-0743-6
Biemont JC, Chaibou M, Pouzat J (1992) Localization and fine structure of the female sex pheromone-producing glands in Bruchidius atrolineatus (Pic) (Coleoptera: Bruchidae). Int J Insect Morphol Embryol 21(3):251–262
Blossey B (1995) Coexistence of two leaf-beetles in the same fundamental niche. Distribution, adult phenology and oviposition. Oikos 74:225–234. https://doi.org/10.2307/3545652
Blum MS, Brand JM, Wallace JB, Fales HM (1972) Chemical characterization of the defensive secretion of a chrysomelid larva. Life Sci 11(II):525–551
Bocak L, Barton C, Crampton-Platt A, Chesters D, Ahrens D, Vogler AP (2014) Building the Coleoptera tree-of-life for >8000 species: composition of public DNA data and fit with Linnaean classification. Syst Entomol 39(1):97–110. https://doi.org/10.1111/syen.12037
Bolter CJ, Dicke M, Van Loon JJA, Visser JH, Posthumus MA (1997) Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J Chem Ecol 23(4):1003–1023. https://doi.org/10.1023/B:JOEC.0000006385.70652.5e
Borghuis A, Van Groenendael J, Madsen O, Ouborg J (2009) Phylogenetic analyses of the leaf beetle genus Galerucella: Evidence for host switching at speciation? Mol Phylogenet Evol 53(2):361–367. https://doi.org/10.1016/j.ympev.2009.07.005
Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CHC, Newton AF, Reid CAM, Schmitt M, Ślipiński SA (2011) Family-group names in Coleoptera (Insecta). ZooKeys 88:1–972. https://doi.org/10.3897/zookeys.88.807
Brezolin AN, Martinazzo J, Muenchen DK, Cezaro AM, Rigo AA, Steffens C, Steffens J, Blassioli-Moraes MC, Borges M (2018) Tools for detecting insect semiochemicals: a review. Anal Bioanal Chem 410:4091–4108. https://doi.org/10.1007/s00216-018-1118-3
Bruce TJ, Martin JL, Smart LE, Pickett JA (2011) Development of semiochemical attractants for monitoring bean seed beetle, Bruchus rufimanus. Pest Manag Sci 67:1303–1308. https://doi.org/10.1002/ps.2186
Bukejs A, Schmitt M (2016) Lilioceris groehni sp. n.: the first authentic species of Criocerinae (Coleoptera, Chrysomelidae) from Baltic amber. Zookeys 618:67–77. https://doi.org/10.3897/zookeys.618.10085
Bush RM (2001) Predicting adaptive evolution. Nat Rev Genet 2:387–392. https://doi.org/10.1038/35072023
Butler C (1609) The Feminine Monarchie: or the Historie of Bees. Oxford
Cai C, Tihelka E, Giacomelli M, Lawrence JF, Ślipiński A, Kundrata R, Yamamoto S, Thayer MK, Newton AF, Leschen RAB, Gimmel ML, Lü L, Engel MS, Bouchard P, Huang D, Pisani D, Donoghue PCJ (2022) Integrated phylogenomics and fossil data illuminate the evolution of beetles. R Soc Open Sci 9:211771. https://doi.org/10.1098/rsos.211771
Carruthers RI, Franc MK, Gee WS, Cosse AA, Grewell BJ, Beck JJ (2011) Volatile emissions from the flea beetle Altica litigata (Coleoptera: Chrysomelidae) associated with invasive Ludwigia hexapetala. Chemoecology 21:253–259. https://doi.org/10.1007/s00049-011-0090-6
Casari AS, Ide S (2012) Coleoptera Linnaeus, 1758. In: Rafael JA, Melo GAR, Carvalho CJB, Casari AS, Constantino R (eds) Insetos do Brasil, Diversidade e Taxonomia. Ribeirão Preto, Holos, pp 453–521
Caxambú MG, Almeida LM (1999) Descrição dos estágios imaturos e redescrição de Lamprosoma azureum Germar (Chrysomelidae, Lamprosomatinae). Revista Brasileira de Zoologia 16(1):243–256. https://doi.org/10.1590/S0101-81751999000500017
Chaboo CS (2007) Biology and phylogeny of the Cassidinae Gyllenhal sensu lato (tortoise and leaf-mining beetles) (Coleoptera: Chrysomelidae). Bull Am Mus Nat Hist 305:1–250. https://doi.org/10.1206/00030090(2007)305[1:BAPOTC]2.0.CO;2
Chaibou M, Pierre D, Biemont JC, Pouzat ET (1993) Existence d’une phéromone sexuelle chez Caryedon serratus: attractivé des femelles et réactivité des mâles. Entomol Exp Appl 67:253–262. https://doi.org/10.1111/j.1570-7458.1993.tb01676.x
Chamorro ML (2014) Lamprosomatinae Lacordaire, 1848. In: Leschen RAB, Beutel RG (eds) Handbook of Zoology. Coleoptera, Beetles. Morphology and Systematics, vol 3. De Gruyter, Berlim, Boston, pp 81–87
Chiluwal K, Kim J, Bae SD, Maharjan R, Park CG (2017) Attractiveness of male azuki bean beetle to the synthetic blends of 2E- and 2Z-homofarnesals. J Asia Pac Entomol 20(4):1183–1189. https://doi.org/10.1016/j.aspen.2017.09.003
Chuman T, Cuss PL, Doolittle RE, McLaughlin JR, Krysan JL, Schalk JM, Tumlinson JH (1987) Identification of female-produced sex pheromone from banded cucumber beetle, Diabrotica balteata LeConte (Coleoptera: Chrysomelidae). J Chem Ecol 13(7):1601–1615
Cork A, Hall DR, Blaney WM, Simmonds MSJ (1991) Identification of a component of the female sex pheromone of Callosobruchus analis (Coleoptera: Bruchidae). Tetrahedron Lett 32:129–132. https://doi.org/10.1016/S0040-4039(00)71236-6
Cossé AA, Bartelt RJ, Zilkowski BW (2002) Identification and electrophysiological activity of a novel hydroxy ketone emitted by male cereal leaf beetles. J Nat Prod 65:1156–1160. https://doi.org/10.1021/np020063q
Cossé AA, Bartelt RJ, Zilkowski BW, Bean DW, Petroski RJ (2005) The aggregation pheromone of Diorhabda elongata, a biological control agent of saltcedar (Tamarix spp.): identification of two behaviorally active components. J Chem Ecol 31(3):657–70. https://doi.org/10.1007/s10886-005-2053-2
Daloze D, Braekman JC, Pasteels JM (1986) A toxic dipeptide from the defense glands of the Colorado beetle. Science 233(4760):221–223. https://doi.org/10.1126/science.233.4760.221
Darwin CR (1871) The descent of man and selection in relation to sex, vol 2, 1st edn. John Murray, London
Derunkov A, Konstantinov A (2013) Taxonomic changes in the genus Diabrotica Chevrolat (Coleoptera: Chrysomelidae: Galerucinae): results of a synopsis of North and Central America Diabrotica species. Zootaxa 3686(3):301–325. https://doi.org/10.11646/zootaxa.3686.3.1
Derunkov A, Prado LR, Tishechkin AK, Konstantinov AS (2015) New species of Diabrotica Chevrolat (Coleoptera: Chrysomelidae: Galerucinae) and a key to Diabrotica and related genera: results of a synopsis of North and Central American Diabrotica species. J Insect Biodiversity 3(2):1–55
Dettner K, Schwinger G (1987) Chemical defence in the larvae of the leaf beetle Gonioctena viminalis L. (Coleoptera: Chrysomelidae). Experientia 43. Birkhäuser Verlag. CH-4010. Basel/Switzerland
DeWilde J, Lambers-Suverkropp KHR, Van Tol A (1969) Responses to airflow and airborne plant odour in the Colorado beetle. Neth J Plant Pathol 75:53–57. https://doi.org/10.1007/BF02137193
Dickens JC (1999) Predator-prey interactions olfactory adaptations of generalist and specialist predators. Agric For Entomol 1:47–54. https://doi.org/10.1046/j.1461-9563.1999.00007
