Abstract
Local environmental conditions can facilitate or preclude the development of eusocial colonies in insects that facultatively express behavioural-caste polyphenism. To explore how environmental variability relates to the expression of social behaviour, we collected 120 nests of the facultatively social sweat bee, Megalopta genalis (Halictidae: Augochlorini), along a nearly twofold rainfall gradient in central Panama. Brood rearing activity of bees in seasonal neotropical forests should track flowering phenologies, which are typically set by rainfall and phylogenetic patterns. Nests were collected at roughly similar times of year from three sites comprising wet, moist and dry lowland tropical forests. There were significant differences in ovarian development, brood production and body size across sites for some comparisons, but no effect on the proportion of social colonies collected at each site. Results show that phenotypes of M. genalis relevant to social behaviour (ovarian development, brood production, body size) may be responsive to variation in local environment over distances of <20 km.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social arthropods that facultatively switch between solitary and eusocial-group living provide opportunities to explore the factors thought to be influential in the development and maintenance of societies (reviewed by Michener, 1974; West-Eberhard, 1996; Wcislo, 1997; Schwarz et al., 2007; Purcell, 2011). Comparisons of social polyphenism (sensu Michener, 1961) within and among natural populations permit an understanding of how local environmental conditions shape social phenotypes (e.g. Wcislo, 1996; Richards, 2004). In addition to shaping seasonal activity, environmental factors can also influence body size, which often has fitness repercussions relating to social hierarchies, reproductive asymmetries, and other traits (Roulston and Cane, 2000; Wcislo, 2000; Richards, 2004; Cronin et al., 2011; Kapheim et al., 2011; Smith et al., 2012).
The physical environment can preclude or promote the expression of social behaviour in populations of facultatively eusocial bees. Eusocial societies are characterized by an overlap of generations among nest-mates (Michener, 1974), which require a growing season sufficiently long to permit the development of at least two broods (Wcislo and Danforth, 1997). For example, in temperate montane sites, brood rearing seasons may be short relative to egg-to-adult developmental rates, and an overlap of generations would be precluded due to insufficient time to rear a second brood. Thus in some bee species, populations that live at low elevations express eusocial behaviour, while those living at high elevations, are solitary (Sakagami and Munukata, 1972; Eickwort et al., 1996; Wcislo, 1996; Soucy, 2002). Comparable patterns are known for latitudinal gradients (e.g. Richards, 2004; reviewed by Wcislo, 1997; Purcell, 2011) and have been demonstrated experimentally using reciprocal transplants of nests from southern and northern populations of a sweat bee, Halictus rubicundus (Halictidae: Halictini) (Field et al., 2010, 2012).
In contrast to temperate zone bees, the relationships between seasonality of resource availability, brood production and sociality are less well understood in the tropics. Colonies of a Costa Rican sweat bee, Lasioglossum (Dialictus) umbripenne (Halictidae: Halictini), for example, showed markedly different patterns in seasonal activity, social structure, and caste-related morphology, when comparing a highly seasonal Pacific coast population (Wille and Orozco, 1970), with a less seasonal Caribbean slope population (Eickwort and Eickwort, 1971), although bees were studied at different times of the year. Understanding such patterns is complicated by the fact that flowering phenology can vary dramatically even over relatively short geographic distances (Janzen, 1967; Roubik, 1989; Wright and Calderon, 1995; Frankie et al., 2004), and the species composition of forest communities can vary as well (Pyke et al., 2001; Condit et al., 2005).
Here, we examine whether expression of eusociality varies with respect to variation in local environmental conditions, using three populations of the sweat bee Megalopta genalis Meade-Waldo (Halictidae: Augochlorini), along a rainfall gradient parallel to the Panama Canal in central Panama. We used a 50-km north–south transect as a proxy for variation in resource availability. We used nest collections, census data, and dissections to provide snap-shots of social colony structure, assessed as both the proportion of social colonies and colony size within a population. We also used morphometric data to compare body size with ovarian development.
Methods
Précis of natural history
Megalopta is a genus of nocturnally foraging bees that nest within dead tree branches, lianas or vines (Wcislo et al., 2004; Tierney et al., 2008a; Wcislo and Tierney, 2009; Santos et al., 2010). In central Panama the main brood rearing period occurs during the dry season (December to May). The dry season begins slightly earlier (2–3 weeks) on the Pacific coast but ends at roughly the same time at all three sites in mid- to late-April (Condit et al., 2000). Brood rearing continues through the early- to mid-wet season (until August), tapering off as the rains intensify, with little or no foraging activity at the end of the wet season (Wcislo et al., 2004). The egg-to-adult developmental rate of M. genalis in central Panama is approximately 35 days (Wcislo et al., 2004; Wcislo and Gonzalez, 2006). On Barro Colorado Island, bees collect pollen from at least 64 species of angiosperms, but rely most heavily on about five species (Smith et al., 2012).
