Abstract
Queen pheromones evolved independently in multiple eusocial insect lineages, in which they mediate reproductive conflict by inhibiting worker ovarian development. Although fundamentally important for reproductive division of labor – the hallmark of eusociality – their evolutionary origins are enigmatic. Here, we analyze cuticular and Dufour’s gland chemistries across alternative social and reproductive phenotypes in Megalopta genalis bees (tribe Augochlorini, family Halictidae) that facultatively express simple eusociality. Reproductive bees have distinct overall glandular and cuticular chemical phenotypes compared with non-reproductive workers. On the cuticle, a likely site of signal transmission, reproductives are enriched for certain alkenes, most linear alkanes, and are heavily enriched for all methyl-branched alkanes. Chemicals belonging to these compound classes are known to function as fertility signals in other eusocial insect taxa. Some macrocyclic lactones, compounds that serve as queen pheromones in the other eusocial halictid tribe (Halictini), are also enriched among reproductives relative to workers. The intra-population facultative eusociality of M. genalis permits direct comparisons between individuals expressing alternative reproductive phenotypes – females that reproduce alone (solitary reproductives) and social queens – to highlight traits in the latter that may be important mediators of eusociality. Compared with solitary reproductives, the cuticular chemistries of queens are more strongly differentiated from those of workers, and furthermore are especially enriched for methyl-branched alkanes. Determining the pheromonal function(s) and information content of the candidate signaling compounds we identify will help illuminate the early evolutionary history of queen pheromones, chemical signals central to the organization of insect eusocial behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The switch from solitary to eusocial reproductive organization is among the major transitions in evolutionary history (Maynard Smith and Szathmáry 1995). Eusociality is defined by a multigenerational division of reproductive labor between reproductive ‘queen’ and non-reproductive ‘worker’ castes, the latter working co-operatively to rear the queen’s offspring (Michener 1974; Wilson 1971). Coordination of activities between the members of these eusocial societies is critical, and is achieved in part by an increased investment in the sending and receiving of signals (Billen and Morgan 1998; Wittwer et al. 2017; Zhou et al. 2015). Insects rely heavily on chemical communication as a means of guiding behavior, and it follows that many of the signals important for regulating eusocial societies are pheromonal (Wyatt 2014). Analogous to the role of inter-cellular chemical messengers in multicellular organisms (i.e. hormones), chemical communication is critical for the organization of defense, resource acquisition, and partitioning of reproductive activity in social insect societies (Leonhardt et al. 2016). So-called ‘queen pheromones’ are of particular interest, given their central role in the establishment and maintenance of the reproductive skew that defines eusociality. These substances are produced solely or in relative excess by queens, and have short-term behavioral (“releaser”) and longer-term physiological (“primer”) effects on workers that include the inhibition of worker reproductive development (Conte and Hefetz 2008; Liebig 2010). While the chemical identities of the compounds involved vary across taxa, their repeated functional evolution across multiple independent evolutionary origins of eusociality in insects belies their critical role. Despite the importance of queen pheromones in facilitating eusocial organization, their early origins and evolution remain obscure.
Early interpretations of queen pheromone primer effects in advanced eusocial species tended to characterize their inhibition of subordinate reproduction as a sign of manipulative queen ‘control’ over workers’ reproductive best interests (Alexander 1974; Fletcher and Ross 1985). More recently it has been argued that queen pheromones function instead as honest signals communicating the queens’ reproductive quality to the worker caste, though the mechanisms that ensure honest signaling are still not fully understood (Holman 2012; Keller and Nonacs 1993; Kocher and Grozinger 2011; Oi et al. 2015; Orlova et al. 2020; Peso et al. 2015). The core distinction between the ‘queen control’ and ‘honest signal’ hypotheses is that the worker response to pheromones is predicted to maximize their inclusive fitness only in the latter case. In contrast, ‘controlling’ pheromones are predicted to induce suboptimal responses from the worker fitness perspective. While evidence for honest fertility signaling has accumulated for some advanced eusocial species (e.g., Holman 2012), it is possible that ‘queen control’ and ‘queen signal’ adaptive functions could vary with social complexity. In nascent eusocial societies with small colony sizes and minimal queen-worker skew in reproductive potential, comparatively high levels of reproductive conflict may tend to favor dishonest signaling or ‘control’ mechanisms given the relatively divergent reproductive interests of queens and their workers (Bourke 1999). Honest signaling of queen reproductive potential may alternatively be more strongly enforced in relatively simple eusocial societies, in which the relative benefits to workers of pursuing direct versus indirect fitness are likely to be highly dependent on queen condition (Kocher and Grozinger 2011).
Additional unresolved questions surrounding the early origin of queen pheromones include a) the ancestral functions of compounds that later gain queen pheromone functionality in evolutionary transitions to eusociality (Leonhardt et al. 2016), b) the level of eusocial complexity at which queen pheromone ‘signals’ evolve from antecedent ‘cues’ (Michener and Brothers 1974; Wyatt 2014), and c) whether chemical fertility signals guide the expression of conditional worker helping behavior at incipient stages in eusocial evolution (Holman 2014). Insect lineages in which eusociality is evolutionarily nascent and variable in its expression both within (i.e. facultative) and between species are particularly well suited to address these questions. However, chemical signaling of dominance and fertility has been studied mainly in taxa for which eusociality is both advanced and an evolutionarily conserved, ancient, and obligate trait (see Van Oystaeyen et al. 2014). Representatives of these taxa are ideal for questions about the current function of queen pheromones, but are less apt for addressing questions surrounding their evolutionary origins.
Facultatively eusocial insects provide a powerful means of assessing the ecological factors that favor the evolution of eusociality (Purcell 2011; Wcislo 1997), as well as the genetic and physiological mechanisms that underpin its expression (Jones et al. 2017; Schwarz et al. 2007). As yet, however, queen pheromones have not been identified for any facultatively eusocial species. In Hymenoptera, facultative eusociality is characteristic of the wasp subfamily Stenogastrinae and the bee subfamilies Xylocopinae and Halictinae (Field 2008; Rehan and Toth 2015; Schwarz et al. 2007; Wcislo and Fewell 2017). Intra-specific variation in social behaviour is especially well documented among the Halictinae (e.g. Cronin and Hirata 2003; Eickwort et al. 1996; Field et al. 2010; Packer 1990; Richards et al. 2003; Soucy and Danforth 2002). Both bee subfamilies are particularly well suited to comparative studies because they also include reversals to solitary behavior, and thus span the full range of nesting behaviors from solitary to eusocial (Kocher and Paxton 2014; Schwarz et al. 2007; Wcislo and Danforth 1997). These taxa also include the most recent origins of eusociality in Hymenoptera, evolving once ~53 million years ago (mya) in Xylocopinae and twice in Halictinae: once in the tribe Halictini ~35 mya and once in the tribe Augochlorini ~17 mya (Cardinal and Danforth 2011; Gibbs et al. 2012; Rehan et al. 2012). The transition to eusocial nesting Augochlorini is the most recent known among Hymenopteran insects (Kocher and Paxton 2014).
The two independent origins of eusociality in Halictinae provide promising avenues for studying the origin and evolution of queen pheromones. Early studies focused primarily on the role of queen aggressive behavior in the repression of worker ovarian development among primitively eusocial halictid species (Brothers and Michener 1974), but pheromonal signals have more recently been shown to play an important, likely complementary, function. In the genus Lasioglossum (tribe Halictini), macrocyclic lactone compounds present both in Dufour’s glands and on the cuticular surface were shown to exert primer and releaser queen pheromone effects in L. malachurum, and to have similar releaser functions in L. pauxillum (both obligately eusocial: Steitz et al. 2019; Steitz and Ayasse 2020).
