Abstract
Males of Acanthoscelides obtectus (Coleoptera: Chrysomelidae, Bruchinae) emit methyl (E,R)-2,4,5-tetradecatrienoate that attracts females for mating. This study identified further roles for this compound in the sexual behavior of A. obtectus. Earlier observations revealed that males touched females with their antennae while tandem-running with them and initiated mounting and copulation, whereas they showed no such behavior toward other males. A series of subsequent laboratory choice tests were set up to establish if certain cuticular compounds aid contact sex recognition in A. obtectus. Males chose virgin females over other males. The activity toward females could be eliminated by rinsing with hexane, but was regained by application of female extract onto previously rinsed females. Gas chromatographic (GC) comparison of hexane extracts revealed the presence of two male-specific compounds, methyl (E,R)-2,4,5-tetradecatrienoate and octadecanal, which were absent from the behaviorally active female samples. Of the two compounds, methyl (E,R)-2,4,5-tetradecatrienoate was found to be responsible for the inhibition of male sexual behavior, similar to that observed with crude male extracts applied to virgin females. Furthermore, males preferred virgin over mated females. GC analyses revealed the presence of methyl (E,R)-2,4,5-tetradecatrienoate in mated females in amounts sufficient to curtail mating attempts. It appears that methyl (E,R)-2,4,5-tetradecatrienoate, besides being a male-produced sex pheromone, acts as a male-recognition signal in A. obtectus. Males also transfer it onto females during mating, resulting in mated females being avoided by courting males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dried bean beetle, Acanthoscelides obtectus Say (Coleoptera: Chrysomelidae, Bruchinae), is a widely distributed oligophagous seed predator specializing on Phaseolus spp. (Fabaceae) (Imura 1990). Mainly due to its status as a pest of stored products (Abate and Ampofo 1996; Southgate 1979), A. obtectus received attention from chemical ecologists in search of novel semiochemical-based management methods. An unusual allenic ester, methyl (E,R)-2,4,5-tetradecatrienoate, was identified as a male-produced sex pheromone more than 40 years ago (Halstead 1973; Hope et al. 1967; Horler 1970). More recently, octadecanal was described from males and found to synergize the activity of the ester as a sex attractant for females (Annoscia et al. 2010). Other studies on A. obtectus suggested that males use contact chemoreception to distinguish the sexes. They actively tap conspecifics with their antennae, which results in a sequence of chasing, mounting, and copulation with females (Halstead 1973; Á. Szentesi unpublished). Savković et al. (2012) and Stojković et al. (2014) found differences in the cuticular hydrocarbon profiles of A. obtectus females and males, and proposed the role of hydrocarbon compounds in mate recognition. In the closely related Callosobruchus spp., for example, female beetles produce contact sex pheromones, comprising a blend of C25–C35 straight chain and methyl-branched hydrocarbons and dicarboxylic acids, which elicit male sexual behavior (Nojima et al. 2007; Tanaka et al. 1981).
As the process of mate recognition is poorly understood in A. obtectus, we aimed to shed light on this communication system, assuming a role for contact chemical signals on the body surface of beetles. We used a series of bioassays to test this assumption, as well as gas chromatography (GC) and coupled gas chromatography-mass spectrometry (GC/MS) to analyze cuticular extracts of A. obtectus. Our findings may become important for developing management strategies that interfere with the sexual behavior of A. obtectus, thereby leading to increased control efficacy.
Methods and Materials
Insects Acanthoscelides obtectus beetles for the tests were obtained from a laboratory population in Hungary, and reared on dry Phaseolus vulgaris ‘Cannellini’ beans. The original laboratory population was from a natural infestation on Ph. vulgaris in Hungary. Maintenance conditions were the same at the Rothamsted and Hungarian laboratories (artificial lighting with a 16:8 h L:D photoperiod, 20 °C, 60 % RH). In order to obtain virgins, seeds were kept individually in wells of an Eppendorf rack and covered with a piece of transparent acetate sheet until beetle emergence, at which time the sexes were separated immediately for use in the experiments.
Preparation of Extracts
We extracted surface chemicals (for Tests 3, 4, and 6, see below) from 20 female and male A. obtectus. Samples were prepared by first freezing the beetles on dry ice, then soaking them in 200 μl freshly distilled hexane (Fisher Scientific, UK) for 10 min. The extracts were filtered through a glass wool plug, transferred into a 1.1 ml conical vial (Kinesis, UK), and evaporated under a gentle nitrogen stream to the appropriate volumes to obtain ca. 1 beetle equivalent per 2 μl, which was determined by GC. Samples were kept at −20 °C until use.