Dickens JC (2000) Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agric For Entomol 2:167–172. https://doi.org/10.1046/j.1461-9563.2000.00065.x
Dickens JC, Oliver JE, Hollister B, Davis JC, Klun JA (2002) Breaking a paradigm: male-produced aggregation pheromone for the Colorado potato beetle. J Exp Biol 205(13):1925–1933. https://doi.org/10.1242/jeb.205.13.1925
Dickinson JL (1995) Trade-offs between postcopulatory riding and mate location in the blue milkweed beetle. Behav Ecol 6(3):280–286. https://doi.org/10.1093/beheco/6.3.280
Dobler S, Farrell BD (1999) Host use evolution in Chrysochus milkweed beetles: evidence from behaviour, population genetics and phylogeny. Mol Ecol 8(8):1297–1307. https://doi.org/10.1046/j.1365-294x.1999.00693.x
Dobler S, Haberer W, Witte L, Hartmann T (2000) Selective sequestration of pyrrolizidine alkaloids from diverse host plants by Longitarsus flea beetle. J Chem Ecol 26(5):1281–1298
Dobzhansky T (1940) Speciation as a stage in evolutionary divergence. Am Nat 74(753):312–321. https://doi.org/10.1086/280899
Douglas HB, Konstantinov AS, Brunke AJ, Moseyko AG, Chapados JT, Eyres J, Richter R, Savard K, Sears E, Prathapan KD, Ruan Y, Dettman JR (2023) Phylogeny of the flea beetles (Galerucinae: Alticini) and the position of Aulacothorax elucidated through anchored phylogenomics (Coleoptera: Chrysomelidae: Alticini). Syst Entomol: 1–26. https://doi.org/10.1111/syen.12582
Dubis E, Malíski E, Dubis A, Szafranrk J, Nawrot J, Poplawski J, Wróbel JT (1987) Sex dependent composition of cuticular hydrocarbons of the Colorado beetle Leptinotarsa decemlineata Say. Comp Biochem Physiol 87A(4):839–843
Duckett CN, Gillespie JJ, Kjer KM (2004) Relationships among the subfamilies of Chrysomelidae inferred from small subunit ribosomal DNA and morphology, with special emphasis on the relationship among the flea beetles and the Galerucinae. In: Jolivet P, Santiago-Blay JA, Schmitt M (eds) New developments in the biology of Chrysomelidae. SPB Academic Publishing, The Hague, pp 3–18
Dussourd DE, Eisner T (1987) Vein-cutting behavior: insect counterploy to the latex defense of plants. Science 237(4817):898–901. https://doi.org/10.1126/science.3616620
Eben A (2022) Ecology and evolutionary history of Diabrotica beetles - overview and update. Insects 13(156):1–11. https://doi.org/10.3390/insects13020156
Eben A, Espinosa de Los Monteros A (2013) Tempo and mode of evolutionary radiation in Diabroticina beetles (genera Acalymma, Cerotoma, and Diabrotica). Zookeys 19(332):207–321. https://doi.org/10.3897/zookeys.332.5220
Ehmke A, Rahier M, Pasteels JM, Theuring C, Hartmann T (1999) Sequestration, maintenance, and tissue distribution of pyrrolizidine alkaloid N-oxides in larvae of two Oreina species. J Chem Ecol 25(10):2385–2395
Endler JA (1993) Some general comments on the evolution and design of animal communication systems. Phil Trans R Soc B: Biol Sci 340:215–225. https://doi.org/10.1098/rstb.1993.0060
Evans PH, Becerra JX, Venable DL, Bowers W (2000) Chemical analysis of squirt-gun defense in Bursera and counter defense by Chrysomelid beetles. J Chem Ecol 26(3):745–754. https://doi.org/10.1023/A:1005436523770
Fabre J-H (1879–1907) Souvenirs Entomologiques: Études sur l'instinct et les murs des insectes. Septième Série. Delagrave, Paris, pp 363–374
Farrell BD (1998) ‘“Inordinate fondness”’ explained: Why are there so many beetles? Science 281(5376):555–559. https://doi.org/10.1126/science.281.5376.555
Farrell BD, Sequeira AS (2004) Evolutionary rates in the adaptive radiation of beetles on plants. Evolution 58(9):1984–2001
Fonseca MG, Zarbin PHG (2009) Mating behaviour and evidence for sex-specific pheromones in Hedypathes betulinus (Coleoptera: Cerambycidae: Lamiinae). J Appl Entomol 133(9–10):695–701. https://doi.org/10.1111/j.1439-0418.2009.01424.x
Fonseca MG, Vidal DM, Zarbin PH (2010) Male produced sex-pheromone of the Cerambycidae beetle Hedypathes betulinus: chemical identification and biological activity. J Chem Ecol 36(11):36–1139. https://doi.org/10.1007/s10886-010-9850-y
Fors L, Liblikas I, Andersson P, Borg-Karlson A-K, Cabezas N, Mozuraitis R, Hambäck PA (2015) Chemical communication and host search in Galerucella leaf beetles. Chemoecology 25:33–45. https://doi.org/10.1007/s00049-014-0174-1
Frérot B, Leppik E (2016) Composition attractive pour la bruche de la féverole. FR3035775A1
Frérot B, Taupin P, Lefranc M (2015) Bruche de la fève sur féverole: des messages chimiques décryptés. Perspect Agric: 60–63
Fukumori K, Oguchi K, Ikeda H, Shinohara T, Tanahashi M, Moriyama M, Koga R, Fukatsu T (2022) Evolutionary dynamics of host organs for microbial symbiosis in tortoise leaf beetles (Coleoptera: Chrysomelidae: Cassidinae). Am Soc Microbiol 13(1):1–17. https://doi.org/10.1128/mbio.03691-21. (e03691-21)
Gassmann A, Schroeder D, Maw E, Sommer G (1996) Biology, ecology, and host specificity of European Aphthona spp. (Coleoptera, Chrysomelidae) used as biocontrol agents for leafy spurge, Euphorbia esula (Euphorbiaceae), in North America. Biol Control 6(1):105–113
Geiselhardt S, Otte T, Hilker M (2009) The role of cuticular hydrocarbons in male mating behavior of the mustard leaf beetle, Phaedon cochleariae (F.). J Chem Ecol 35:1162–1171. https://doi.org/10.1007/s10886-009-9704-7
Geiselhardt SF, Geiselhardt S, Peschke K (2011) Congruence of epicuticular hydrocarbons and tarsal secretions as a principle in beetles. Chemoecology 21:181–186. https://doi.org/10.1007/s00049-011-0077-3
Goloboff PA (2008) Calculating SPR distances between trees. Cladistics 24(4):591–597. https://doi.org/10.1111/j.1096-0031.2007.00189.x
Gómez SR, Gil-Tapetado D, García-Gila J, Blasco-Aróstegui J, Polidori C (2021) The leaf beetle Labidostomis lusitanica (Coleoptera: Chrysomelidae) as an Iberian pistachio pest: projecting risky areas. Pest Manag Sci 78(1):217–229. https://doi.org/10.1002/ps.6624
Goméz-Rodríguez C, Crampton-Platt A, Timmermans MJTN, Baselga A, Vogler AP (2015) Validating the power of mitochondrial metagenomics for community ecology and phylogenetics of complex assemblages. Methods Ecol Evol 6(8):883–894. https://doi.org/10.1111/2041-210X.12376
Gómez-Zurita J, Hunt T, Vogler AP (2008) Multilocus ribosomal RNA phylogeny of the leaf beetles (Chrysomelidae). Cladistics 24:34–50. https://doi.org/10.1111/j.1096-003
Gonçalves RB, Meira OM, Rosa BB (2022) Total-evidence dating and morphological partitioning: a novel approach to understand the phylogeny and biogeography of augochlorine bees (Hymenoptera: Apoidea). Zool J Linn Soc 195(4):1390–1406. https://doi.org/10.1093/zoolinnean/zlab098
Greenfield MD (2002) Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford University Press, p 432