Here, we characterize the Dufour’s gland and cuticular chemistries of the tropical augochlorine bee M. genalis, a facultatively eusocial species with a totipotent worker caste that expresses helping behavior conditionally. Social (and solitary) insects make extensive use of cuticular hydrocarbons and other lipids to communicate information such as individual identity, fertility status, sex, nest membership, kinship, and task allocation (reviewed in Ginzel and Blomquist 2016). Among the aculeate Hymenoptera, the Dufour’s gland has repeatedly evolved a comparable social signaling function (reviewed in Mitra 2013). Because the emission site of potential signaling compounds is unknown for M. genalis, we compare both cuticular and glandular chemical profiles of adult females expressing alternate reproductive phenotypes (workers, solitary reproductives, and queens), with the aim of highlighting candidate fertility cues or queen pheromone signals. While all compounds biased towards reproductive castes are potential candidates for these roles, the unique reproductive polyphenism of M. genalis enables further refinement of promising substances through the contrast of sympatric and contemporaneous queens and solitary reproductives. Compounds likely to be relevant for social signaling are predicted to be especially enriched in the chemical profiles of queens relative to solitary reproductives, particularly if those compounds are dedicated (and potentially costly) signals rather than constitutively produced cues of reproductive activity.
Methods and Materials
Megalopta genalis Social Behavior Synopsis
Megalopta genalis is unusual amongst facultatively eusocial Halictinae for its intra-population social polyphenism that is apparently stable over a wide geographical range (Tierney et al. 2013). Foundress females disperse from natal nests, become singly mated (Kapheim et al. 2012) and excavate a nest tunnel in the pith of a suspended branch in the forest understory. Foundresses then pursue one of two alternative strategies: about one third produce all-male first broods (despite having mated) and provision nests alone as ‘solitary reproductives’. The other two thirds produce at least one female in the first brood, and these daughters may remain in the nests as non-reproductive workers. Social nests generally contain a single queen and 1–3 workers (mode = 1) who conduct the majority of foraging trips and mate (<5% worker matedness) and lay eggs (~2–3% worker-laid eggs) only very rarely (Kapheim et al. 2012, 2013). The physiological factors determining the pursuit of alternative foundress strategies are not completely understood, but relative to solitary reproductives the body sizes, ovary sizes, vitellogenin titers, and juvenile hormone titers of queens are greater (Kapheim et al. 2012, 2013; Smith et al. 2013). Allelic variation at some genetic loci may also influence this life history bifurcation (Kapheim et al. 2020). M. genalis also exhibit facultative expression of the worker phenotype: while first brood females are typically smaller than their foundress mothers, a consequence of maternal manipulation of brood cell provisions, only three quarters of these apparently ‘worker-destined’ first brood female offspring remain in the nests beyond 10 days as non-reproductive workers (Kapheim et al. 2011, 2012, 2013). Among workers remaining in natal nests, the incipient queen-worker dominance hierarchies are established during the course of this 10 d ‘dispersal window,’ during which time queens feed incipient workers by trophallaxis (Wcislo and Gonzalez 2006) but are also aggressive towards them, eliciting worker subordinate behaviors (Kapheim et al. 2016).
Study Site
All fieldwork and collections were performed on Barro Colorado Island (BCI, 9°09′ N, 79°51′ W) in Panamá Province, the Republic of Panamá. BCI is a tropical semi-deciduous moist forest with pronounced wet and dry seasons and a mean annual temperature of 27 °C; peak reproductive activity of Megalopta occurs during a short dry season (<100 mm monthly rainfall) between January and April (Leigh 1999; Smith et al. 2019).
Nest Collection and Monitoring
Wild M. genalis nests (Fig. 1) were collected in the forest understory on BCI during daylight hours, when all adult bees are present. Nests were opened the same day, and all brood cells were placed in 24-well plates (Corning Inc., Corning, NY) where brood development continued at ambient temperature and humidity. Brood plates were monitored daily between 24-January and 11-May of 2015, and freshly-eclosed females were moved each morning into observation nests consisting of a slab (½” × 3″ × 9″) of extra-light density balsa wood (Specialized Balsa Wood, LLC, Loveland, CO) sandwiched between acrylic plates and held together with binder clips (Wcislo and Gonzalez 2006; pictured in Smith et al. 2013). A 5″ tunnel with a single exit was pre-drilled in the balsa slab, and the ‘foundress’ was plugged inside using a cotton ball partly dipped in a dilute (~30%) solution of honey water. This plug was removed after 24 h, and the observation nest was placed in the forest understory below a plastic rainproof hood ~1 m above the ground. The construction and closure of brood cells by each foundress was monitored every 3 d during daylight hours: on the morning of the 15th day following the first brood cell closure, the foundress was removed and marked on the left wing with a white DecoColor® oil-based paint pen. Beginning 30 d after the closure of the first brood cell (egg to adult development in Megalopta takes 35 d), nests were monitored daily to determine the exact emergence date of the first offspring. The dates of first offspring emergence were recorded, and the day after emergence any newly-eclosed females (potential workers) were marked on the right wing with the paint pen. Nest activity – cell openings, closures, and number of adult males and females – was monitored daily until the 10th day after eclosion of the first offspring, when all adult bees were sampled (29-Mar to 11-Aug 2015).
Physical Measurements and Chemical Sample Collections
For sampling, bees were coaxed from their nests into pre-weighed 15 ml glass vials previously cleaned with acetone, hexane, and baked at 150 °C to remove contaminants. This vial was then re-weighed to 0.1 mg to obtain mass, and the thorax of the bee was photographed dorsally with a stereoscope camera at 6.4x magnification to measure intertegular distance, a metric of body size (Cane 1987). Bees were anaesthetized by placing the vials on ice for 90 s, and the head width was measured with digital calipers (Mitutoyo Corp., Japan) to 0.1 mm. Wings were removed to prevent contamination by paint-derived compounds. Cuticular extracts were collected by submerging the entire bee in 2 ml pentane (HPLC-grade, 34,956, Sigma Aldrich) in a chemically cleaned 15 ml glass vial that was swirled for 60 s before the bee was removed with clean forceps. Immediately following the collection of cuticular extracts, the abdomen was dissected under double-distilled water. The coloration (clear or amber) of the Dufour’s gland contents was noted, and the whole excised gland was photographed dorsally at 6.4x magnification. The gland was removed from solution, briefly dried with a clean absorbent tissue, and torn from the sting apparatus before being immediately submerged in 0.5 ml pentane for 24 h. The ovaries were separated from the abdominal cavity and photographed at 10x magnification from above. The overhead surface areas of the Dufour’s glands and ovaries (mm2), as well as the lengths of Dufour’s glands and the intertegular distance, were measured in ImageJ (v1.51).
Chemical Analysis
Cuticular extracts were evaporated under gentle nitrogen stream to 200 μl, and Dufour’s gland extracts were adjusted to 500 μl. Both sample types were spiked with 740 ng undecane (analytical standard, 94,000, Sigma Aldrich) internal standard dissolved in HPLC-grade heptane (5 μl of 148 ng/μl stock solution). An Agilent autosampler was used to inject 1 μl of each sample into an Agilent 6890 N gas chromatograph (GC) fitted with an HP5-MS capillary column (Agilent, 0.25 mm × 30 m × 0.25 μm). The GC injector was operated in splitless mode using ultra-high purity helium as the carrier gas, at a constant flow rate of 1 ml/min. The injection port temperature was held at 250 °C and the temperature program of the oven was as follows: 50 °C for 1 min, ramp of 10 °C/min to 310 °C, then held for 9 min before dropping at 10 °C/min to 300 °C where the temperature was held for a final 4 min (40 min total runtime). The GC oven was linked to an Agilent 5973 N mass selective detector operated in electron ionization mode with the transfer line at 280 °C and the ion source at 230 °C.