Field sites
We examined M. genalis nesting biology at three lowland (<200 m elevation) sites along a transect in central Panama, roughly in parallel with the transoceanic Canal (see Table S1 for details on site descriptions and meteorological data):
(1) Caribbean site Santa Rita Arriba (SRA) [9°19′48″N, 79°46′48″W] is near the Atlantic coast and has mean annual precipitation of 3,054 mm. Some rain falls in all months of the year, and at the Atlantic entrance to the Canal the dry season lasts approximately 102 days (Condit et al., 2000). The forest is diverse with 162 species of trees and shrubs in a 1-ha plot having a diameter at breast height (dbh) ≥10 cm (Condit et al., 2005).
(2) Mid-isthmus site Barro Colorado Island (BCI) [9°9′0″N, 79°51′36″W] has mean annual precipitation of 2,581 mm. The forest is intermediate in diversity with 90 species in a 1-ha plot having a dbh ≥10 cm (Condit et al. 2005).
(3) Pacific site Parque Natural Metropolitano (PNM) [8°59′34″N, 79°32′24″W] is near the Pacific terminus of the Canal, where there is a distinct dry season from December through late April to early May (approximately 129 days), and on average the area receives <1,875 mm of rain per year, nearly all of this during the wet season (see Table S1). Surrounding Pacific forests are generally less diverse with a mean of 57 species in a 1-ha plot having a dbh ≥10 cm (Condit et al., 2005), around 80 species of trees and lianas have been recorded from PNM (Kalacska et al., 2007).
In central Panama there are 1,142 tree and shrub morphospecies. Of these, 492 are restricted to wet forest only, such as the SRA site, 87 are restricted to dry sites, such as PNM, and 197 species are widespread and occur across the isthmus (Pyke et al., 2001; Condit et al., 2005).
Nest collections
Between 1 April and 17 June 2009, 40 nests were collected at each site on the following dates: SRA, 1–13 April (n = 12) and 1–16 June (n = 28); BCI, 8–22 May (n = 20) and 10–11 June (n = 20); PNM, 4–25 May (n = 33) and 9–17 June (n = 7). Nests were collected between 0800 and 1600 hours, when bees were not foraging and all residents were assumed to be present. Nests were transported back to the laboratory, split longitudinally and then all contents were recorded as a snap-shot of nesting biology and social structure for each nest. Nest architecture was noted and brood were categorized as follows: egg, small larva (first and second instars), medium larva (third and early fourth instar), large larva (late fourth instar), pre-pupa (post-defecating fourth instar), pupa, or callow adult. We recorded the number of sealed and open brood cells; open brood cells that only contained pollen were presumed to represent provisions for future egg-laying.
Dissections and morphometrics
Adult females were measured and dissected under a stereomicroscope to record the following morphometrics on body size and reproductive condition. Intertegular width (Cane, 1987; Tierney et al., 2008b) and forewing length (axillary sclerites to the base of the stigma) provided measures of body size. Wing wear was scored by the number of nicks in the distal wing margin, which provides an estimate of foraging effort or age (Mueller and Wolf-Mueller, 1993; Tierney and Schwarz, 2009). Spermathecae were examined for presence of sperm as evidence of mating. The lengths of the three largest terminal oocytes were summed, as a measure of ovarian development. We used an ANCOVA on solitarily nesting females to assess potentially confounding body size scaling effects on ovary size, prior to conducting other analyses. Females were ranked within each nest according to summed oocyte length, as a proxy for their reproductive caste; ranks lower than fourth were excluded from analyses due to small sample sizes. We subsequently compared means of this metric within and between sites, and used ovarian rank to assess relationships between body size and wing wear.
Re-sampling to account for asynchronous nest initiation
Ovarian development is associated with social status within a nest (e.g., Smith et al., 2008), and our analyses are potentially confounded by the fact that nest initiation is not necessarily synchronous among females within a population, or among populations. To address this problem, we used a re-sampling procedure (1,000 replicates) to calculate the mean difference in ovary size between randomly selected pairs of individuals, drawn from the pool of recorded individual values (Rehan et al., 2009; Tierney and Schwarz, 2009). We then calculated the observed mean difference and identified its percentile position within a simulated stochastic distribution. This result gives an indication of the deviation of observed data from a random assortment of pairs. We ran analyses for all multi-female colonies pooled, then independently for each site, and repeated these for two-female colonies only. We also used two resampling procedures, whereby the sampled pairs were either (1) excluded from or (2) returned to the pool.