Grimaldi D, Engel MS (2005) Evolution of the Insects, 1st edn. Cambridge University Press, Cambridge
Guss PL (1976) The sex pheromone of the western corn rootworm (Diabrotica virgifera). Environ Entomol 5(2):219–223
Guss PL, Tumlinson JH, Sonnet PE, Proveaux AT (1982) Identification of a female-produced sex pheromone of the western corn rootworm. J Chem Ecol 8(2):545–556
Guss PL, Carney RL, Sonnet PE, Tumlinson H (1983a) Stereospecific sex attractant for Diabrotica cristata (Harris) (Coleoptera: Chrysomelidae). Environ Entomol 12:1296–1297
Guss PL, Tumlinson JH, Sonnet PE, McLaughlin JR (1983b) Identification of a female-produced sex. J Chem Ecol 9(9):1363–1375
Guss PL, Sonnet PE, Carney RL, Branson TF, Tumlinson JH (1984) Response of Diabrotica virgifera virgifera, D. v. zeae, and D. porracea to stereoisomers of 8-methyl-2-decyl propanoate. J Chem Ecol 10(7):1123–1131
Haddad S, McKenna DD (2016) Phylogeny and evolution of the superfamily Chrysomeloidea (Coleoptera: Cucujiformia). Syst Entomol 41(4):697–716. https://doi.org/10.1111/syen.12179
Hambäck PA, Weingartner E, Ericson L, Fors L, Cassel-Lundhagen A, Stenberg JA, Bergsten J (2013) Bayesian species delimitation reveals generalist and specialist parasitic wasps on Galerucella beetles (Chrysomelidae): sorting by herbivore or plant host. BMC Evol Biol 13:1–14. https://doi.org/10.1186/1471-2148-13-92
Hight SD, Blossey B, Laing J, Declerck-Floate R (1995) Establishment of insect biological control agents from Europe against Lythrum salicaria in North America. Environ Entomol 24(4):967–977. https://doi.org/10.1093/ee/24.4.967
Hilker M, Schulz S (1991) Anthraquinones in different developmental stages of Galeruca tanaceti (Coleoptera, Chrysomelidae). J Chem Ecol 17:2323–2332. https://doi.org/10.1007/BF00988011
Hilker M, Eschbach U, Dettneret K (1992) Occurrence of anthraquinones in eggs and larvae of several Galerucinae. Sci Nat 79(6):271–274. https://doi.org/10.1007/BF01175394
Hinchliff CE, Smith SA, Allman JF, Burleigh JG, Chaudhary R, Coghill LM, Crandall KA, Dengc J, Drew BT, Gazis R, Gude K, Hibbett DS, Katzi LA, Dail Laughinghouse H IV, McTavish EJ, Midford PE, Owen CL, Ree RH, Rees JA, Soltis DE, Williamsm T, Cranstonk KA (2015) Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Evolution 11(41):12764–12769. https://doi.org/10.1073/pnas.1423041112
Hollande MA-C (1909) Sur la fonction d’excretionchez les insectes salicicoles et en particulier sur l’existence des derives salicyles. Annales de l’Université de Grenoble 21:459–517
Honda H, Yamamoto I (1976) Evidence for and chemical nature of a sex pheromone present in azuki bean weevil, Callosobruchus chinensis L. Proceedings Symposium on Insect Pheromones and their Applications (Nagaoka and Tokyo), 164
Hope JA, Horler DF, Rowlands DG (1967) A possible pheromone of the bruchid Acanthoscelides obtectus (Say). J Stored Prod Res 3:387–388
Horler DF (1970) (–) Methyl n-tetradeca-trans-2,4,5-trienoate, an allenic ester produced by the male Dried Bean beetle, Acanthoscelides obtectus (Say. J Chem Soc C: Organic 6:859–862. https://doi.org/10.1039/J39700000859
Howard RW, Jackson LL, Banse H, Blows MW (2003) Cuticular hydrocarbons of Drosophila birchii and D. serrata: identification and role in mate choice in D. serrata. J Chem Ecol 29(4):961–976. https://doi.org/10.1023/a:1022992002239
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23(2):254–267. https://doi.org/10.1093/molbev/msj030
Islami I, Nikbakhtzadeh MR (2009) New records of canthariphily among beetles (Coleoptera) from Iran. Turk J Entomol 33(4):243–251
Johnson CD (1981) Interactions between bruchid (Coleoptera) feeding guilds and behavioral patterns of pods of the Leguminosae. Environ Entomol 10:249–253
Johnson CD (1990) Systematics of the seed beetle genus Acanthoscelides (Bruchidae) of northern South America. Trans Am Entomol Soc 116:297–618
Johnson CD, Romero-Nápoles JJ (2004) A review of evolution of ovoposition guilds in the Bruchidae (Coleoptera). Rev Bras Entomol 48(3):401–408. https://doi.org/10.1590/S0085-56262004000300017
Johnson CD, Romero JJ, Raimúndez-Urrutia E (2001) Ecology of Amblycerus crassipunctatus Ribeiro-Costa (Coleoptera: Bruchidae) in seeds of Humiriaceae, a new host family for Bruchids, with an ecological comparision to other species of Amblycerus. Coleopt Bull 55(1):37–48
Johnson CD (1989) Adaptive radiation of Acanthoscelides in seeds: examples of Legume-Bruchid Interactions. In: Shirton CH, Zarucchi JL (eds) Advances in Legume Biology. Monographs in Systematic Botany from the Missouri Botanical Garden, pp 747–779
Jolivet P, Lawrence JF, Verma KK, Ślipiński A (2014) Eumolpinae C. G. Thomson, 1859. In: Leschen RAB, Beutel RG (eds) Handbook of Zoology. Coleoptera, Beetles. Morphology and systematics, vol 3. Walter de Gruyter, Berlin, pp 217–225
Jurenka R (2004) Insect pheromone biosynthesis. In: Schulz S (ed) The Chemistry of Pheromones and Other Semiochemicals I. Topics in Current Chemistry 239. Springer, Berlin Heidelberg. https://doi.org/10.1007/b95450
Jyothi KN, Prasuna AL, Prasad AR, Tewari H (2014) Electrophysiological and behavioural responses of groundnut seed beetle Caryedon serratus (Olivier) (Coleoptera: Chrysomelidae) to conspecific and groundnut seed chemical cues. Afr Entomol 22(3):505–511. https://doi.org/10.4001/003.022.0309
Kawazu K, Ichiki RT, Dang DT, Nakamura S (2011) Mating sequence and evidence for the existence of a female contact sex pheromone in Brontispa longissima (Coleoptera: Chrysomelidae). Jpn Agric Res Q 45(1):99–106. https://doi.org/10.6090/jarq.45.99
Kergoat GJ, Le Ru BP, Sadeghi SE, Tuda M, Reid CA, György Z, Genson G, Ribeiro-Costa CS, Delobel A (2015) Evolution of Spermophagus seed beetles (Coleoptera, Bruchinae, Amblycerini) indicates both synchronous and delayed colonizations of host plants. Mol Phylogenet Evol 89:91–103. https://doi.org/10.1016/j.ympev.2015.04.014
Kingsolver JM (2002) Bruchidae Latreille 1802. In: Arnett RH, Thomas MC, Skelley PE, Frank JH (eds) Amarican Beetles. Polyphaga: Scarabaeoidea though Curculionoidea. CRC, Boca Raton, pp 602–608
Kocienski PJ, Cernigliaro G, Feldstein G (1977) A Synthesis of (±)-Methyl n-Tetradeca-trans-2,4,5-trienoate, an Allenic Ester Produced by the Male Dried Bean Beetle Acanthoscelides obtectus (Say). J Org Chem 42(2):353–355
Konstantinov AS (1998) Revision of the Paleartic species of Aphthona Chevrolat and cladistic classification of the Aphthonini (Coleoptera, Chrysomelidae, Alticinae). Memoirs on Entomology, International. Am Entomol Inst 11:429