Compound identifications were first tentatively assigned by comparison of mass spectra and Kovats retention indices (KI) with those of published database standards (NIST 2014 mass spectral library). Tentative identifications of compounds belonging to the following chemical classes were further refined by comparison of KI values and mass spectra with those reported in the literature: methylalkanes (Carlson et al. 1998), sesquiterpene esters (Tengö and Bergström 1975), sesquiterpenes (Adams 2007; Krasulová et al. 2012), ketones (Strohm et al. 2008; Yasui et al. 2003), macrocyclic lactones (Ayasse et al. 1990; Duffield et al. 1981; Peram et al. 2017; Schulz et al. 2017; Soro et al. 2011), isopentenyl esters (Ayasse et al. 1990; Duffield et al. 1981) and ethyl esters (Ayasse et al. 1990). We use the term ‘terpenoid esters’ to refer to sesquiterpene esters only (isopentenyl esters are hemiterpene esters). Macrocyclic lactones, which were detected in both cuticular and Dufour’s extracts, were of particular a priori interest given their signal function in other halictid species (Steitz et al. 2019; Steitz and Ayasse 2020). In addition to saturated unbranched and monomethyl-branched lactones of carbon ring sizes between 16 and 28, we identified a series of unsaturated lactones (18 to 26 carbons) eluting immediately after the saturated lactone of corresponding ring size. The derivatization procedure used to determine the double-bond positions of unsaturated lactones is described in the online resources. Determination of alkene double bond positions was not attempted, but previous work on cuticular and glandular chemistries of halictid bees indicates that (Z)-9 and (Z)-7 alkenes predominate (Steitz et al. 2018). Determination of linear alkanes, methylalkanes, and saturated unbranched macrocyclic lactones was confirmed by co-injection of synthetic reference standards; all other compound identifications should be considered tentative.
Peak areas for all compounds were determined by integration using Chemstation software (Agilent Technologies, Inc.) and aligned manually across samples by visual inspection of the corresponding spectra. The integration parameters used are provided in the online resources. Only compounds present in ≥50% of individuals from at least one of the three castes were retained for further analysis (Dufour’s and cuticular extracts considered separately). The absolute amount of all compounds was estimated for each sample extract by dividing the total area of all remaining peaks by the internal standard peak area and multiplying by the amount of standard present (740 ng).
Statistical Analyses
Differences in overall cuticular and glandular chemical profiles (relative abundance) between workers, queens, and solitaries were visualized with non-metric multidimensional scaling (NMDS) of Bray-Curtis similarity index values. NMDS multivariate visualization was selected because it is suitable for datasets with large numbers of variables relative to sample size, presence of zero values, and non-normally distributed variables (Brückner and Heethoff 2017). Stress values of <0.2 are considered to reliably reflect multivariate differences (Kruskal 1964). Bray-Curtis values were also used to compare overall chemical phenotypes between castes using non-parametric one-way analysis of similarities tests (ANOSIM, 10,000 permutations, post-hoc pairwise tests with sequential Bonferroni corrections). NMDS and ANOSIM tests were implemented in PAST (v. 3.24, Hammer et al. 2001).
Differences in mean ovary area, body mass, head width, Dufour’s gland size, intertegular distance, and total number of compounds were compared across castes with ANOVA followed by post-hoc Bonferroni-corrected t-tests. Mean age at sampling of solitaries and queens was compared using a Mann-Whitney U-test. Disparities between members of the three alternative social phenotypes in relative amounts of individual compounds, summed compounds classes, and total absolute amounts were determined with non-parametric tests (Kruskal-Wallis tests with post-hoc pairwise Bonferroni-corrected Dunn’s tests). Individual compounds or compound classes were labeled as queen-biased, solitary-biased, or worker-biased if members of the indicated caste had significantly increased mean relative abundance values compared to members of other castes. Many compound relative abundance values were overrepresented in both queens and solitaries as compared with workers: those compounds and compound classes were labeled as ‘reproductive-biased’. We observed differing patterns of caste-biases between large (≥24 carbon) and small (≤ 22 carbon) ring size macrocyclic lactones, compounds of special interest given their queen pheromone function in other halictid bees (Steitz and Ayasse 2020). To further explore this trend, we examined inter-correlations between macrocyclic lactones of various ring-sizes in the Dufour’s glands of all bees in the dataset (n = 37) using non-parametric Spearman’s correlations. Small and large ring-size lactones were found to co-vary independently, indicative of separate biosynthetic regulation; we therefore compared the summed relative abundance values for each lactone subclass separately between castes. All univariate statistical tests were implemented in SPSS (v. 26).
To visualize contributions of individual chemical compounds to chemical caste differences, we generated heatmaps using the pheatmap package in R (v. 3.6.1, R Core Team 2019) following methods adapted from Princen et al. (2019). Individual bees are depicted as columns in the heatmap and are hierarchically clustered based on an unsupervised weighted pair group method with arithmetic mean (WPGMA), using one minus the Pearson correlation as the distance metric. In calculating the distance metric, relative peak areas were log10-transformed to better approximate a normal distribution. Heatmap colors were generated using z-scores (constrained between −2 and 2) calculated for each compound (i.e., row) from transformed relative peak areas to illustrate the degree of departure from the row average. Though all compounds contribute to WPGMA clustering, heatmaps for each analysis (cuticular and glandular profiles) were manually clipped to show only those compounds that differed significantly between castes.
Results
Social Phenotype (Caste) Characteristics
Observation nest collections totaled 11 social nests (queen-worker pairs) and 16 solitary nests. Age at sampling did not differ between queens and solitaries (Mann-Whitney U = 68.5, P = 0.342; Table S1). All workers were sampled at 10 days post-eclosion. Both reproductive phenotypes (queens and solitaries) had larger ovaries and Dufour’s glands than those of workers, and queens – but not solitaries – were heavier than workers (Table S1).
Cuticular and Glandular Chemical Diversity
110 compounds were present across all individuals and both extract types (cuticular and glandular). After removing compounds detected in less than 50% of individuals in all three castes, 89 compounds remained: 63 of these occurred in Dufour’s glands (18 specific to gland samples) and 71 on the cuticle (26 specific to cuticular samples). Across both sample types, identified compounds could be grouped into 11 classes: linear alkanes, methylalkanes, alkenes, ketones, macrocyclic lactones (MLs), ethyl esters, isopentenyl esters, isopropyl esters, terpenoid (sesquiterpene) esters, sesquiterpenes, and the triterpenoid squalene (Table S2). While at least one compound from each class was represented on the cuticle, Dufour’s glands lacked all 12 methylalkanes, both ketones, and squalene. Nine compounds could not be tentatively or definitively identified. Four could not be reliably assigned to any chemical class, two had retention indices and mass spectra characteristic of sesquiterpenes, and three were determined to be likely terpenoid esters (Tables S2 & S3). All unknowns were low abundance compounds (<1% in all castes).
Cuticular and glandular samples differed in their relative representations of the different compound classes. The three classes of cuticular hydrocarbons – linear alkanes, methylalkanes, and alkenes – occurred at higher relative amounts in cuticular compared with Dufour’s extracts, though linear alkanes and alkenes were also present in Dufour’s glands. Sesquiterpenes and all ester classes (including ML ‘cyclic esters’) were relatively more abundant in glands than cuticles. Correlation analysis of unbranched, saturated MLs of varying ring size (16–28 carbons) in the Dufour’s glands revealed co-varying subsets of MLs. 18-octadecanolide varied independently of other lactones, 20-eicosanolide and 22-docosanolide abundances were correlated, and 24-tetracosanolide co-varied with the low abundance 26 and 28 membered lactones (Table S4). Detailed information on the double bond and methyl-branch positions of the lower-abundance unsaturated and branched MLs is provided in the online resources.