To determine the amount of methyl (E,R)-2,4,5-tetradecatrienoate from males, 10 males were extracted individually in 50 μl hexane for 10 min., and the extracts analyzed by GC. Ten mated females were similarly extracted in hexane immediately after the 24 h copulation period. Another two groups of 10 females were separated from males after 24 h, and extracted either 72 or 144 h later. An extract from 10 virgin females served as a control.
To estimate the amount of methyl (E,R)-2,4,5-tetradecatrienoate on different parts of the male A. obtectus cuticle, the heads (with antennae and palpi), thoraxes (with legs), elytrae and abdominal tergites, pygidia and sternites of 10 males were soaked in 50 μl hexane in a 1.1 ml conical vial for 10 min. Extracts were filtered through a glass wool plug, transferred into a 1.1 ml conical vial and evaporated under nitrogen to 20 μl. They were kept at −20 °C until used for GC analyses.
GC and GC/Mass Spectrometry (GC/MS) Analyses of Extracts
For analysis of extracts, an Agilent 6890A GC (Agilent Technologies, USA), equipped with a cool on-column injector, flame ionization detector (FID), and a 50 m × 0.32 mm i.d. HP-1 column, was used. The oven temperature was maintained at 30 °C for 0.1 min, then programmed to increase at 10 °C.min−1 to 250 °C, and then held for 38 min.
For GC/MS (Micromass Autospec Ultima, Waters/Micromass, USA) compound identification, an HP-1 capillary GC column (50 m × 0.32 mm i.d. × 0.52 μm film thickness), equipped with a cool on-column injector, was used. Ionization was by electron impact at 70 eV, 250 °C. The oven temperature was maintained at 30 °C for 5 min and then programmed at 5 °C.min−1 to 250 °C. Tentative identification by GC/MS was confirmed by comparing retention indices of peaks with those of synthetic standards and by peak enhancement on GC by co-injection with authentic compounds (Pickett 1990), using an Agilent 6890A GC with 50 m × 0.32 mm i.d. HP-1 column, as well as a 30 m × 0.32 mm i.d. DB-WAX column. The carrier gas was helium. Quantification of compounds was achieved using known amounts of external standards (a series of C7-C22 alkanes).
Chemicals
For behavioral assays of the two male-specific compounds, enantiomerically pure methyl (E,R)-2,4,5-tetradecatrienoate was synthesized following the protocol of Mori (2012). Octadecanal was synthesized from octadecanol by TPAP-mediated oxidation (Griffith et al. 1987).
Behavioral Assays
All experiments were carried out under the same conditions used for the maintenance of bruchid cultures. Petri dishes (55 mm diam., 13 mm height), with a 55 mm diam. filter paper on the bottom, served as test arenas. Two pencil marks, 35 mm apart on the filter paper, indicated the places for the test (t) and control (c) A. obtectus individuals (3–8-d-old). These were freeze-killed on dry ice before use in experiments, and were laid on the side. If a beetle was soaked in hexane, the solvent was allowed to evaporate prior to testing. One male A. obtectus (3–11-d-old) was put in each Petri dish arena (representing 1 replication), and the number of copulation attempts (mounting and penis extruded) toward the test and control freeze-killed individuals was recorded. Beetles were observed for 20 min. Ten replications of each test were carried out.
Test 1 was carried out to confirm earlier observations on male mating preference for female conspecifics. Freeze-killed virgin females (t) and males (c) were compared.
Test 2 investigated if the mounting and copulation-initiating cues are chemicals found on the surface of females. Virgin freeze-killed females (t) were tested against females soaked in hexane for 10 min (c) (termed as ‘hexane-washed’ hereafter).
Test 3 assessed the effect of treating hexane-washed females with female extract (t). Hexane-washed females served as controls (c). Test females were coated with 2 μl (ca. 1 female equivalent) of a hexane extract of 20 females using glass micropipettes (Brand GmbH, Germany), by evenly spreading the extract on the entire dorsal surface of an insect.
Test 4 compared the activity toward virgin freeze-killed females treated with male extract (t) vs. hexane-washed females (c). Test females were coated with 2 μl (ca. 1 male equivalent) hexane extract of 20 males.