Kuhar TP, Mori K, Dickens JC (2006) Potential of a synthetic aggregation pheromone for integrated pest management of Colorado potato beetle. Agric For Entomol 8:77–81. https://doi.org/10.1111/j.1461-9555.2006.00286.x
Kumano-Nomura Y, Yamaoka R (2009) Beetle visitations, and associations with quantitative variation of attractants in floral odors of Homalomena propinqua (Araceae). J Plant Res 122:183–192. https://doi.org/10.1007/s10265-008-0204-6
Landor PD, Landor SR, Mukasa S (1971) Synthesis of (±)-methyl tetradeca-trans-2,4,5-trienoate, the allenic sex pheromone produced by the male dried bean beetle. J Chem Soc D 0(24):1638–1639. https://doi.org/10.1039/c29710001638
Laurent P, Braekman J-C, Daloze D, Pasteels JM (2003) An ecdysteroid (22-acetyl-20-hydroxyecdysone) from the defense gland secretion of an insect: Chrysolina carnifex (Coleoptera: Chrysomelidae). Chemoecology 13:109–111. https://doi.org/10.1007/s00049-003-0235-3
Lawrence JF, Lawrence CAM (2014) Sagrinae Leach, 1815. In: Leschen RAB, Beutel RG (eds) Handbook of Zoology. Coleoptera, Beetles. Morphology and systematics, vol 3. Walter de Gruyter, Berlin, pp 264–270
Leal WS, Bento JMS, Vilela EF, Della Lucia TMC (1994) Female sex pheromone of the longhorn beetle Migdolus fryanus Westwood: N-(2’S)-methylbutanoyl 2-methylbutylamine. Experientia 50(9):853–856. https://doi.org/10.1007/BF01956471
Leschen RAB, Beutel RG (2014) Handbook of Zoology: Arthropoda: Insecta: Coleoptera: Volume 3: Morphology and Systematics (Phytophaga). De Gruyter, Berlin, München, Boston. https://doi.org/10.1515/9783110274462
Liebregts W, Chapman K (2004) Impact and control of the coconut hispine beetle, Brontispa longissima Gestro (Coleoptera: Chrysomelidae), pp 19–34. In Report of the Expert Consultation on Coconut Beetle Outbreak in APPPC Member Countries. FAO Regional Office for Asia and the Pacific
López S, Rodrigo-Gómez S, Fernández-Carrillo E, Corbella-Martorell C, Quero C (2022) 2-Isobutyl-3-methoxypyrazine as a Putative Male-Specific Aggregation Pheromone in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae). Preprints 2022120343. https://doi.org/10.20944/preprints202212.0343.v1
Manguin S, White R, Blossey B, Hight SD (1993) Genetics, taxonomy, and ecology of certain species of Galerucella (Coleoptera: Chrysomelidae). Ann Entomol Soc Am 86(4):397–410. https://doi.org/10.1093/aesa/86.4.397
Marques FDA, Wendler EP, Macedo A, Wosch CL, Maia BHS, Mikami AY, Arruda-Gatti IC, Pissinati A, Mingotte FLC, Alves A, Ventura MU (2009) Response of Diabrotica speciosa (Coleoptera: Chrysomelidae) to 1,4-dimethoxybenzene and analogs in common bean crop. Braz Arch Biol Technol 52(6):1333–1340
Martin S, Drijfhout F (2009) A review of ant cuticular hydrocarbons. J Chem Ecol 35:1151–1161. https://doi.org/10.1007/s10886-009-9695-4
Marvaldi AE, Duckett CN, Kjer KM, Gillespie JJ (2009) Structural alignment of 18S and 28S rDNA sequences provides insights into the phylogeny of Phytophaga and related beetles (Coleoptera: Cucujiformia). Zool Scr 38:63–7710
Matsuda K (1976) Flavonoids as feeding stimulants of the beetles attacking the polygonaceous plants. Tohoku J Agric Res 27(3–4):115–121
Matsuda K (1978) Feeding stimulation of flavonoids for various leaf beetles (Coleoptera, Chrysomelidae). Appl Entomol Zool 13(3):228–230
Matsuda K, Matsumoto Y (1975) Feeding stimulation of the organic acids characteristic to the polygonaceous plants in four species of Chrysomelidae. Jpn J Appl Entomol Zool 19:281–284
Matsuda K, Sugawara F (1980) Defensive secretion of chrysomelid larvae Chrysomela vigintipunctata costella (Marseul), C. populi L. and Gastrolina depressa Baly (Coleoptera: Chrysomelidae). Appl Entomol Zool 15(3):316–320
McIndoo NE (1926) An insect olfactometer. J Econ Entomol 19(3):545–571. https://doi.org/10.1093/jee/19.3.545
McKenna DD, Sequeira AS, Marvaldi AE, Farrell BD (2009) Temporal lags and overlap in the diversification of weevils and flowering plants. Proc Natl Acad Sci USA 106:7083–7088. https://doi.org/10.1073/pnas.0810618106
McKenna DD, Shin S, Ahrens D, Balke M, Beza-Beza C, Clarke DJ, Donath A, Escalona HE, Friedrich F, Letsch H, Liuj S, Maddisonk D, Mayere C, Misofe B, Murina PJ, Niehuisg O, Petersc RS, Podsiadlowskie L, Pohll H, Scullym ED, Yanl EV, Zhouo X, Ślipiński A, Beutel RG (2019) The evolution and genomic basis of beetle diversity. Proceedings of the National Academy of Sciences of the United States of America 116(49):24729–24737. https://doi.org/10.1073/pnas.1909655116
McKenna DD, Wild AL, Kanda K, Bellamy CL, Beutel RG, Caterino MS, Farnum CW, Hawks DC, Ivie MA, Jameson ML, Leschen RAB, Marvaldi AE, Mchugh JV, Newton AF, Robertson JA, Thayer MK, Whiting MF, Lawrence JF, Ślipiński A, Maddison DR, Farrell BD (2015) The beetle tree of life reveals Coleoptera survived end Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst Entomol 40:835–880. https://doi.org/10.1111/syen.12132
Mclaughlin JR, Tumlinson JH, Mori K (1991) Responses of Male Diabrotica balteata to Stereoisomers of the Sex Pheromone 6,12-dimethylpentadecan-2-one. J Econ Entomol 84(1):99–102
Meinwald J, Jones TH, Eisner T, Hicks K (1977) New methylcyclopentanoid terpenes from the larval defensive secretion of a chrysomelid beetle (Plagiodera versicolora). Proc Natl Acad Sci USA 74(6):2189–2193
Metcalf RL, Metcalf RA, Rhodes AM (1980) Cucurbitacins as kairomones for diabroticite beetles. Proc Natl Acad Sci USA 77(7):3769–3772
Metcalf RL (1994) Chemical ecology of diabroticites. In: Jolivet PH, Cox ML, Petitpierre E (eds) Novel aspects of the biology of Chrysomelidae. Series Entomologica 50, pp 153–166
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA
Moore BP (1967) Hydrogen cyanide in the defensive secretions of larval Paropsini (Coleoptera, Chrysomelidae). J Aust Entomol Soc 6:36–38
Mori K (2005) Synthesis of (R)-ar-turmerone and its conversion to (R)-ar-himachalene, a pheromone component of the flea beetle: (R)-ar-himachalene is dextrorotatory in hexane, while levorotatory in chloroform. Tetrahedron: Asymmetry 16(3):685–692. https://doi.org/10.1016/j.tetasy.2004.11.077
Mori K, Nukada T, Ebata T (1981) Synthesis methyl of optically active forms of (E)-2,4,5-tetradecatrienoate, the pheromone of the male dried bean beetle. Tetrahedron 37(7):1343–1347. https://doi.org/10.1016/S0040-4020(01)92450-0
Morris BD, Smyth RR, Foster SP, Hoffmann MP, Roelofs WL, Franke S, Francke W (2005) Vittatalactone, a ꞵ-Lactone from the Striped Cucumber Beetle, Acalymma vittatum. J Nat Prod 68(1):26–30. https://doi.org/10.1021/np049751v
Morse GE (2014) Bruchinae Latreille, 1802. In: Leschen RAB, Beutel RG (eds) Handbook of Zoology. Coleoptera, Beetles. Morphology and systematics, vol 3. De Gruyter, Berlim, Boston, pp 189–197