Caste Differences in Overall Cuticular Chemistry
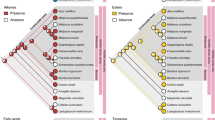
The number of cuticular compounds per individual was greater in reproductive queens (Q) and solitaries (S) than among workers (W) (mean ± SE: Q = 63 ± 2, S = 60 ± 2, W = 53 ± 2; one-way ANOVA, F = 6.329, P = 0.005; Table S2). However, the absolute amount of cuticular lipids did not differ between castes (Kruskal-Wallis test, W = 0.95, P = 0.622, Table S2). Overall cuticular profile was significantly associated with caste membership (ANOSIM: global R = 0.160, P = 0.006, Fig. 2A). Representative chromatograms for each caste are provided in the online resources (Fig. S1). NMDS visualization of cuticular profiles shows that workers and queens were strongly differentiated, and that each were tightly clustered, while solitaries had comparatively dispersed profiles which overlapped with both queen and worker profiles in chemical space (Fig. 2A). While the cuticular profiles of queens and workers diverged markedly (post-hoc pairwise ANOSIM: R = 0.361, P = 0.004), the difference between solitary and worker profiles was borderline insignificant (post-hoc pairwise ANOSIM: R = 0.176, P = 0.062). Queen and solitary cuticular profiles did not differ (post-hoc pairwise ANOSIM: R = 0.022, P = 0.871). Hierarchical clustering likewise clearly distinguished queen and worker profiles, but solitaries were interspersed between both queen and worker clusters: twelve solitaries clustered with queens and the remaining four with workers (Fig. 3).
A heatmap of cuticular compound relative amounts across adult female social phenotypes (castes). Individual bees, represented as columns, are hierarchically clustered based on a distance metric calculated from correlations between log10-transformed relative compound abundance values. Standardized differences (z-scores) in transformed relative peak areas compared to row averages are indicated in the heatmap for compounds (rows) exhibiting caste biases (assessed with Kruskal-Wallis tests); the direction of bias (queen, worker, or reproductive-skewed) is indicated on the left. Compounds with no significant differences (n = 36) between castes are omitted, but relative amounts of all cuticular compounds (71 total) contribute to the WPGMA clustering. Asterisks indicate the compounds illustrated for each major class
Caste Differences in Cuticular Chemicals
Worker cuticles presented relatively few compounds (a total of 4) occurring at high relative abundance compared with reproductive bees. The compound with the starkest worker bias is a heptacosadiene that constitutes nearly one quarter (22.44 ± 4.37%, mean ± SE) of the worker cuticular chemical profile (Fig. 3, Table S2). Biases towards workers were also observed for the isopentenyl ester 3-methyl-3-butenyl docosanoate, and for two low abundance cuticular compounds: a pentacosene and an unknown with a KI of 2751. We tentatively identify the KI 2751 compound as nonenyl octadecyl ether, based on its mass spectral fragmentation pattern and KI comparable to structurally similar ethers (Fig. S2). The relative abundances of many (28) individual cuticular compounds were biased towards both reproductive castes, including 7 linear alkanes, 6 alkenes, 5 MLs, an isopropyl ester, and all 12 methylalkanes (Table S2). Alkenes of carbon chain lengths C25-C27 were skewed towards workers, while heavier alkenes with chain lengths of C29-C35 were more abundant on the cuticles of reproductive bees. The abundance of small ring size MLs (≤22 carbons) tended to be conserved across castes, while inter-correlated large ring size MLs (≥24 carbons) were more abundant on reproductives (Table S2, Fig. S3). The summed relative abundances of four chemical classes differed with respect to caste. Isopentenyl esters were relatively more abundant on workers than solitaries, isopropyl esters (present in trace amounts across all castes) were relatively more abundant on queens than workers, and alkanes (both linear and methyl-branched) were relatively more abundant among reproductives than workers (Table S2, Fig. 4).
Mean (±SEM) relative amounts of all major compound classes in cuticular extracts across three adult female social phenotypes (castes). Major classes are those with >2% combined relative amount in at least one caste. Statistical significance of caste differences is determined with pairwise Bonferroni corrected U-tests: * = P < 0.05, ** = P < 0.01, *** = P < 0.001. The difference in linear alkane relative amounts between workers and queens meets only borderline statistical significance after correction (P = 0.058)
Chemical Differentiation of Social (Queen) and Solitary Reproductives
Methylalkanes had an extreme bias towards reproductives, being 78 times more abundant on queen and 34 times more abundant on solitary cuticles compared with workers. Queens had nearly double the relative amount of total monomethylalkanes than age-matched solitaries, but this distinction was not significant following Bonferroni correction (post-hoc Dunn’s test, Z = 8.08, P = 0.168, Table S2, Fig. 4). While all methylalkanes were enriched on the cuticles of queens relative to age-matched solitaries, centrally branched nonacosanes were the only compounds significantly biased towards queens compared with both workers and solitaries after correction for multiple testing (Table S2).
Caste Differences in Overall Dufour’s Gland Chemistry
As on the cuticles, mean compound diversity was higher in the Dufour’s glands of queens and solitaries compared with those of workers (mean ± SE: Q = 49 ± 4, S = 45 ± 4, W = 22 ± 5; one-way ANOVA, F = 10.276, P < 0.001). The absolute amount of glandular exudate was higher in reproductive Dufour’s glands than in the smaller glands of workers (Kruskal-Wallis test, W = 11.87, P = 0.002; post-hoc Dunn’s tests, Q > W, Z = 15.78, P = 0.003, S > W, Z = 11.35, P = 0.028, Table S2). Overall Dufour’s gland chemical makeup differed between castes (ANOSIM: global R = 0.225, P < 0.001, Fig. 2B). Caste-specific Dufour’s gland profiles differed primarily along a reproductive axis, with queen and solitary glands undifferentiated (pairwise ANOSIM, R = -0.012, P = 1) and worker glands differing from both reproductive castes (pairwise ANOSIM: Q-W R = 0.500, P < 0.001; S-W R = 0.290, P = 0.008). Cluster analysis of Dufour’s glands generated the same pattern, largely (but incompletely) separating individuals by their reproductive state (Fig. S4). One cluster included bees (mainly workers) producing a low diversity of Dufour’s gland chemicals, a second cluster included bees from all three reproductive phenotypes also producing reproductive-biased esters and MLs, and a final cluster included only reproductive bees with high biosynthetic diversity producing sesquiterpenes, terpenoid esters, and the large ring size MLs 26-hexacos-(Z)-17-enolide and 28-octadecanolide (Fig. S4, Table S2).
Caste Differences in Dufour’s Gland Chemicals
A majority of caste-biased Dufour’s gland compounds, including representatives from all chemical classes, were biased towards reproductives. Only one compound, 20-eicosanolide, was relatively more abundant in glands of workers (Kruskal-Wallis test, W = 15.7, P < 0.001; post-hoc Dunn’s tests: W > S, Z = -14.4, P = 0.003; W > Q, Z = -17.4, P = 0.001; Q = S, Z = -3.0, P = 1, Table S2). The total relative abundances of three major (>2% abundance in all castes) chemical classes were caste-biased: isopentenyl esters and linear alkanes were reproductive-biased, while ML abundance was greater among workers (Fig. S5). This skew was driven by the presence of relatively more small ring size MLs in worker glands (Kruskal-Wallis test, W = 14.59, P = 0.001; post-hoc Dunn’s tests, W > S, Z = -14.48, P = 0.003; W > Q, Z = -16.28, P = 0.002, Fig. S3). In contrast, the inter-correlated large ring size MLs were more abundant in reproductive bee glands (Kruskal-Wallis test, W = 11.18, P = 0.004; post-hoc Dunn’s tests, S > W, Z = 12.58, P = 0.012; Q > W, Z = 14.34, P = 0.007; Q = S, Z = 1.76, P = 1, Fig. S3).
Discussion
The comparison of chemical profiles across three distinct adult female phenotypes in facultatively eusocial M. genalis bees revealed substantial differentiation between the chemistries of reproductive (mated queen and solitary) and non-reproductive (unmated worker) castes, highlighting candidate compounds that may mediate inter-caste fertility signaling. Among these, cuticular methylalkanes stand out as especially promising candidates due to their increased abundance among queens relative to solitary reproductive females within the species’ intra-population reproductive polyphenism, as well as their near-absence among workers.