Statistics
Re-sampling analyses were performed using R (R Development Core Team, 2011); code is available from the first author. All other statistical analyses were performed with SPSS© v11. Data were tested for normality (Kolmogorov–Smirnov or Shapiro–Wilk tests) prior to analyses, and Levene’s homogeneity of variance tests were undertaken prior to all parametric analyses. When assumptions were violated we used non-parametric tests, as indicated in the text, and alpha values for multivariate tests were corrected using the sequential Bonferroni technique.
Results
Nest censuses
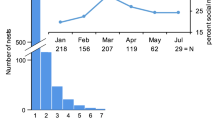
Colonies contained up to five adult females and nine brood (Table 1). The modal group size was one female per nest, with 25–33 % of colonies containing two or more females. The number of adult females per nest did not differ among collection sites (Table 1; Fig. S1). There were significant differences among sites in total brood numbers (see Table 1; Fig. 1). Colonies from BCI contained significantly more total brood than both SRA (U = 520.5, P = 0.006) and PNM (U = 494, P < 0.002); these patterns were maintained when classes of brood were analyzed separately as eggs or larvae, but were not significant for pupae. When total brood numbers were considered separately for solitary nests and pooled multi-female colonies, then the effect of site on brood variables was upheld for solitary nests, but not for multi-female colonies from different sites (Table 1). An analysis of open brood cells that were actively being provisioned also showed an effect of collection site (Table 1), with greater numbers of such cells in solitary nests from BCI than at SRA (U = 256.5, P = 0.014), but not between BCI and PNM (U = 313, P = 0.06).
Brood production summarized by developmental stage. Mean number of open brood cells being actively provisioned (active provisions), and cells with eggs, larvae and pupae per nest are summarized for each collection site: SRA Santa Rita Arriba, BCI Barro Colorado Island, PNM Parque Natural Metropolitano
Body size
There was a highly significant effect of collection site on intertegular width (one-way ANOVA, F 2,191 = 14.5, P < 0.001), and post hoc Tukey tests showed that BCI females were significantly larger than females at both SRA (P = 0.003) and PNM (P < 0.001), whereas females from SRA and PNM did not differ (Fig. 2). Comparing only solitary females again showed a significant effect of site on body size (F 2,79 = 15.3, P < 0.001): BCI females were larger than SRA (P = 0.005) and PNM (P < 0.001) females, with no difference between the latter two sites (Fig. 4).
Mean body size for our populations did not differ over the period of our sampling dates. Independent Kruskal–Wallis tests for each site showed no significant differences between intertegular width as a function of collection week (SRA H 4 = 4.54, P = 0.338; BCI H 3 = 1.76, P = 0.624; PNM H 5 = 5.37, P = 0.372).
Multi-female colonies
Morphometrics for individuals from multi-female colonies are summarized in Table S2. In all but one colony, the female exhibiting the greatest ovarian development was fertilized, and in the majority of cases this individual was also the largest female within the nest (pooled sites 73 %; SRA 69 %; BCI 62 %; PNM 91 %). In most (76 %) nests more than one female were inseminated. Nine of 36 (25 %) multi-female colonies contained a sole inseminated female accompanied by un-mated female(s) with lesser ovarian development. In one multi-female nest (PNM 8) females were uninseminated, had small ovaries and no wing wear, suggesting that the nest may have been recently orphaned. We statistically assessed the relationships between body size and ovarian development at the population level in greater detail below (see among-site comparisons).
Ovarian development
Body size scaling effects
An ANCOVA on solitary females, with the sum of oocyte lengths as the dependent variable, collection site as the fixed factor and intertegular width as a covariate, indicated no interaction between collection site and intertegular width (F 2,74 = 1.17, P = 0.317). With the interaction removed from the model we found no evidence of a body size scaling effect on ovarian development, while controlling for site (F 1,76 = 1.5, P = 0.225). There was, however, a significant effect of site on ovarian development when the covariate (body size) was controlled for (F 2,76 = 5.3, P = 0.007).