Muto S, Bando M, Mori K (2004) Synthesis and stereochemistry of the four himachalene-type sesquiterpenes isolated from the flea beetle (Aphthona flava) as pheromone candidates. Eur J Org Chem 9:1946–1952. https://doi.org/10.1002/ejoc.200300812
Nadein K, Bezděk J (2014) Galerucinae Latreille, 1802. In: Leschen RAB, Beutel RG (eds) Handbook of Zoology. Coleoptera, Beetles. Morphology and systematics, vol 3. Walter de Gruyter, Berlin/Boston, pp 251–259
Nakamura S, Konishi K, Takasu K (2006) Invasion of the Coconut Hispine Beetle, Brontispa longissima: Current Situation and Control Measures in Southeast Asia. Proceedings of International Workshop on Development of Database (APASD) for Biological Invasion 3:1–9
Nammour D, Huignard J, Pouzat J (1988) A female sex pheromone in Bruchidius atrolineatus (Pic) (Coleoptera, Bruchidae): analysis of the factors affecting production or emission of this pheromone. Physiol Entomol 13:185–192
Nápoles JR (2016) Systematics of the seed beetle genus Decellebruchus Borowiec, 1987 (Coleoptera, Bruchidae). Zookeys. 579:59–81. https://doi.org/10.3897/zookeys.579.7716
Nie RE, Xue HJ, Hua Y, Yang XK, Vogler AP (2012) Distinct species or colour polymorphism? Life history, morphology and sequence data separate two Pyrrhalta elm beetles (Coleoptera: Chrysomelidae). Syst Biodivers 10(2):133–146. https://doi.org/10.1080/14772000.2012.687783
Nie RE, Breeschoten T, Timmermans MJTN, Nadein K, Xue H-J, Bai M, Huang Y, Yang X-K, Vogler AP (2018) The phylogeny of Galerucinae (Coleoptera: Chrysomelidae) and the performance of mitochondrial genomes in phylogenetic inference compared to nuclear rRNA genes. Cladistics 34(2):113–130. https://doi.org/10.1111/cla.12196
Nie RE, Andujar C, Gómez-Rodriguez C, Bai M, Xue HJ, Tang M, Yang CT, Tang P, Yang XK, Vogler AP (2020a) The phylogeny of leaf beetles (Chrysomelidae) inferred from mitochondrial genomes. Syst Entomol 45(1):188–204. https://doi.org/10.1111/syen.12387
Nie RE, Vogler AP, Yang XK, Lin M (2020b) Higher-level phylogeny of longhorn beetles (Coleoptera: Chrysomeloidea) inferred from mitochondrial genomes. Syst Entomol 46(1):56–70. https://doi.org/10.1111/syen.12447
Nielsen JK (1978) Host plant discrimination within Cruciferae, feeding responses of four leaf beetles (Coleoptera: Chrysomelidae) to glucosinolates, cucurbitacins and cardenolides. Entomol Exp Appl 24:41–54
Nixon KC (1999–2004) Winclada ver. ASADO 1.61. TNT-MRBAYES SLAVER. Published by the author, Ithaca, New York. Avaible at: http://www.cladistics.com/. Acessed Nov 2022
Nojima S, Shimomura K, Honda H, Yamamoto I, Ohsawa K (2007) Contact sex pheromone components of the Cowpea Weevil, Callosobruchus maculatus. J Chem Ecol 33:923–933. https://doi.org/10.1007/s10886-007-9266-5
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583
Oliver JE, Dickens JC, Glass TE (2002) (S)-3,7-Dimethyl-2-oxo-6-octene-1,3-diol: an aggregation pheromone of the Colorado potato beetle, Leptinotarsa decemlineata (Say). Tetrahedron Lett 43:2641–2643. https://doi.org/10.1242/jeb.205.13.1925
Page RDM (2001) Nexus data editor 0.5.0. Program distributed by the author, Institute of Biomedical and Life Sciences. University of Glasgow, Glasgow. Available at rod/NDE/nde.html
Panday G, Bhatt PA, Kanaujia S, Kanaujia K, Jyothi KN, Prasuna AL (2011) Studies on electrophysiology, olfactometric response and chemical analysis of groundnut extracts against groundnut bruchid (Caryedon serratus). Int J Agric Technol 7(5):1265–1273
Pasteels JM, Daloze D, van Dorsser W, Roba J (1979) Cardiac glycosides in the defensive secretion of Chrysolina herbacea (Coleoptera, Chrysomelidae). Identification, biological role and pharmacological activity. Comp Biochem Physiol 63C:117–121
Pasteels JM, Rowell-Rahier M, Braekman JC, Dupont A (1983) Salicin from host plant as precursor of salicylaldehyde in defensive secretion of secretion of Chrysomeline larvae. Physiol Entomol 8:307–314
Pasteels JM, Rowell-Rahier M, Randoux T, Braekman JC, Daloze D (1988) Pyrrolizidine alkaloids of probable host-plant origin in the pronotal and elytral secretion of the leaf beetle Oreina cacaliae. Entomol Exp Appl 49:55–58. https://doi.org/10.1007/BF00188238
Pasteels JM, Termonia A, Windsor DM, Witte L, Theuring C, Hartmann T (2001) Pyrrolizidine alkaloids and pentacyclic triterpene saponins in the defensive secretions of Platyphora leaf beetles. Chemoecology 11:113–120. https://doi.org/10.1007/pl00001840
Pauls P, Becker T, Rahfeld P, Gretscher RR, Paetz C, Pasteels J, von Reuss SH, Burse A, Boland W (2016) Two defensive lines in juvenile leaf beetles; Esters of 3-nitropropionic acid in the hemolymph and aposematic warning. J Chem Ecol 42:240–248. https://doi.org/10.1007/s10886-016-0684-0
Peacock L, Lewis M, Powers S (2001) Volatile compounds from Salix spp. varieties differing in susceptibility to three willow beetle species. J Chem Ecol 27(10):1943–1951. https://doi.org/10.1023/a:1012278417424
Pedersen HÆ, Watthana S, Kocyan A, Srimuang K-O (2013) Pollination biology of Luisia curtisii (Orchidaceae): indications of a deceptive system operated by beetles. Plant Syst Evol 299:177–185. https://doi.org/10.1007/s00606-012-0713-6
Peng C, Weiss MJ (1992) Evidence of an aggregation pheromone in the flea beetle, Phyllotreta cruciferae (Goeze) (Coleoptera: Chrysomelidae). J Chem Ecol 18(6):875–884. https://doi.org/10.1007/BF00988328
Peng C, Bartelt RJ, Weiss MJ (1999) Male crucifer flea beetles produce an aggregation pheromone. Physiol Entomol 24:98–99
Peterson MA, Honchak BM, Locke SE, Beeman TE, Mendoza J, Green J, Buckingham KJ, White MA, Monsen KJ (2005) Relative abundance and the species-specific reinforcement of male mating preference in the Chrysochus (Coleoptera: Chrysomelidae) hybrid zone. Evolution 59(12):2639–2655. https://doi.org/10.1111/j.0014-3820.2005.tb00976.x
Peterson MA, Dobler S, Larson EL, Juárez D, Schlarbaum T, Monsen KJ, Francke W (2007) Profiles of cuticular hydrocarbons mediate male mate choice and sexual isolation between hybridising Chrysochus (Coleoptera: Chrysomelidae). Chemoecology 17:87–96. https://doi.org/10.1007/s00049-007-0366-z
Phillips JK, Walgenbach CA, Klein JA, Burkholder WE, Schmuff NR, Fales HM (1985) (R*, S*)-5-hydroxy-4-methyl-3-heptanone: male-produced aggregation pheromone of Sitophilus oryzae (L.) and S. zeamais Motsch. J Chem Ecol 11:1263–1274. https://doi.org/10.1007/BF01024114
Phillips TW, Phillips JK, Webster FX, Tang R, Burkholder WE (1996) Identification of sex pheromones from cowpea weevil, Callosobruchus maculatus, and related studies with C. analis (Coleoptera: Bruchidae). J Chem Ecol 22(12):2233–2249. https://doi.org/10.1007/BF02029543