The diverse cuticular and glandular chemical repertoires of M. genalis consist principally of compounds belonging to classes previously reported in halictid bees, including taxonomically widespread cuticular hydrocarbons (CHs) but also esters, lactones, ketones, and terpenoids. Steitz et al. (2018) partially described the cuticular chemistry of M. genalis and its congener M. amoena. We build on that preliminary description by identifying additional compounds (e.g, methylalkanes), resolving most of the remaining unknowns, and by characterizing the Dufour’s gland chemistry. Megalopta genalis (and M. amoena) are so far the only species of Augochlorini for which cuticular chemistry has been described. The CHs of M. genalis are typical of Hymenoptera, consisting of linear alkanes, methylalkanes, and odd chain-length alkenes (Kather and Martin 2015). The production of macrocyclic lactones (MLs), by far the most abundant compounds in cuticular and glandular extracts of all castes, is a characteristic of the bee families Colletidae and Halictidae (Mitra 2013). One exception is a singular report (Cane 1983) of MLs of 18, 20, and 22 carbon ring sizes in the Dufour’s glands of Protoxaea gloriosa in the family Andrenidae. MLs are critical for the nest architecture of halictids and colletids, and perhaps certain andrenids, polymerizing to form the waterproof polyester layer that lines the interior of brood cells (Hefetz et al. 1979).
The ancestral connection between reproductive activity and ML biosynthesis in halictid bees may have underpinned their secondary co-option as queen pheromones in the tribe Halictini (genus Lasioglossum). The same saturated lactones (18–24 carbons) we find to be predominant in M. genalis were previously shown to repress worker ovarian development in L. malachurum, and to induce worker-characteristic ‘backing’ behavior in L. malachurum and L. pauxillum (Steitz et al. 2019; Steitz and Ayasse 2020). Although a queen pheromone function has not yet been established for L. zephyrum, lactones do influence dominance interactions between nest-founding gynes (Smith and Weller 1989). Consistent with their role as pheromones in these obligately eusocial Lasioglossum species, L. malachurum and L. pauxillum queens have roughly double and L. zephyrum queens roughly 10x the relative amount of total cuticular MLs compared with workers (Steitz et al. 2018). Our finding that total cuticular ML is the same, or marginally higher, among M. genalis workers compared with queens is at odds with these patterns. When individual compounds were grouped into chemical classes, only saturated alkanes (linear and especially methyl-branched) were relatively more abundant among reproductives. Within their respective classes, subsets of heavier alkenes (≥29 carbon, predominantly C29:1 and C31:1) and larger ring size MLs (24–28 carbons) also exhibited such a bias. We suggest that these four groups should be considered as candidates in future efforts to assay fertility signal and queen pheromone functionality in Megalopta. Concordant with our results, and further supporting their separate biosynthetic regulation, small and large ring-size MLs were found to co-vary independently in L. zephyrum (Smith and Wenzel 1988).
The stark difference in caste bias patterns between Lasioglossum in the tribe Halictini and Megalopta in the tribe Augochlorini may indicate alternate trajectories of signal evolution in these two independently eusocial lineages, which diverged approximately 64mya (53-76mya, Brady et al. 2006). The bias towards saturated alkane CHs among Megalopta reproductive castes aligns more closely with the deeply rooted and phylogenetically conserved role of these compounds as correlates of reproductive activity across the order Hymenoptera (Liebig 2010; Monnin 2006; Van Oystaeyen et al. 2014). The extensive variability in eusocial behavior within the subfamily Halictinae, which includes both Augochlorini and Halictini, would provide ample opportunity to test alternate hypotheses explaining these apparently divergent trajectories. In the evolutionary sequence suggested by Kocher and Grozinger (2011), for instance, reliable fertility cues or signals are reinforced (but not replaced) in more advanced eusocial taxa by pheromonal signals of queen presence lacking detailed information on queen fertility. Thus, the patterns so far observed could be explained if CH fertility cues were reinforced by ML queen pheromones in the evolution of advanced, obligate eusociality in Halictidae. This scenario would predict a) adoption of ML queen pheromones only among obligate eusocial species with larger colony sizes, b) widespread presence of CH fertility signals in eusocial Halictinae, and c) persistent correlations between fertility and CHs, but not MLs. While available data is still insufficient for effective tests of these predictions, queens of facultatively eusocial Halictini (Megalopta spp., Halictus rubicundus, and Lasioglossum albipes) at least do not appear to have the same increase in cuticular macrocyclic lactone characteristic of obligately eusocial species (Steitz et al. 2018; Wittwer et al. 2017; the present study). While the information content of individual cuticular chemicals has not been explicitly tested for any halictid, the chemical distance between queens and workers is linked to queen ovarian development among facultatively eusocial species only, and not among obligate eusocial species (Steitz et al. 2018).
We suggest that future studies of obligately eusocial Lasioglossum and other Halictini should consider the possibility that CHs may complement the established queen pheromone function(s) of MLs. In Lasioglossum, bioassays have so far suggested that linear alkane CHs, at least, do not exhibit queen pheromone primer or releaser effects (Steitz et al. 2019; Steitz and Ayasse 2020). While methylalkanes were not tested in those experiments, Wittwer et al. (2017) found no bias in methylalkane production towards reproductives of L. albipes. Also in contrast to our observations in Megalopta, Polidori et al. (2020) found large amounts of methylalkane on the cuticles of foraging workers in facultatively eusocial L. calceatum (13% of total profile) as well as obligately eusocial L. malachurum (14%) and L. politum (7%). Data are lacking for queens in the fully eusocial phase, however. Sampling of queen-destined foundresses as substitutes for active queens (as in Wittwer et al. 2017) may lead to promising compounds being overlooked, as these compounds may be produced only in the appropriate social context.
In this vein, we found that the overall cuticular profiles of queens, which inhabit a socio-environmental context necessitating inter-caste communication, differ subtly from the profiles of solitary reproductives that otherwise have shared morphological and physiological characteristics (Table S1). Queen profiles overall clearly differed from those of workers, yet solitary reproductive profiles were intermediate and more variable. Some solitaries exhibited queen-like profiles, others more worker-like profiles, and others intermediate profiles. Our analysis of individual compounds and compound classes showed that queens produce substantially more (by a factor of ~2) methylalkanes on their cuticles than solitaries. This class includes the only set of compounds that differed significantly between queens and solitaries, a co-eluting mixture of methylnonacosane positional isomers that was twice as abundant in queens. Methylalkanes were also the most divergent chemical class between the four solitaries grouped with workers and the remaining twelve grouped with queens in our hierarchical cluster analysis (>2x more abundant among the latter). Queens and solitary reproductives did not exhibit a similar divergence in the chemistry of their Dufour’s glands, where methylalkanes are absent.
Chemical changes associated with queenship per se in facultatively eusocial species may indicate the presence of dedicated communicative signals, distinct from simple constitutive cues of ovarian activity among solitary reproductives. It should be noted that alterations to or embellishments of these chemical cues among queens do not constitute evidence of dishonest or manipulative signaling. The ‘honest signal’ and ‘queen control’ alternatives are adaptive arguments that make predictions regarding the effects of attending to queen pheromones on workers’ fitness, a point which has sometimes been a source of confusion (summarized in Peso et al. 2015). Elaborations in the queen chemical repertoires of these species may instead reflect a degree of signal ‘ritualization’ from antecedent cues, yet may still communicate queen fertility honestly (Steiger et al. 2011; Stökl and Steiger 2017). The honest transmission of information on queen fertility is thought to be maintained by costs and/or constraints on signal production (Holman 2012; Wyatt 2014). The use of costly signals for inter-caste communication may select for social context-dependent production, analogous to plastic (e.g., seasonal) investment in costly sexual ornamentations used in mate choice (Badyaev and Duckworth 2003).