In comparisons among collection sites, BCI solitary females had significantly greater ovarian development than solitary females at the other two sites (Fig. 3). A one-way ANOVA (covariate removed) indicated a highly significant site effect (F 2,77 = 9.5, P < 0.001). BCI solitary females showed significantly greater mean ovarian development than either SRA (P = 0.002) or PNM (P < 0.001) solitary females, and the means of the latter two sites did not differ.
The mean length of summed oocytes (with 95 % confidence intervals) is given as a function of ovarian rank for females in solitary nests, and those in multi-female colonies: 1st ranks are the putative reproductively dominant queens; 2nd to 4th ranks are supernumerary females. Collection sites are coded: SRA black, BCI grey, PNM white
Social female ovarian state
Among-site comparisons There were no differences between sites in ovary size for first rank social females (Fig. 3). For second rank females, however, those of BCI had larger ovaries than females from the other two sites, but differences were marginally non-significant with a Bonferroni correction (adjusted α = 0.025); SRA (U = 41, P = 0.026) and PNM (U = 33, P = 0.026), which is the same pattern as found in solitary females (above). Ovarian development of other lower ranked females did not differ among collection sites.
Within-site comparisons When each collection site was analyzed independently, we found a significant population level effect of within-nest ovarian rank on mean oocyte length (SRA H 2 = 21.5, P = 0.001; BCI H 2 = 23.9, P = 0.001; PNM H 2 = 14.9, P = 0.002). Within each site, first-ranked females from SRA and BCI had significantly larger ovaries than all other (second to fourth rank) supernumerary females (Mann–Whitney, Bonferroni adjusted α = 0.0083: SRA P ≤ 0.003; BCI P ≤ 0.005); while first-ranked females from PNM had larger ovaries than second and third rank females only (P ≤ 0.004). A comparison of supernumerary females revealed that BCI second rank females displayed significantly different ovary development than BCI third rank females (U = 8.5 P = 0.003), but no other pairwise tests were significant.
Re-sampled pairs to account for reproductive asynchrony Among females sharing the same nest, observed pairwise differences in oocyte length were not significantly different from re-sampled distributions, where females were paired at random (Table S3). At BCI observed differences ranged across the mid-level percentiles (44th–63rd) of the re-sampled distribution, while at SRA and PNM observed differences placed into the upper percentiles of the re-sampled distribution (SRA 73rd–96th percentile range; PNM 69th–77th percentile range). Results did not differ greatly among procedures that either (1) replaced or (2) excluded previously sampled pairs from the sampling pool.
Ovarian state of first-ranked females versus solitary females Within each site, we also compared ovary size between first rank females of multi-female colonies and those of solitary females. Analyses showed that first rank females from colonies at SRA and PNM, respectively, had more developed ovaries than solitary females at SRA (U = 84.5, P = 0.007) and PNM (U = 90.0, P = 0.035). On BCI, however, ovary size of solitary females did not statistically differ from first-ranked females from multi-female colonies (Fig. 3).
Ovarian differentiation and body size
We tested for evidence of size-dependent reproductive differentiation, comparing differences in mean body size (intertegular width) among ovarian ranks of multi-female colonies (Fig. S2), conducting analyses independently for each site. To present these findings in a clearer manner, we grouped all supernumerary females and compared this mean with that of first-ranked females, the putative queens (Fig. 4). BCI colonies showed no significant difference in mean body size among ovarian ranks. SRA colonies showed a marginal effect of ovarian rank (F 3,35 = 2.9, P = 0.047), but post hoc tests did not indicate any pair-wise differences between ranks; although a t test comparing queens with pooled supernumerary females was significant (t 37 = 3.04, P = 0.004). There was a significant difference in body size among ovarian ranks only for PNM colonies (F 3,28 = 8.8, P < 0.001), such that first-ranked females were significantly larger than all other ovarian ranks (all post hoc Tukey comparisons with P ≤ 0.035); this finding was substantiated by a t test when supernumerary females were pooled and compared with queens (t 32 = 5.01, P < 0.001).
Body size as a function of social status. Mean intertegular width (with 95 % confidence intervals) as a measure of body size, given as a function of nesting habit and ovarian rank for females in ‘solitary’ nests, and those in multi-female colonies: 1st ranks are the putative reproductively dominant ‘queens’; 2nd to 5th ranks are designated as ‘supernumerary’ females (mean body size for all supernumerary ranks are provided in Fig. S2). Collection sites are coded: SRA black, BCI grey, PNM white
Mean body size and confidence intervals of first rank females (queens) from all sites were virtually the same (Fig. 4), despite highly significant site effects on body size at the population level (see Fig. 2). However, the population level trends of mean body size at each collection site were paralleled in both (1) mean body size of solitary females and (2) supernumerary females (compare Figs. 2; 4). The general trend is that BCI females were biggest, followed by SRA, and PNM females were always smallest.