Pierce AM, Pierce HD Jr, Oehlschlager AC, Borden JH (1985) Macrolide aggregation pheromones in Oryzaephilus surinamensis and Oryzaephilus mercator (Coleoptera: Cucujidae). J Agric Food Chem 33(5):848–852
Pimbert M, Pouzat J (1988) Electroantennogram responses of Zabrotes subfasciatus to odours of the sexual partner. Entomol Exp Appl 47(1):49–53. https://doi.org/10.1111/j.1570-7458.1988.tb02281.x
Pirkle WH, Boeder CW (1978) Synthesis and Absolute Configuration of (–)-Methyl (E)-2,4,5- tetradecatrienoate, the Sex Attractant of the Male Dried Bean Weevil. J Org Chem 43(11):2091–2093
Pouzat J, Nammour D (1989) Electrophysiological investigations of sex pheromone reception and release in Bruchidius atrolineatus. Physiol Entomol 14:319–324
Qadir I, Qamar A, Paul B, Mir AH (2021) Cuticular hydrocarbons C14–C36 are potential contact pheromonal elements modulating some behaviors in Zygogramma bicolorata (Coleoptera: Chrysomelidae). Biologia 76:123–132. https://doi.org/10.2478/s11756-020-00515-w
Rambaut A, Suchard M, Drummond A (2013) Tracer 1.6. Available in: http://tree.bio.ed.ac.uk/software/tracer/
Rao S, Cossé AA, Zilkowski BW, Bartelt RJ (2003) Aggregation pheromone of the cereal leaf beetle. J Chem Ecol 29(9):2165–2175. https://doi.org/10.1023/a:1025698821635
Ray AM, Swift IP, McElfresh JS, Alten RL, Millar JG (2012) R)-desmolactone, a female-produced sex pheromone component of the cerambycid beetle Desmocerus californicus californicus (subfamily Lepturinae. J Chem Ecol 38:157–167. https://doi.org/10.1007/s10886-012-0070-5
Rees D (2007) Insects of stored grain. Australia, Collingwood VIC, 3066
Reid CAM (1995) A cladistic analysis of subfamilial relationships in the Chrysomelidae sensu lato (Chrysomeloidea). In: Pakaluk J, Ślipiński SA (eds) Biology, Phylogeny and Classification of Coleoptera: papers celebrating the 80th birthday of Roy A. Crowson. Muzeum i Instytut Zoologii PAN, Warszawa, pp 559–631
Reid CAM (2000) Spilopyrinae Chapuis: a new subfamily in the Chrysomelidae and its systematic placement (Coleoptera). Invertebr Taxon 14(6):837–862
Reid CAM (2006) A taxonomic revision of the Australian Chrysomelinae, with a key to the genera (Coleoptera: Chrysomelidae). Zootaxa 1292:1–119. https://doi.org/10.11646/ZOOTAXA.1292.1.1
Reinhard J (2004) Insect chemical communication. Chemosense 6(4):2–6
Riley EG, Clark SM, Flowers RW, Gilbert AJ (2002) Family 124. Chrysomelidae Latreille 1802. In: Arnett RH, Thomas MC, Skelley PE, Frank JH (eds) Chrysomelinae, in American Beetles, Volume 2. Polyphaga: Scarabaeoidea through Curculionoidea. CRC Press, Boca Raton, pp 648–653
Robertson JA, Ślipiński A, Moulton M, Shockley FW, Giorgi A, Lord NP, Mckenna D, Tomaszewska W, Forrester J, Miller KB, Whiting MF, Mchugh JV (2015) Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst Entomol 40:745–778. https://doi.org/10.1111/syen.12138
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rosa BB, Melo GAR, Barbeitos MS (2019) Parsimony-based partitioning outperforms alternatives in Bayesian analysis of discrete morphological data. Syst Biol 68(4):657–671. https://doi.org/10.1093/sysbio/syz001
Rup PJ (1986) Mating and its attendant behaviour in Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J Stored Prod Res 22(2):77–79
Rup PJ, Sharma SP (1978) Behavioural response of males and females of Callosobruchus maculatus (F.) to the sex pheromone. Indian J Ecol 1:72–76
Rutledge C, Silk PJ, Mayo P (2014) Use of contact chemical cues in prey discrimination by Cerceris fumipennis. Entomol Exp Appl 153:93–105. https://doi.org/10.1111/eea.12233
Santos AT, Ribeiro-Costa CS (2019) Rearrangements in some species groups of Amblycerus Thunberg, 1815 (Coleoptera: Chrysomelidae: Bruchinae) including keys, description of a new species, new host plant and distributional records. Zootaxa 4701(2):101–148. https://doi.org/10.11646/zootaxa.4701.2.1
Sari LT, Ribeiro-Costa CS, Pereira PRVS (2003) Biological aspects of Zabrotes subfasciatus (Bohemann, 1833) (Coleoptera, Bruchidae) on Phaseolus vulgaris L., cv. Carioca (Fabaceae), under laboratory conditions. Rev Bras Entomol 47(4):621–624. https://doi.org/10.1590/S0085-56262003000400014
Schanz M (1953) Der Geruchssinn des Kartoffelkäfers (Leptinotarsa decemlineata Say). Z Vgl Physiol 35:353–379. https://doi.org/10.1007/BF00297882
Schütz S, Weißbecker B, Klein A, Hummel HE (1997) Host plant selection of the Colorado potato beetle as influenced by damage induced volatiles of the potato plant. Naturwissenschaften 84:212–217. https://doi.org/10.1007/s001140050381
Sears ALW, Smiley JT, Hilker M, Müller F, Rank NE (2001) Nesting behavior and prey use in two geographically separated populations of the specialist wasp Symmorphus cristatus (Vespidae: Eumeninae). Am Midl Nat 145(2):233–246. https://doi.org/10.1674/0003-0031(2001)145[0233:NBAPUI]2.0.CO;2
Segers A, Megido RC, Lognay G, Francis F (2021) Overview of Bruchus rufimanus BOHEMAN 1833 (Coleoptera: Chrysomelidae): biology, chemical ecology and semiochemical opportunities in integrated pest management programs. Crop Protect 140:105411. https://doi.org/10.1016/j.cropro.2020.105411
Sereno PC (2007) Logical basis for morphological characters in phylogenetics. Cladistics 23(6):565–587. https://doi.org/10.1111/j.1096-0031.2007.00161.x
Shimomura K, Nojima S, Yajima S, Ohsawa K (2008) Homofarnesals female sex attractant pheromone components of the southern cowpea weevil, Callosobruchus chinensis. J Chem Ecol 34:467–477. https://doi.org/10.1007/s10886-008-9451-1
Shimomura K, Mimura T, Ishikawa S, Yajima S, Ohsawa K (2010a) Variation in mate recognition specificities among four Callosobruchus seed beetles. Entomol Exp Appl 135(3):315–322. https://doi.org/10.1111/j.1570-7458.2010.00994.x
Shimomura K, Akasaka K, Yajima A, Mimura T, Yajima S, Ohsawa K (2010b) Contact sex pheromone components of the seed beetle, Callosobruchus analis (F.). J Chem Ecol 36:955–965. https://doi.org/10.1007/s10886-010-9841-z
Shimomura K, Matsui S, Ohsawa K, Yajima S (2016) Saltational evolution of contact sex pheromone compounds of Callosobruchus rhodesianus (Pic). Chemoecology 26:15–23. https://doi.org/10.1007/s00049-015-0204-7
Shimomura K, Koshino H, Yajima A, Matsumoto N, Yajima S, Ohsawa K (2010c) A new sesquiterpenoid produced by female Callosobruchus rhodesianus (Pic): a possible component of the sex attractant pheromone. Tetrahedron Lett 51:6860–6862. https://doi.org/10.1016/j.tetlet.2010.10.100
Shu S, Mbata GN, Cork A, Ramaswamy SB (1999) Sex pheromone of Callosobruchus subinnotatus. J Chem Ecol 25(12):2715–2727. https://doi.org/10.1023/A:1020899407497