In Test 5, to eliminate the effect of solvent possibly moving compounds around the cuticle of otherwise stimulatory females (Coates and Langley 1982), thereby mimicking the inhibitory effect of male extract, virgin freeze-killed females were treated with 2 μl hexane (t). Hexane-washed females served as a control (c).
Additionally, in Test 6, to ensure that the inhibitory effect of the male extract was not a result of unnaturally high doses of cuticular compounds present in extracts of both sexes, unwashed females were treated with 2 μl (ca.1 beetle equivalent) of female extract (t), thereby doubling the concentration of compounds normally present on the cuticle of a female. Hexane-washed females were used as controls (c). The female extract was prepared from 20 individuals.
We then focused on two male-specific cuticular compounds to assess their behavioral roles. Test 7 compared the behavioral activity to virgin freeze-killed females treated with ca. 1 male equivalent of methyl (E,R)-2,4,5-tetradecatrienoate (1000 ng) plus octadecanal (200 ng) dissolved in 2 μl hexane (t) against hexane-washed females (c).
Further tests assessed male sexual activity to the effect of treating virgin freeze-killed females with either 200 ng octadecanal or 1000 ng methyl (E,R)-2,4,5-tetradecatrienoate in 2 μl hexane (t) (Tests 8 and 9, respectively), using hexane-washed females as controls (c).
There are reports of insects for the reduction of female attractiveness to males after mating due to (i) the delivery of certain compounds via contact from the male cuticle onto the female cuticle during courtship or after mating, or (ii) transfer of compounds via the spermatophore or mating plug into the female (Thomas 2011). As a mechanism similar to (i) could occur also in A. obtectus, in light of the mating-inhibitory compound methyl (E,R)-2,4,5-tetradecatrienoate, male sexual responses to freeze-killed virgin (t) and mated (c) females were compared directly in Test 10. Mated females were obtained by pairing a virgin female and male A. obtectus in a glass vial (10 mm diam. and 50 mm length), closed with a cotton wool plug, for 24 h to ensure copulation, which usually occurred within 30 min. from the start of pairing.
Subsequent tests compared male sexual activity toward virgin freeze-killed females treated with ca. 1 mated female equivalent of either methyl (E,R)-2,4,5-tetradecatrienoate (100 ng) or octadecanal (20 ng) in 2 μl hexane (t) with that to hexane-washed females (c) (Tests 11 and 12, respectively).
Statistics
To analyze the effect of treatment in each behavioral experiment separately, the difference in count was taken for each of the ten replicate arenas. These values were modeled assuming a Poisson distribution with a log-link function, fitting a generalized linear model (McCullagh and Nelder 1989) of the form
where μ is a constant and i = 1,…10. We tested whether the mean difference in counts was different (at α = 0.05) from 0, on 9° of freedom (being the number of arenas used less 1). The predicted mean difference was then output with appropriate standard error.
Results
After an initial period of 1–2 min., A. obtectus males started to explore the arena and investigated both freeze-killed conspecifics with intensive antennation. In Test 1, males did not attempt to copulate with freeze-killed males, but readily mounted freeze-killed virgin females. Motion was not required to elicit mating (Fig. 1). Males did not copulate with virgin females that had their cuticular chemicals removed by solvent (Test 2, Fig. 1). However, adding back female extract in amounts similar to that found on a female stimulated male copulation attempts (Test 3, Fig. 1).
Results of Petri dish arena bioassays. The behavior of one male Acanthoscelides obtectus/arena was observed for 20 min and the number of copulation attempts (mounting and penis extruded) towards each freeze-killed individual was counted. (no. replications/test = 10). Ester = methyl (E,R)-2,4,5-tetradecatrienoate, Oct = octadecanal, P.m.d. = Predicted mean difference, n.s. = not significant, P > 0.05 (α = 0.05)
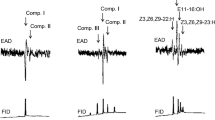
Comparative GC and GC/MS analyses of hexane extracts of both sexes revealed a high level of similarity (Supplementary material), apart from two compounds that were specific to males (Fig. 2). These were identified by GC/MS and co-injection with authentic standards as methyl (E,R)-2,4,5-tetradecatrienoate and octadecanal (999.5 ± 263.8 and 205.5 ± 51.72 ng/male, mean ± SE, respectively).