Raw data scattergrams plotting intertegular width against summed oocyte length are provided in the ESM (Fig. S3). These plots indicate that there were substantial overlaps in body size ranges both among populations and behavioural categories (solitary, queens and supernumerary females). Only five supernumerary females were smaller than the smallest queens (compare Figs. S3c and S3d).
Supplementary results
Data concerning nest architecture (Table S4), colony size (Fig. S1), dissection data (Table S2), resampled ovarian differentiation between nest-mates (Table S3), site effects on body size and ovarian development (Figs. S2, S3), wing wear (Fig. S4) and sex ratio are provided in the ESM.
Discussion
Environmental effects on social organization
Halictines are among the most frequently studied social bees with regard to environmental factors and their influence in shaping the expression of social behaviour (Michener, 1990; Wcislo, 1997, 2000; Richards, 2004; Schwarz et al., 2007; Field et al., 2010). In our study there were no differences in either the proportion or the size of multi-female colonies, across a rainfall gradient. At a finer spatial scale (BCI 50-ha forest plot), Smith et al. (2012) found there was no correlation between the spatial distribution of floral resources and the spatial distribution of M. genalis social or solitary nests, indicating that both solitary and social nests have access to the same resources at this spatial scale. Such observations suggest that resource availability is not a decisive factor in the expression of social nesting in M. genalis. Interpreting the among-site behavioural differences in the context of local variation in resource availability is complicated by the fact that the sites differ significantly in tree species composition (Condit et al., 2005), and hence are predicted to have somewhat different phenologies due to phylogeny (Wright and Calderon, 1995), regardless of local variation in rainfall patterns.
Variation in local climate at the same site across annual cycles has been shown to influence sociality in a temperate zone halictine. Increased rainfall during brood rearing periods of temperate Halictus ligatus (Halictidae: Halictini) is associated with production of smaller and fewer workers, as compared to drier brood rearing seasons when there are more workers that are larger, mated and actively reproducing (Richards and Packer, 1995, 1996; Richards et al., 1995, Richards, 2004). While the proportions of M. genalis multi-female colonies did not differ across our sites, colonies were most productive at the mid-point (BCI) of our rainfall transect (Fig. 2) and supernumerary females (putative workers) were smallest at the driest site (with the longest dry season), PNM on the Pacific coast (Fig. 4).
Environmental effects on colony productivity and body size
Results suggest that M. genalis may show evidence of seasonal difference in brood productivity and body size at different sites that vary in rainfall. Studies of two populations of L. umbripenne in Costa Rica demonstrated an influence of seasonality on colony size and degree of worker-queen body size differences (Wille and Orozco, 1970; Eickwort and Eickwort, 1971). In contrast, different populations of M. genalis showed no observable differences in colony size and there is no indication of diapause in any of our samples. The reproductive dominants and supernumerary females in our study differed most significantly in body size in nests from the Pacific site (PNM, Fig. 4), but the greatest observed differences in nest-mate ovarian development were found among colonies at the Caribbean site (SRA, Fig. 3), which is the least seasonal site in terms of rainfall.
Megalopta genalis were physically larger and more fecund at the mid-isthmus site (BCI) than at either extreme of the isthmian transect, in our samples from an 11-week period spanning the transition from dry to rainy seasons. BCI was the only site where the ovarian development of reproductive dominants (ovarian first rank) from social nests was not significantly greater than that of solitary females, at the population mean level (Fig. 3). This finding contradicts results from previous studies of M. genalis from BCI (Smith et al., 2008, 2009; Kapheim et al., 2012), in which social queens displayed greater ovarian enlargement than solitarily nesting females and workers in social nests. Kapheim et al. (2012) set up artificial observation nests collected from roughly the same period of the same season as our study, although these nests were seeded with newly eclosed callow females that might or might not have founded nests in their own right. Our results also differ from modified natural nests observed by Smith et al. (2009), but these were collected earlier in the dry season (February–March 2004). It is possible that some of our solitary nests may have eventually developed into social nests, and that this is masked by our sampling procedures; however, the same site-related patterns of ovarian development observed among solitary females are also maintained among second ovarian-rank females from social nests (Fig. 3).