Complementing our findings in M. genalis, similar queen-specific chemical shifts seem to occur in facultatively eusocial L. albipes, in which social and solitary populations can be distinguished on the basis of their cuticular (and Dufour’s gland) profiles (Wittwer et al. 2017). In the primitively eusocial paper wasp Ropalidia marginata, the Dufour’s gland chemical composition of solitary foundresses prior to the eusocial phase is intermediate between those of workers and social queens (Mitra and Gadagkar 2012). R. marginata queens produce a mixture of long-chain saturated hydrocarbons in their Dufour’s glands, with certain monomethylalkanes increased compared with workers and also correlated with ovarian development (Mitra and Gadagkar 2011). Methylalkanes are frequently overrepresented in the cuticular profiles of reproductives within Hymenoptera (Monnin 2006), and have repeatedly been identified as bioactive queen substances reliably linked to ovarian developmental state ( Cuvillier-Hot et al. 2001; d'Ettorre et al. 2004; Holman et al. 2010a, 2010b; Yagound et al. 2014, 2015; Oi et al. 2016). Production and maintenance costs imposed by the greater volatility (Gibbs 2002) and biosynthetic requirements (Holman 2012) of methylalkanes may explain the frequency with which they have evolved a signaling function. The absence of methylalkanes from M. genalis Dufour’s glands could perhaps reflect their costliness, given that the contents of this gland are used for architectural purposes and would be ‘lost’ to incorporation in the cell lining if secreted there. Methylalkanes are a major component of the R. marginata Dufour’s secretion, but the gland is much smaller and appears to have a purely communicative function (Mitra 2013).
Many of the compounds present on the M. genalis cuticle – MLs, sesquiterpenes, and esters – likely originate in the Dufour’s gland. M. genalis frequently rub the hindmost legs together at the base of the sting, where the Dufour’s is located, and then rub their cuticular surface. Active application of glandular secretions to the cuticle may be important step for broadcasting glandular chemical cues and signals (Hefetz et al. 2001). Among the behaviorally active Dufour’s gland components in Halictini (e.g., MLs), isopentenyl esters of unsaturated fatty acids stimulate male mating attempts and are more abundant in young unmated gynes than queens (Ayasse et al. 1993, 1999). All sampled M. genalis castes had only trace amounts of a single unsaturated isopentenyl ester, 3-methyl-3-butenyl docos-(Z)-13-enoate. None of the sampled castes are expected to be receptive to mating, however, and if the sex pheromone function were conserved in M. genalis this pattern would be concordant. Our putative identifications of isopropyl esters, terpenoid (sesquiterpenes) esters, and sesquiterpenes in M. genalis are the first for Halictidae. Farnesyl and geranyl esters are the major components of Andrena (family: Andrenidae) Dufour’s gland secretions, and are also present in their cell linings and pollen balls (Cane 1981). Interestingly, these esters are also present in the mandibular gland of male Nomada bees, the females of which parasitize Andrena (Tengö and Bergström 1975). Farnesyl esters inhibit male mating efforts in A. nigroaenea, and are more abundant in the glands of older mated females (Schiestl and Ayasse 2000). While the function of terpenoids in Megalopta is still unknown, terpenoids and their fatty acid derivatives produced in the mandibular glands of many solitary bees are known to inhibit fungal and bacterial growth in nests (Cane et al. 1983).
The utility of halictid bees for studying the evolution of eusociality and associated traits (e.g. queen pheromones) has long been recognized (Michener 1974; Schwarz et al. 2007; Kocher and Grozinger 2011). Here we provide the first detailed information on chemical caste differentiation in a flexibly eusocial halictid bee, in a species which also exhibits facultative helping behavior in the worker caste. The eusocial behavior of M. genalis closely resembles the life history conditions modeled by Holman (2014), who showed that reliable fertility signals could facilitate the adoption of facultative worker behavior and thus ease the evolutionary transition from solitary to eusocial nesting behavior. Further study of pheromonal communication in M. genalis, and the socially diverse family Halictidae in which it is nested, is likely to provide valuable insight into the evolutionary origins of queen pheromones and of eusociality itself.
Data Availability
The chemical data generated for this study, and the R scripts used for heatmap generation, are available on Cornell University’s eCommons repository (https://doi.org/10.7298/9qym-kw90).
References
Adams, RP (2007) Identification of essential oil components by gas chromatography / mass spectrometry (4th ed.). Allured Publishing Corp, Carol Stream, IL
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Ayasse M, Engels W, Hefetz A, Lübke G, Francke W (1990) Ontogenetic patterns in amounts and proportions of Dufour’s gland volatile secretions in virgin and nesting queens of Lasioglossum malachurum (Hymenoptera: Halictidae). Z Naturforsch 45:709–714
Ayasse M, Engels W, Hefetz A, Tengö J, Lübke G, Francke W (1993) Ontogenetic patterns of volatiles identified in Dufour’s gland extracts from queens and workers of the primitively eusocial halictine bee, Lasioglossum malachurum (Hymenoptera: Halictidae). Insect Soc 40:41–58
Ayasse M, Engels W, Lübke G, Taghizadeh T, Francke W (1999) Mating expenditures reduced via female sex pheromone modulation in the primitively eusocial halictine bee, Lasioglossum (Evylaeus) malachurum (Hymenoptera: Halictidae). Behav Ecol Sociobiol 45:95–106
Badyaev AV, Duckworth RA (2003) Context-dependent sexual advertisement: plasticity in development of sexual ornamentation throughout the lifetime of a passerine bird. J Evol Biol 16:1065–1076
Billen J, Morgan ED (1998) Pheromone communication in social insects: sources and secretions. In: Vander Meer RK, Breed MD, Espelie KE, Winston M (eds) Pheromone communication in social insects: ants, wasps, bees and termites. Westview Press, Boulder
Bourke AFG (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12:245–257
Brady SG, Sipes S, Pearson A, Danforth BN (2006) Recent and simultaneous origins of eusociality in halictid bees. Proc Royal Soc B 273:1643–1649
Brothers DJ, Michener CD (1974) Interactions in colonies of primitively social bees: III. Ethometry of division of labor in Lasioglossum zephyrum (Hymenoptera: Halictidae). J Comp Physiol 90:129–168
Brückner A, Heethoff M (2017) A chemo-ecologists’ practical guide to compositional data analysis. Chemoecology 27:33–46
Cane JH (1981) Dufour’s gland secretion in the cell linings of bees (Hymenoptera: Apoidea). J Chem Ecol 7:403–410
Cane JH (1983) Chemical evolution and chemosystematics of the Dufour’s gland secretions of the lactone-producing bees (Hymenoptera: Colletidae, Halictidae, and Oxaeidae). Evolution 37:657–674
Cane JH (1987) Estimation of bee size using intertegular span (Apoidea). J Kansas Entomol Soc 60:145–147
Cane JH, Gerdin S, Wife G (1983) Mandibular gland secretions of solitary bees (Hymenoptera: Apoidea): potential for nest cell disinfection. J Kansas Entomol Soc 56:199–204
Cardinal S, Danforth BN (2011) The antiquity and evolutionary history of social behavior in bees. PLoS One 6:e21086