Gas chromatographic flame ionization detection (FID) chromatograms of Acanthoscelides obtectus hexane extracts. Beetles were extracted in 50 μl hexane for 10 min, then samples compared by GC (50 m × 0.32 mm i.d. HP-1 column). Chromatograms are on the same scale for comparison. Amounts (mean ± SE) of methyl (E,R)-2,4,5-tetradecatrienoate and octadecanal from males: 999.5 ± 263.8 and 205.5 ± 51.72 ng, respectively; on mated females, 102.3 ± 15.67 and 20 ± 4.1 ng, respectively
When male extract was applied in physiologically relevant amounts onto unwashed females in Test 4, no male courtship was observed (Fig. 1). Hexane treatment of unwashed females resulted in no reduction of male sexual behavior (Test 5, Fig. 1). Additionally, males showed the same type of response to females with and increased dose of female cuticular chemicals that was observed to females at the naturally occurring concentrations (Test 6, Fig. 1).
Total inhibition of mating behavior was observed when methyl (E,R)-2,4,5-tetradecatrienoate and octadecanal were added to unwashed females (Test 7, Fig. 1). However, when these chemicals were tested individually, octadecanal did not prevent male mating attempts (Test 8, Fig. 1), whereas methyl (E,R)-2,4,5-tetradecatrienoate completely inhibited this behavior (Test 9, Fig. 1).
The distribution of methyl (E,R)-2,4,5-tetradecatrienoate on the body of male A. obtectus was as follows: head 3.4 %, thorax (with legs only) 37.5, elytrae 39.1 %, abdominal tergites 0 %, pygidium 6.4 %, abdominal sternites 13.6 %, of a total amount per insect of ca. 1000 ng.
Males attempted to mate with virgin females more than with mated ones (Test 10, Fig. 1). Comparison of GC profiles of hexane extracts of females 24 h after mating revealed the presence of both methyl (E,R)-2,4,5-tetradecatrienoate and octadecanal, with 102.3 ± 15.67 and 20 ± 4.1 (mean ± SE) ng/female, respectively, while these compounds were absent in virgin females (Fig. 1). In Test 11, application of methyl (E,R)-2,4,5-tetradecatrienoate, in an amount similar to that extracted from one mated female, to virgin females eliminated male mating attempts completely (Fig. 1), whereas application of octadecanal at this level had no such effect (Test 12, Fig. 1). GC analyses of hexane extracts of mated females, 72 or 144 h after the initial 24 h copulation period, revealed the absence of methyl (E,R)-2,4,5-tetradecatrienoate and octadecanal.
Discussion
We demonstrated that A. obtectus males recognize other males, and thus reduce the frequency of same-sex mating attempts, by utilizing a male-specific chemical signal, methyl (E,R)-2,4,5-tetradecatrienoate, found on the cuticle, which is perceived through contact chemoreception. The absence of this compound indicates the presence of a virgin female and allows mating to commence.
Methyl (E,R)-2,4,5-tetradecatrienoate is known as a male-produced sex pheromone of A. obtectus that attracts virgin females (Halstead 1973; Hope et al. 1967; Horler 1970). Its role as a contact signal that contributes to sex recognition and aids mate choice is somewhat unexpected, as contact pheromones with more than 20 carbons were identified previously (Buckner 2010). Methyl (E,R)-2,4,5-tetradecatrienoate can be obtained from the cuticle after wiping the surface of male A. obtectus with silica gel, then extracting the silica with diethyl ether (Vuts et al. unpublished). GC profiles of these extracts show qualitative and quantitative similarities to those of direct solvent extracts, indicating that methyl (E,R)-2,4,5-tetradecatrienoate is present on the cuticle. Interestingly, spiders utilize the saturated structural analog, methyl tetradecanoate, as a contact sex pheromone (e.g., Prouvost et al. 1999), although further conclusions about any functional analogy are problematic between such distant taxa. Johansson and Jones (2007) suggest that “signals used in species recognition could evolve from signals with mate recognition or mate assessment functions”. Long-distance signaling by highly specific female-emitted sex pheromones aids reproductive isolation in many insects, even among closely related species (Löfstedt 1993; Smadja and Butlin 2009). However, pheromones also can be used to fine-tune the reproductive behavior of individuals of a given species. Some of these, unlike other sex pheromones, are predicted not to be under high selective pressure to evolve species-specificity (Brent and Byers 2011), and can comprise ubiquitous chemicals active in a number of biological systems. In light of this, male-produced methyl (E,R)-2,4,5-tetradecatrienoate may have been utilized originally only for mate recognition, but a new role of species recognition has emerged.