Silfverberg H (2010) Family Orsodacnidae C. G. Thomson, 1859. In: Löbl I, Smetana A (eds) Catalogue of Palaearctic Coleoptera. Volume 6. Chrysomeloidea. Apollo Books, Stenstrup
Silk PJ, Sweeney J, Wu JP, Price J, Gutowski JM, Kettela EG (2007) Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby) (Coleoptera: Cerambycidae). Naturwissenschaften 94:697–701. https://doi.org/10.1007/s00114-007-0244-0
Silk PJ, Sweeney J, Wu J, Sopow S, Mayo PD, Magee D (2011) Contact sex pheromones identified for two species of longhorned beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the subfamily Spondylidinae. Environ Entomol 40:714–726. https://doi.org/10.1603/EN10213
Silva WD, Millar JG, Hanks LM, Bento JMS (2016) (6E,8Z)-6,8-Pentadecadienal, a novel attractant pheromone produced by males of the cerambycid beetles Chlorida festiva and Chlorida costata. J Chem Ecol 42:1082–1085. https://doi.org/10.1007/s10886-016-0742-7
Sollai G, Solari P (2022) An overview of “Insect Biodiversity.” Diversity 14(2):134. https://doi.org/10.3390/d14020134
Soroka JJ, Bartelt RJ, Zilkowski BW, Cossé AA (2005) Responses of flea beetle Phyllotreta cruciferae to synthetic aggregation pheromone components and host plant volatiles in field trials. J Chem Ecol 31(8):1829–1843. https://doi.org/10.1007/s10886-005-5929-2
Spikes AE, Paschen MA, Millar JG, Moreira JA, Hamel PB, Schiff NM, Ginzel MD (2010) First contact pheromone identified for a longhorned beetle (Coleoptera: Cerambycidae) in the subfamily Prioninae. J Chem Ecol 36:943–954. https://doi.org/10.1007/s10886-010-9837-8
Stojković B, Savković U, Đorđević M, Tucić N (2014) Host-shift effects on mating behavior and incipient pre-mating isolation in seed beetle. Behav Ecol 25:553–564. https://doi.org/10.1093/beheco/aru015
Stork NE (2018) How many species of insects and other terrestrial arthropods are there on Earth? Annu Rev Entomol 63:31–45. https://doi.org/10.1146/annurev-ento-020117-043348
Sugawara F, Matsuda K, Kobayashi A, Yamashita K (1979) Defensive secretion of chrysomelid larvae Linaeidea aenea Linné and Plagiodera versicolora distincta Baly. J Chem Ecol 5(6):929–934
Sugeno W, Hori M, Matsuda K (2006) Identification of the contact sex pheromone of Gastrophysa atrocyanea (Coleoptera, Chrysomelidae). Appl Entomol Zool 41(2):269–276. https://doi.org/10.1303/aez.2006.269
Sun X, Zhang X, Wu G, Li X, Liu F, Xin Z, Zhang J (2017) n-Pentacosane acts as both contact and volatile pheromone in the tea weevil, Myllocerinus aurolineatus. J Chem Ecol 43:557–562. https://doi.org/10.1007/s10886-017-0857-5
Tanaka K, Ohsawa K, Honda H, Yamamoto Y (1981) Copulation release pheromone, erectin, from the azuki bean weevil (Callosobruchus chinensis L.). J Pestic Sci 6:75–82
Tanaka K, Ohsawa K, Honda H, Yamamoto Y (1982) Synthesis of Erectin, a copulation release pheromone of the Azuki Bean Weevil, Callosobruchus chinensis L. J Pestic Sci 7:535–537
Tanton MT (1965) Agar and chemostimulant concentrations and their effect on intake of synthetic food by larvae of the mustard beetle Phaedon cochleariae Fab. Entomol Exp Appl 8:74–82
Tarasov S, Génier F (2015) Innovative Bayesian and parsimony phylogeny of dung beetles (Coleoptera, Scarabaeidae, Scarabaeinae) enhanced by ontology-based partitioning of morphological characters. PLoS ONE 10(3):e0116671. https://doi.org/10.1371/journal.pone.0116671
Tarasov SI, Solodovnikov AY (2011) Phylogenetic analyses reveal reliable morphological markers to classify mega-diversity in Onthophagini dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Cladistics 27(5):490–528. https://doi.org/10.1111/j.1096-0031.2011.00351.x
Termonia A, Pasteels JM (1999) Larval chemical defence and evolution of host shifts in Chrysomela leaf beetles. Chemoecology 9:13–23
Tihelka E, Cai C, Giacomelli M, Lozano-Fernandez J, Rota-Stabelli O, Huang D, Engel MS, Donoghue PCJ, Pisani D (2021) The evolution of insect biodiversity. Curr Biol 31(19):R1299–R1311. https://doi.org/10.1016/j.cub.2021.08.057
Timmermans M, Randoux T, Daloze D, Braekman J-C, Pasteels JM, Lesage L (1992) The chemical defense of Doryphorina beetles (Coleoptera: Chrysomelidae). Biochem Syst Ecol 20(4):343–349
Timmermans MJTN, Barton C, Haran J, Ahrens D, Culverwell CL, Ollikainen A, Dodsworth S, Foster PG, Bocak L, Vogler AP (2015) Family-level sampling of mitochondrial genomes in Coleoptera: compositional heterogeneity and phylogenetics. Genome Biol Evol 8(1):161–175. https://doi.org/10.1093/gbe/evv241
Tóth M, Csonka E, Bartelt RJ, Cossé AA, Zilkowski BW, Muto S, Mori K (2005) Pheromonal activity of compounds identified from male Phyllotreta cruciferae: Field tests of racemic mixtures, pure enantiomers, and combinations with allyl isothiocyanate. J Chem Ecol 31(11):2705–2720. https://doi.org/10.1007/s10886-005-7621-y
Tóth M, Csonka E, Bartelt RJ, Cossé AA, Zilkowski BW (2011) Similarities in pheromonal communication of flea beetles Phyllotreta cruciferae Goeze and Ph. vittula Redtenbacher (Coleoptera, Chrysomelidae). J Appl Entomol 136(9):688–697. https://doi.org/10.1111/j.1439-0418.2011.01702.x
Tuda M, Rönn J, Buranapanichpan S, Wasanos N, Arnqvist G (2006) Evolutionary diversification of the bean beetle genus Callosobruchus (Coleoptera: Bruchidae): traits associated with stored-product pest status. Mol Ecol 15(12):3541–3551. https://doi.org/10.1111/j.1365-294X.2006.03030.x
U.S. Department of Agriculture (n.d.) https://www.fas.usda.gov/2021-export-overview
Ueno T, Kuwahara Y, Fujii K, Taper ML, Toquenaga Y, Suzuki T (1990) D-catechin. an oviposition stimulant of azuki bean weevil Callosobruchus chinensis in the host azuki bean. J Pestic Sci 15:573–578
Valkama H, Raty M, Niemela P (1997) Catches of Ips duplicatus and other non-target Coleoptera by Ips typographus pheromone trapping. Entomol Fennica 8:153–159. https://doi.org/10.33338/ef.83934
Vencl FV, Leschen RAB (2014) Criocerinae Lattreille 1807. In: Leschen RAB, Beutel RG (eds) Handbook of Zoology. Coleoptera, Beetles. Morphology and Systematics (Phytophaga), vol 3. Walter de Gruyter, Berlin, pp 237–242
Ventura MU, Martins MC, Pasini A (2000) Responses of Diabrotica speciosa and Cerotoma arcuata tingomariana (Coleoptera: Chrysomelidae) to volatile attractants. Fla Entomol 83:403–410. https://doi.org/10.2307/3496715
Viana JH (2016) Nomenclatural changes and lectotype designations in the seed-beetle genus Sennius Bridwell: with the synonymization of Megasennius Whitehead & Kingsolver (Coleoptera: Chrysomelidae: Bruchinae). Zootaxa 4175(3):249–260. https://doi.org/10.11646/zootaxa.4175.3.4
Vidal DM, Fonseca MG, Zarbin PHG (2010) Enantioselective synthesis and absolute configuration of the sex pheromone of Hedypathes betulinus (Coleoptera: Cerambycidae). Tetrahedron Lett 51:6704–6706. https://doi.org/10.1016/j.tetlet.2010.10.024