Carlson DA, Bernier UR, Sutton BD (1998) Elution patterns from capillary GC for methyl-branched alkanes. J Chem Ecol 24:1845–1865
Conte YL, Hefetz A (2008) Primer pheromones in social Hymenoptera. Annu Rev Entomol 53:523–542
Cronin AL, Hirata M (2003) Social polymorphism in the sweat bee Lasioglossum (Evylaeus) baleicum (Cockerell) (Hymenoptera, Halictidae) in Hokkaido, northern Japan. Insect Soc 50:379–386
Cuvillier-Hot V, Cobb M, Malosse C, Peeters C (2001) Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. J Insect Physiol 47:485–493
d'Ettorre P, Heinze J, Schulz C, Francke W, Ayasse M (2004) Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J Exp Biol 207:1085–1091
Duffield RM, Fernandes A, Lamb C, Wheeler JW, Eickwort GC (1981) Macrocyclic lactones and isopentenyl esters in the Dufour’s gland secretion of halictine bees (Hymenoptera: Halictidae). J Chem Ecol 7:319–331
Eickwort GC, Eickwort JM, Gordon J, Eickwort MA, Wcislo WT (1996) Solitary behavior in a high-altitude population of the social sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Behav Ecol and Sociobiol 38:227–233
Field J (2008) The ecology and evolution of helping in hover wasps (Hymenoptera: Stenogastrinae). In: Korb J, Heinze J (eds) Ecology of social evolution. Springer-Verlag, Berlin
Field J, Paxton RJ, Soro A, Bridge C (2010) Cryptic plasticity underlies a major evolutionary transition. Curr Biol 20:2028–2031
Fletcher DJC, Ross KG (1985) Regulation of reproduction in eusocial Hymenoptera. Annu Rev Entomol 30:319–343
Gibbs AG (2002) Lipid melting and cuticular permeability: new insights into an old problem. J Insect Physiol 48:391–400
Gibbs J, Brady SG, Kanda K, Danforth BN (2012) Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae). Mol Phylogenet Evol 65:926–939
Ginzel, MD, and Blomquist, GJ (2016) Insect hydrocarbons: biochemistry and chemical ecology. In: Cohen, E, and Moussian, B (eds.) Extracellular composite matrices in arthropods. Springer International Publishing, Switzerland
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:9
Hefetz A, Fales HM, Batra SWT (1979) Natural polyesters: Dufour’s gland macrocyclic lactones form laminesters in Colletes bees. Science 204:415–417
Hefetz A, Soroker V, Dahbi A, Malherbe MC, Fresneau D (2001) The front basitarsal brush in Pachycondyla apicalis and its role in hydrocarbon circulation. Chemoecology 11:17–24
Holman L (2012) Costs and constraints conspire to produce honest signaling: insights from an ant queen pheromone. Evolution 66:2094–2105
Holman L (2014) Conditional helping and evolutionary transitions to eusociality and cooperative breeding. Behav Ecol 25:1173–1182
Holman L, Dreier S, d'Ettorre P (2010a) Selfish strategies and honest signalling: reproductive conflicts in ant queen associations. Proc Royal Soc B 277:2007–2015
Holman L, Jørgensen CG, Nielsen J, d'Ettorre P (2010b) Identification of an ant queen pheromone regulating worker sterility. Proc Royal Soc B 277:3793–3800
Jones BM, Kingwell C, Wcislo WT, Robinson GE (2017) Caste-biased gene expression in a facultatively eusocial bee suggests a role for genetic accommodation in the evolution of eusociality. Proc Royal Soc B 284:20162228
Kapheim KM, Bernal SP, Smith AR, Nonacs P, Wcislo WT (2011) Support for maternal manipulation of developmental nutrition in a facultatively eusocial sweat bee, Megalopta genalis (Halictidae). Behav Ecol Sociobiol 65:1179–1190
Kapheim KM, Smith AR, Ihle KE, Amdam GV, Nonacs P, Wcislo WT (2012) Physiological variation as a mechanism for developmental caste-biasing in a facultatively eusocial sweat bee. Proc R Soc B 279:1437–1446
Kapheim KM, Smith AR, Nonacs P, Wcislo WT, Wayne RK (2013) Foundress polyphenism and the origins of eusociality in a facultatively eusocial sweat bee, Megalopta genalis (Halictidae). Behav Ecol Sociobiol 67:331–340
Kapheim KM, Chan T-Y, Smith AR, Wcislo WT, Nonacs P (2016) Ontogeny of division of labor in a facultatively eusocial sweat bee Megalopta genalis. Insect Soc 63:185–191
Kapheim KM, Jones BM, Pan H, Li C, Harpur BA, Kent CF, Zayed A, Ioannidis P, Waterhouse RM, Kingwell C, Stolle E, Avalos A, Zhang G, McMillan WO, Wcislo WT (2020) Developmental plasticity shapes social traits and selection in a facultatively eusocial bee. PNAS 117:13615–13625
Kather R, Martin SJ (2015) Evolution of cuticular hydrocarbons in the Hymenoptera: a meta-analysis. J Chem Ecol 41:871–883
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Anim Behav 45:787–794
Kocher SD, Grozinger CM (2011) Cooperation, conflict, and the evolution of queen pheromones. J Chem Ecol 37:1263–1275
Kocher SD, Paxton RJ (2014) Comparative methods offer powerful insights into social evolution in bees. Apidologie 45:289–305
Krasulová J, Hanus R, Kutalová K, Šobotník J, Sillam-Dussès D, Tichý M, Valterová I (2012) Chemistry and anatomy of the frontal gland in soldiers of the sand termite Psammotermes hybostoma. J Chem Ecol 38:557–565
Kruskal JB (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129
Leigh EG (1999) Tropical forest ecology: a view from Barro Colorado Island. Oxford University Press, Oxford
Leonhardt SD, Menzel F, Nehring V, Schmitt T (2016) Ecology and evolution of communication in social insects. Cell 164:1277–1287
Liebig J (2010) Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In: Blomquist GJ, Bagnères A-G (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge
Maynard Smith J, Szathmáry E (1995) The major transitions in evolution. Oxford University Press, Oxford
Michener CD (1974) The social behavior of the bees: a comparative study. Harvard University Press, Cambridge, MA
Michener CD, Brothers DJ (1974) Were workers of eusocial Hymenoptera initially altruistic or oppressed? PNAS 71:671–674
Mitra A (2013) Function of the Dufour’s gland in solitary and social Hymenoptera. J Hymenopt Res 35:33–58
Mitra A, Gadagkar R (2011) Can Dufour’s gland compounds honestly signal fertility in the primitively eusocial wasp Ropalidia marginata? Naturwissenschaften 98:157–161
Mitra A, Gadagkar R (2012) Queen signal should be honest to be involved in maintenance of eusociality: chemical correlates of fertility in Ropalidia marginata. Insect Soc 59:251–255
Monnin T (2006) Chemical recognition of reproductive status in social insects. Ann Zool Fenn 43:531–549
Oi CA, van Zweden JS, Oliveira RC, Van Oystaeyen A, Nascimento FS, Wenseleers T (2015) The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. BioEssays 37:808–821
Oi CA, Millar JG, van Zweden JS, Wenseleers T (2016) Conservation of queen pheromones across two species of vespine wasps. J Chem Ecol 42:1175–1180
Orlova M, Malka O, Hefetz A (2020) Choosing the best: honeybee workers can assess reproductive quality of the queen through pheromonal signalling in simultaneous choice assays. Apidologie 51:291–306
Packer L (1990) Solitary and eusocial nests in a population of Augochlorella striata (Provaneher) (Hymenoptera; Halictidae) at the northern edge of its range. Behav Ecol Sociobiol 27:339–344
Peram PS, Vences M, Schulz S (2017) A synthetic dodecanolide library for the identification of putative semiochemicals emitted by mantellid frogs. Org Biomol Chem 15:6967–6977
Peso M, Elgar MA, Barron AB (2015) Pheromonal control: reconciling physiological mechanism with signalling theory. Biol Rev 90:542–559
Polidori C, Geyer M, Schmitt T (2020) Do Sphecodes cuckoo bees use chemical insignificance to invade the nests of the social Lasioglossum bee hosts? Apidologie 51:147–162