Contact sex pheromones, mostly comprising hydrocarbons, have been described from females of a number of insects (Howard and Blomquist 2005), including two bruchid beetles, in which females produce mating elicitors that guide male sexual behavior (Nojima et al. 2007; Tanaka et al. 1981;). To our knowledge, only a few reports exist of male-produced contact compounds that play a role in sexual communication. These chemicals, termed as abstinons, block mating attempts by conspecific males, and have been identified from tsetse flies, Glossina spp., and the house fly, Musca domestica L. (Diptera) (Carlson and Schlein 1991; Nelson et al. 1981; Schlein et al. 1980). Kim et al. (1993) described a blend of saturated and unsaturated hydrocarbons that serves as an abstinon in males of the longhorn beetle Semanotus japonicus Lacordaire (Coleoptera: Cerambycidae).
In addition to the new role as a male-recognition signal, methyl (E,R)-2,4,5-tetradecatrienoate appears to be used in A. obtectus for rendering mated females unattractive to males. It was isolated from mated, but not virgin, females, suggesting that it is transferred onto the female cuticle during mating, most probably through physical contact, similar to that for tsetse flies (Carlson and Schlein 1991) and crickets (Weddle et al. 2013). In the latter, it is females that mark males during mating with cuticular hydrocarbons to identify previous mates, thus avoiding them for future mating. Nonetheless, the mechanism itself might be similar in A. obtectus. Males mate with more than one female during their lifetime, whereas females show a pronounced resistance to re-mating, kicking off males during mounting attempts, similar to that in another bruchid, the cowpea seed beetle (Callosobruchus maculatus Fabricius) (Crudgington and Siva-Jothy 2000; Huignard 1974). This conflict between the sexes could be due partially to a toxic seminal compound in the spermatophore that is transferred during mating and which shortens female longevity (Das et al. 1980), or to direct physical injuries to females during copulation (Crudgington and Siva-Jothy 2000; Lange et al. 2013). The amount of methyl (E,R)-2,4,5-tetradecatrienoate isolated from one female 24 h post-mating was enough to totally inhibit mounting and copulation attempts by males. However, observations of female A. obtectus re-mating 3–4 days after an initial mating (Maklakov et al. 2007) could indicate erosion of female unattractiveness. With regard to this, disappearance of the transferred male anti-aphrodisiac, methyl (E,R)-2,4,5-tetradecatrienoate, ca. 48 h after mating, by an as yet unknown mechanism, was observed in the present study. This phenomenon has also been described in Pieris butterflies (Andersson et al. 2000). Although mated females display resistant behavior to re-mating, they are no longer unattractive to males, which may then perform traumatic mating.
It seems that the costs and benefits of polygamy may differ between the sexes of A. obtectus, which creates potential for sexual conflict due to divergence of interests (Maklakov et al. 2007). The use of male-deposited scent signals on the female cuticle may relax such conflict by conferring selective advantage on both sexes. Multiple mating by females creates an opportunity for sperm competition (Parker and Pizzari 2010), but males could reduce this by marking mated females with methyl (E,R)-2,4,5-tetradecatrienoate, thereby imposing monandry, as well as invoking preferential selection of unmated females. Studies by Maklakov et al. (2007) also suggest that “males that succeed in mating with a virgin female will father all eggs produced by this female for, typically, at least a few days.” Thus, males would be expected to evolve an ability to discriminate female mating status, for which, chemical signals provide a feasible solution (Thomas 2011). Females would benefit from male-produced anti-aphrodisiacs through reduced male harassment, similar to the green-veined white butterfly, Pieris napi L. (Lepidoptera: Pieridae), that uses a male-produced volatile anti-aphrodisiac, methyl salicylate (Andersson et al. 2000), or to the Western tarnished plant bug, Lygus hesperus Knight (Heteroptera: Myridae), which utilizes myristyl acetate (Brent and Byers 2011). Theoretically, A. obtectus females could benefit from re-mating because males also transfer a nutritious substance in their spermatophore during mating (Das et al. 1980) that prolongs female lifespan (Tucić et al. 1996). According to Slatyer et al. (2012), re-mating also could hypothetically increase female reproductive success via the avoidance of inbreeding depression, a factor described in isolated populations of C. maculatus (Tran and Credland 1995). However, studies by Maklakov et al. (2006, 2007) indicate that costs of re-mating outweigh any benefits for females of A. obtectus.