Visser JH, Straten SV, Maarse H (1979) Isolation and identification of volatiles in the foliage of potato, Solanum tuberosum, a host plant of the Colorado beetle, Leptinotarsa decemlineata. J Chem Ecol 5(1):13–25
Vuts J, Powers SJ, Caulfield JC, Pickett JA, Birkett MA (2015a) Multiple roles of a male-specific compound in the sexual behavior of the dried bean beetle, Acanthoscelides Obtectus. J Chem Ecol 41:287–293. https://doi.org/10.1007/s10886-015-0560-3
Vuts J, Francke W, Mori K, Zarbin PHG, Hooper AM, Millar JG, Pickett JA, Tóth M, Chamberlain K, Caulfield JC, Woodcock CM, Tröger AG, Csonka EB, Birkett MA (2015b) Pheromone bouquet of the dried bean beetle, Acanthoscelides obtectus (Col.: Chrysomelidae), Now Complete. Eur J Org Chem 22:4843–4846. https://doi.org/10.1002/ejoc.201500196
Wallace JB, Blum MS (1969) Refined defensive mechanisms in Chrysomela scripta. Ann Entomol Soc Am 62(3):503–506
Warren DL, Geneva AJ, Lanfear R (2017) RWTY (R We There Yet): an R package for examining convergence of Bayesian phylogenetic analyses. Mol Biol Evol 34:1016–1020
Wiley EO, Lieberman BS (2011) Phylogenetics: Theory and Practice of Phylogenetic Systematics, 2nd Edition
Wilgenbusch JC, Warren DL, Swofford DL (2004) AWTY: a system for graphical exploration of MCMC convergence in Bayesian phylogenetic inference. Available in: http://ceb.csit.fsu.edu/awty
Williams CE (1992) Movement of the dogbane beetle, Chrysochus auratus (Coleoptera: Chrysomelidae) in a patchy environment. Banisteria 1:8–10
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge
Xue HJ, Wei JN, Magalhães S, Zhang B, Song KQ, Liu J, Li WZ, Yang XK (2016) Contact pheromones of 2 sympatric beetles are modified by the host plant and affect mate choice. Behav Ecol 27(3):895–902. https://doi.org/10.1093/beheco/arv238
Xue HJ, Segraves KA, Wei J, Zhang B, Nie RE, Li WZ, Yang XK (2018) Chemically mediated sexual signals restrict hybrid speciation in a flea beetle. Behav Ecol 29(6):1462–1471. https://doi.org/10.1093/beheco/ary105
Yajima A, Akasaka K, Yamamoto M, Ohmori S, Nukada T, Yabuta G (2007) Direct Determination of the Stereoisomeric Composition of Callosobruchusic Acid, the Copulation Release Pheromone of the Azuki Bean Weevil, Callosobruchus chinensis L., by the 2D-Ohrui-Akasaka Method. J Chem Ecol 33(7):1328–1335. https://doi.org/10.1007/s10886-007-9311-4
Yang S, Zhang XF, Gao YL, Chen D, She DM, Zhang T, Ning J (2017) Male-produced aggregation pheromone of coffee bean weevil, Araecerus fasciculatus. J Chem Ecol 43:978–985. https://doi.org/10.1007/s10886-017-0894-0
Zavaleta E (2000) The economic value of controlling an invasive shrub. AMBIO: J Hum Environ 29(8):462–467. https://doi.org/10.1579/0044-7447-29.8.462
Zhang Z-Q, McEvoy PB (1994) Attraction of Longitarsus jacobaeae males to cues associated with conspecific females (Coleoptera: Chrysomelidae). Environ Entomol 23(3):732–737. https://doi.org/10.1093/ee/23.3.732
Zhang B, Xue HJ, Song KQ, Liu J, Li WZ, Nie RE, Yang XK (2014) Male mate recognition via cuticular hydrocarbons facilitates sexual isolation between sympatric leaf beetle sister species. J Insect Physiol 70:15–21. https://doi.org/10.1016/j.jinsphys.2014.08.006
Zhang LJ, Wu L, Wei CY, Liu XL, Xue HJ, Yang XK, Nie RE (2018) The complete mitochondrial genome of the cowpea weevil, Callosobruchus maculates (Coleoptera: Chrysomelidae: Bruchinae) and a related phylogenetic analysis of Chrysomelidae. Mitochondrial DNA Part B 3(2):645–647. https://doi.org/10.1080/23802359.2017.1413308
Zhang H, Song N, Yin X (2022) Higher-level phylogeny of Chrysomelidae based on expanded sampling of mitogenomes. PLOS ONE 17(1):e0258587. https://doi.org/10.1371/journal.pone.0258587
Zilkowski BW, Bartelt RJ, Cossé AA, Petroski RJ (2006) Male-produced aggregation pheromone compounds from the eggplant flea beetle (Epitrix fuscula): Identification, synthesis, and field biossays. J Chem Ecol 32:2543–2558. https://doi.org/10.1007/s10886-006-9163-3
Zilkowski BW, Bartelt RJ, Vermillion K (2008) Analysis of 2,4,6-Nonatrienal geometrical isomers from male flea beetles, Epitrix hirtipennis and E. fuscula. J Agric Food Chem 56(13):4982–4986. https://doi.org/10.1021/jf8005273
Zou Y, Millar JG (2015) Chemistry of the pheromones of mealybug and scale insects. Nat Prod Rep 32(7):1067–1113. https://doi.org/10.1039/c4np00143e
Acknowledgements
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Instituto Nacional de Ciências e Tecnologia de Semioquímicos na Agricultura (INCT), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), for financial support. The Academic Publishing Advisory Center (Centro de Assessoria de Publicação Acadêmica, CAPA—https://www.capa.ufpr.br) of the Federal University of Paraná (UFPR) for assistance with English language developmental editing. We would also like to thank Dr. Mário Jardim Cupello for his contribution to the discussion on phylogenetic analysis. Dr. Michael Geiser and Dr. Adelita Maria Linzmeier for sending us literature on the taxonomy of some Chrysomelidae taxa.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico [142496/2019-7, 465511/2014-7], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [88882.344270/2019-01], Fundação Araucária [103/2020], Instituto Nacional de Ciências e Tecnologia de Semioquímicos na Agricultura/Fundação de Amparo à Pesquisa do Estado de São Paulo [50871-0/2014], and FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo (grant # 2020/13943-4 and 2022/09215-9 to B.B. Rosa).
Author information
Authors and Affiliations
Contributions
Aluska Tavares dos Santos: Conceptualization, Data Curation, Investigation, Methodology, Visualization, Writing—Original Draft, Writing—Review & Editing. João Pedro Albuquerque de Souza: Investigation, Methodology, Visualization, Writing—Review & Editing. Isaac Reis Jorge: Formal analysis, Investigation, Methodology, Visualization, Writing—Review & Editing. Samara M. M. Andrade: Investigation, Visualization, Writing—Review & Editing. Mauricio Oswaldo Moura: Formal analysis, Visualization, Writing—Review & Editing. Brunno Bueno Rosa: Formal analysis, Methodology, Visualization, Writing—review & Editing. Paulo Henrique Gorgatti Zarbin: Supervision, Visualization, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dos Santos, A.T., Souza, J.P.A., Jorge, I.R. et al. Can Pheromones Contribute to Phylogenetic Hypotheses? A Case Study of Chrysomelidae. J Chem Ecol 49, 611–641 (2023). https://doi.org/10.1007/s10886-023-01450-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-023-01450-1