Princen SA, Oliveira RC, Ernst UR, Millar JG, van Zweden JS, Wenseleers T (2019) Honeybees possess a structurally diverse and functionally redundant set of queen pheromones. Proc R Soc B 286:20190517
Purcell J (2011) Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol Rev 86:475–491
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. URL: https://www.R-project.org/
Rehan SM, Toth AL (2015) Climbing the social ladder: the molecular evolution of sociality. Trends Ecol Evol 30:426–433
Rehan SM, Leys R, Schwarz MP (2012) A mid-cretaceous origin of sociality in Xylocopine bees with only two origins of true worker castes indicates severe barriers to eusociality. PLoS One 7:e34690
Richards MH, von Wettberg EJ, Rutgers AC (2003) A novel social polymorphism in a primitively eusocial bee. PNAS 100:7175–7180
Schiestl FP, Ayasse M (2000) Post-mating odor in females of the solitary bee, Andrena nigroaenea (Apoidea, Andrenidae), inhibits male mating behavior. Behav Ecol Sociobiol 48:303–307
Schulz S, Peram PS, Menke M, Hötling S, Röpke R, Melnik K, Poth D, Mann F, Henrichsen S, Dreyer K (2017) Mass spectrometry of aliphatic macrolides, important semiochemicals or pheromones. J Nat Prod 80:2572–2582
Schwarz MP, Richards MH, Danforth BN (2007) Changing paradigms in insect social evolution: sights from Halictine and Allodapine bees. Annu Rev Entomol 52:127–150
Smith BH, Weller C (1989) Social competition among gynes in halictine bees: the influence of bee size and pheromones on behavior. J Insect Behav 2:397–411
Smith BH, Wenzel JW (1988) Pheromonal covariation and kinship in social bee Lasioglossum zephyrum (Hymenoptera: Halictidae). J Chem Ecol 14:87–94
Smith AR, Kapheim KM, Pérez-Ortega B, Brent CS, Wcislo WT (2013) Juvenile hormone levels reflect social opportunities in the facultatively eusocial sweat bee Megalopta genalis (Hymenoptera: Halictidae). Horm Behav 63:1–4
Smith AR, Kapheim KM, Wcislo WT (2019) Survival and productivity benefits of sociality vary seasonally in the tropical, facultatively eusocial bee Megalopta genalis. Insect Soc 66:555–568
Soro A, Ayasse M, Zobel MU, Paxton RJ (2011) Kin discriminators in the eusocial sweat bee Lasioglossum malachurum: the reliability of cuticular and Dufour’s gland odours. Behav Ecol Sociobiol 65:641–653
Soucy SL, Danforth BN (2002) Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Evolution 56:330–341
Steiger S, Schmitt T, Schaefer HM (2011) The origin and dynamic evolution of chemical information transfer. Proc Royal Soc B 278:970–979
Steitz I, Ayasse M (2020) Macrocyclic lactones act as a queen pheromone in a primitively eusocial sweat bee. Curr Biol 30:1136–1141
Steitz I, Kingwell C, Paxton RJ, Ayasse M (2018) Evolution of caste-specific chemical profiles in halictid bees. J Chem Ecol 44:827–837
Steitz I, Brandt K, Biefel F, Minat Ä, Ayasse M (2019) Queen recognition signals in two primitively eusocial halictid bees: evolutionary conservation and caste-specific perception. Insects 10:416
Stökl J, Steiger S (2017) Evolutionary origin of insect pheromones. Curr Opin Insect Sci 24:36–42
Strohm E, Herzner G, Kaltenpoth M, Boland W, Schreier P, Geiselhardt S, Peschke K, Schmitt T (2008) The chemistry of the postpharyngeal gland of female European Beewolves. J Chem Ecol 34:575–583
Tengö J, Bergström G (1975) All-trans-farnesyl hexanoate and geranyl octanoate in the Dufour gland secretion of Andrena (Hymenoptera: Apidae). J Chem Ecol 1:253–268
Tierney SM, Fischer CN, Rehan SM, Kapheim KM, Wcislo WT (2013) Frequency of social nesting in the sweat bee Megalopta genalis (Halictidae) does not vary across a rainfall gradient, despite disparity in brood production and body size. Insect Soc 60:163–172
Van Oystaeyen A, Oliveira RC, Holman L, van Zweden JS, Romero C, Oi CA, d’Ettorre P, Khalesi M, Billen J, Wäckers F, Millar JG, Wenseleers T (2014) Conserved class of queen pheromones stops social insect workers from reproducing. Science 343:287–290
Wcislo WT (1997) Behavioral environments of sweat bees (Halictinae) in relation to variability in social organization. In: Choe JC, Crespi BJ (eds) Social behavior in insects and arachnids. Cambridge University Press, Cambridge
Wcislo WT, Danforth BN (1997) Secondarily solitary: the evolutionary loss of social behavior. Trends Ecol Evol 12:468–474
Wcislo WT, Fewell JH (2017) Sociality in bees. In: Rubenstein DR, Abbott P (eds) Comparative Social Evolution. Cambridge University Press, Cambridge
Wcislo WT, Gonzalez VH (2006) Social and ecological contexts of trophallaxis in facultatively social sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera, Halictidae). Insect Soc 53:220–225
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, MA
Wittwer B, Hefetz A, Simon T, Murphy LEK, Elgar MA, Pierce NE, Kocher SD (2017) Solitary bees reduce investment in communication compared with their social relatives. PNAS 114:6569–6574
Wyatt TD (2014) Pheromones and animal behavior: chemical signals and signatures. Cambridge University Press, Cambridge
Yagound B, Blacher P, Fresneau D, Poteaux C, Châline N (2014) Status discrimination through fertility signaling allows ants to regulate reproductive conflicts. Anim Behav 93:25–35
Yagound B, Gouttefarde R, Leroy C, Belibel R, Barbaud C, Fresneau D, Chameron S, Poteaux, Châline N (2015) Fertility signaling and partitioning of reproduction in the ant Neoponera apicalis. J Chem Ecol 41:557–566
Yasui H, Akino T, Yasuda T, Fukaya M, Ono H, Wakamura S (2003) Ketone components in the contact sex pheromone of the white-spotted longicorn beetle, Anoplophora malasiaca, and pheromonal activity of synthetic ketones. Entomol Exp Appl 107:167–176
Zhou X, Rokas A, Berger SL, Liebig J, Ray A, Zwiebel LJ (2015) Chemoreceptor evolution in Hymenoptera and its implications for the evolution of eusociality. Genome Biol Evol 7:2407–2416
Acknowledgements
We thank Stefan Schulz for assistance with interpretation of lactone spectra, Robert Raguso for helpful comments on early drafts of the manuscript, and Jocelyn Millar for guidance with derivatization procedures and methylalkane chemical standards. Yasuharu Yoshimi graciously contributed macrocyclic lactone chemical standards. We also thank Gabriel Trujillo, Isis Lopez, and Esther Velasquez for field assistance, and Barro Colorado Island staff for logistical support in Panamá.
Funding
C. Kingwell was supported by fellowships from the Smithsonian Tropical Research Institute, the Natural Sciences and Engineering Research Council of Canada (NSERC), and Cornell University.
Author information
Authors and Affiliations
Contributions
C. Kingwell, M. Ayasse, and W. Wcislo conceptualized the study. C. Kingwell collected field data, conducted statistical analyses, and wrote the first draft of the manuscript. C. Kingwell, K. Böröczky, M. Ayasse, and I. Steitz determined the identities of chemical compounds. All authors contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Supplementary Information
ESM 1
(PDF 4517 kb)
Rights and permissions
About this article
Cite this article
Kingwell, C., Böröczky, K., Steitz, I. et al. Cuticular and Dufour’s Gland Chemistry Reflect Reproductive and Social State in the Facultatively Eusocial Sweat Bee Megalopta genalis (Hymenoptera: Halictidae). J Chem Ecol 47, 420–432 (2021). https://doi.org/10.1007/s10886-021-01262-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01262-1