The results presented here provide better insight into the sexual behavior of A. obtectus. On the one hand, they confirm the role of cuticular compounds in mate recognition proposed by Savković et al. (2012) and Stojković et al. (2014); on the other, they demonstrate the parsimonious use of a compound in intra-species chemical communication. Further studies are required to elucidate fully the pheromone biology of A. obtectus, thereby creating a platform for investigations of a more applied nature.
References
Abate T, Ampofo JKO (1996) Insect pests of beans in Africa: their ecology and management. Annu Rev Entomol 41:45–73
Andersson J, Borg-Karlson A-K, Wiklund C (2000) Sexual cooperation and conflict in butterflies: a male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc R Soc Lond B 267:1271–1275
Annoscia D, Bruce T, Hooper T, Nazzi F, Pickett J (2010) Biological role of a compound produced by Acanthoscelides obtectus males. International Society of Chemical Ecology 26th Annual Meeting, Tours, France, 2010; P029, p 212
Brent CS, Byers JA (2011) Female attractiveness modulated by a male-derived anti-aphrodisiac pheromone in a plant bug. Anim Behav 82:937–943
Buckner JS (2010) Oxygenated derivatives of hydrocarbons. In: Blomquist GJ, Bagnères A-G (eds) Insect hydrocarbons. Biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge, pp 187–206
Carlson DA, Schlein Y (1991) Unusual polymethyl alkenes in tsetse flies acting as abstinon in Glossina morsitans. J Chem Ecol 17:267–284
Coates TW, Langley PA (1982) The causes of mating abstention in male tsetse flies Glossina morsitans. Physiol Entomol 7:235–242
Crudgington HS, Siva-Jothy MT (2000) Genital damage, kicking and early death: the battle of sexes takes a sinister turn in the bean weevil. Nature 407:855–856
Das AK, Huignard J, Barbier M, Quesneau-Thierry A (1980) Isolation of the two paragonial substances deposited into the spermatophores of Acanthoscelides obtectus (Coleoptera, Bruchidae). Cell Mol Life Sci 36:918–920
Griffith WP, Ley SV, Whitcombe GP, White AD (1987) Preparation and use of tetra-n-butylammonium per-ruthenate (TBAP reagent) and tetra-n-propylammonium per-ruthenate (TPAP reagent) as new catalytic oxidants for alcohols. J Chem Soc, Chem Commun. 1625–1627
Halstead DGH (1973) Preliminary biological studies on the pheromone produced by male Acanthoscelides obtectus (Say) (Coleoptera, Bruchidae). J Stored Prod Res 9:109–117
Hope JA, Horler DF, Rowlands DG (1967) A possible pheromone of the bruchid, Acanthoscelides obtectus (Say). J Stored Prod Res 3:387–388
Horler DF (1970) (−)-Methyl n-tetradeca-trans-2,4,5-trienoate, an allenic ester produced by the male dried bean beetle, Acanthoscelides obtectus (Say). J Chem Soc C 859–862
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Huignard J (1974) Influence de la copμlation sur la function reproductrice femelle Acanthoscelides obtectus (Insecte coléoptère). I. Copμlation et spermatophore. Ann Sci Nat Zool Paris 16:361–434
Imura O (1990) Life histories of stored product insects. In: Fujii K, Gatehouse AMR (eds) Bruchids and legumes: Economics, ecology and coevolution. Kluwer, pp 257–269
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Kim G-H, Takabayashi J, Takahashi S, Tabata K (1993) Function of contact pheromone in the mating behavior of the Cryptomeria bark borer, Semanotus japonicus Lacordaire (Coleoptera: Cerambycidae). Appl Entomol Zool 28:525–535
Lange R, Reinhardt K, Michiels NK, Anthes N (2013) Functions, diversity, and evolution of traumatic mating. Biol Rev 88:585–601
Löfstedt C (1993) Moth pheromone genetics and evolution. Phil Trans R Soc Lond B 340:167–177
Maklakov AA, Kremer N, Arnqvist G (2006) Ageing and the evolution of female resistance to remating in seed beetles. Biol Lett 2:62–6
Maklakov AA, Kremer N, Arnqvist G (2007) The effects of age at mating on female life-history in a seed beetle. Behav Ecol 18:551–555
McCullagh, P, Nelder, JA (1989) Generalized linear models. Chapman & Hall/CRC Monographs on Statistics and Applied Probability, 37, 2nd ed, pp 511
Mori K (2012) Pheromone synthesis. Part 249: Syntheses of methyl (R, E)-2,4,5-tetradecatrienoate and methyl (2E,4Z)-2,4-decadienoate, the pheromone components of the male dried bean beetle, Acanthoscelides obtectus (Say). Tetrahedron 68:1936–1946
Nelson DR, Dillwith JW, Blomquist GJ (1981) Cuticμlar hydrocarbons of the house fly, Musca domestica. Insects Biochem 11:187–197
Nojima S, Shimomura K, Honda H, Yamamoto I, Ohsawa K (2007) Contact sex pheromone components of the cowpea weevil, Callosobruchus maculatus. J Chem Ecol 33:923–933
Parker GA, Pizzari T (2010) Sperm competition and ejaculate economics. Biol Rev 85:897–934
Pickett JA (1990) GC-MS in insect pheromone identification: three extreme case histories. In: McCaffery AR, Wilson ID (eds) Chromatography and isolation of insect hormones and pheromones. Plenum Press, pp 299–309
Prouvost O, Trabalon M, Papke M, Schulz S (1999) Contact sex signals on web and cuticle of Tegenaria atrica (Araneae, Agelenidae). Arch Insect Biochem Physiol 40:194–202
Savković U, Vučković I, Stojković B (2012) The growth on different stored legume species affects the profiles of cuticμlar hydrocarbon (CHC) in Acanthoscelides obtectus (Say). J Stored Prod Res 50:66–72
Schlein Y, Galun R, Ben-Eliahu MN (1980) Abstinons. Male-produced deterrents of mating in flies. J Chem Ecol 7:285–290
Slatyer RA, Mautz BS, Backwell PRY, Jennions MD (2012) Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol Rev 87:1–33
Smadja C, Butlin RK (2009) On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102:77–97
Southgate BJ (1979) Biology of the bruchidae. Annu Rev Entomol 24:449–473
Stojković B, Savković U, Đorđević M, Tucić N (2014) Host-shift effects on mating behavior and incipient pre-mating isolation in seed beetle. Behav Ecol 25:553–564
Tanaka K, Ohsawa K, Honda H, Yamamoto I (1981) Copμlation release pheromone, erectin, from the azuki bean weevil (Callosobruchus chinensis L.). J Pestic Sci 6:75–82
Thomas ML (2011) Detection of female mating status using chemical signals and cues. Biol Rev 86:1–13
Tran BMD, Credland PF (1995) Consequences of inbreeding for the cowpea seed beetle, Callosobruchus macμlatus (F.) (Coleoptera: Bruchidae). Biol J Linn Soc 56:483–503
Tucić N, Cliksman I, Śeślija D, Milanovic D, Mikμljanac D, Stoikovic O (1996) Laboratory evolution of longevity in the bean weevil (Acanthoscelides obtectus). J Evol Biol 52:1713–1725
Weddle CB, Steiger S, Hamaker CG, Ower GD, Mitchell C, Sakaluk SK, Hunt J (2013) Cuticular hydrocarbons as a basis for chemosensory self-referencing in crickets: a potentially universal mechanism facilitating polyandry in insects. Ecol Lett 16:346–353
Acknowledgments
The authors thank Professors Kenji Mori and Wittko Francke for providing enantiomerically pure samples of methyl (E,R)-2,4,5-tetradecatrienoate. The authors are grateful for the comments of Professor Tibor Jermy and Dr Árpád Szentesi, as well as two anonymous reviewers, on an earlier version of the manuscript. This work was in part supported by the Hungarian Scientific Research Fund (OTKA) (grant K81494), by the Research and Technology Innovation Fund (grant OMFB-00609/2010) and by the Royal Society Grant IE111142 to JV. Rothamsted receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 98 kb)
Rights and permissions
About this article
Cite this article
Vuts, J., Powers, S.J., Caulfield, J.C. et al. Multiple Roles of a Male-Specific Compound in the Sexual Behavior of the Dried Bean Beetle, Acanthoscelides Obtectus . J Chem Ecol 41, 287–293 (2015). https://doi.org/10.1007/s10886-015-0560-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0560-3