Reproductive dominants from BCI social colonies were not physically larger than supernumerary females, when assessed at the population level (Fig. 4). Again, this differs from results of the previously mentioned studies of M. genalis from BCI (Smith et al., 2009; Kapheim et al., 2012). Size-based reproductive hierarchies in these bees are determined relative to the body size of nest-mates (Smith et al., 2008, 2009; Tierney et al., 2008a; Kapheim et al., 2012), rather than absolute size. The similarity of mean body size (and variances) of queens across all collection sites (Fig. 4) raises the possibility that a certain threshold is required to maintain a dominance hierarchy; dominant females face an increased risk of nest supersedure if their daughters are equal or nearly equal to them in body size (Kapheim et al., in press). By comparison, the site-related patterns of body size in solitary and supernumerary females imply that there are localized site effects on phenotype. Given that our data are snap-shots of colony phenology we do need to consider the possibility that (1) some supernumerary females may disperse to found their own nests, (2) some solitary nests may develop into social colonies, and (3) some foundresses may have died or been superseded (Kapheim et al., in press). Nevertheless, site-related trends in body size are consistent across all supernumerary females ranks (Fig. S2, summarized in Fig. 4).
The question remains as to whether these site-related phenotypic differences represent a plastic response to environmental variation, as demonstrated for H. rubicundus (Field et al., 2012). In central Panama, the dry season begins earlier and is longer at the Pacific end of the transect (Condit et al., 2000, 2004), so it may be that at the time of sampling BCI offered higher quality or more abundant floral resources than the other sites. However, detailed phenological data across sites are unavailable to test this hypothesis. Times of peak brood rearing and resource availability may vary both spatially and temporally. For example, a study of the BCI population showed that both bee productivity and diversity of pollen within cells was higher in dry season collections (February and March 2007) than in wet season ones (May and July 2007) (Smith et al., 2012).
Pollen protein quality supplied to Lasioglossum bee larvae is more critical than pollen quantity in determining adult body size (Roulston and Cane, 2002), but there is no evidence that bees can judge quality when collecting pollen. Seasonal studies on a tropical stingless bee, Nannotrigona perilampoides (Apidae: Meliponini), show that worker body size and mass directly correspond to seasonal pollen protein content (Quezada-Euán et al., 2011), and that size variation is lowest among cohorts of the same age as opposed to individuals collected across the year. This implies that variance in bee body size is partly determined by temporal variance in resource quality, but our study cannot distinguish pollen use at different sites.
Translocation experiments are an appropriate way to tease apart the relative influence of environment on population phenotypes in facultatively social bees (Cronin, 2001; Field et al., 2010, 2012). While constraints of genetic lineage may need to be considered (Plateaux-Quénu et al., 2000; Soucy and Danforth, 2002; but see Zayed and Packer, 2002), the overlap in body size ranges among populations and behavioural castes of M. genalis presented here suggests social polyphenism.
Conclusions
Based on censuses of nest collections, there were no differences in the frequency of social nesting in populations of Megalopta genalis at three different sites across a rainfall gradient in central Panama. There were, however, significant differences among some sites in body size, ovarian development and brood productivity for some classes of females. Spatial and temporal variation in an environmental factor such as rainfall may partly determine flowering phenology, and hence resource availability for bees. In turn, quantity and quality of available resources may ultimately affect size-based reproductive differentiation and social organization. Most population level studies lack detailed phenological information, and data on resource utilization. The site-related differences identified in this study should provide an incentive to collect comparative data on flowering phenology and quality and quantity of floral resources, to better understand the role of resource availability on bee social organization.
References
Cane J.H. 1987. Estimation of bee size using intertegular width (Apoidea). J. Kansas Entomol. Soc. 60: 145–147
Condit R., Watts K., Bohlman S.A., Rolando P., Foster R.B. and Hubbell S.P. 2000. Quantifying the deciduousness of tropical forest canopies under varying climates. J. Veg. Sci. 11: 649–658
Condit R., Aguilar S., Hernandez A., Perez R., Lao S., Angher G., Hubbell S.P. and Foster R.B. 2004. Tropical forest dynamics across a rainfall gradient and the impact of an El Niño dry season. J. Trop. Ecol. 20: 51–72
Condit R., Pérez R., Lao A., Aguilar S. and Somoza A. 2005. Geographic ranges and b-diversity: Discovering how many tree species there are where. Biol. Skrif. 55: 57–71
Cronin A.L. 2001. Social flexibility in a primitively social allodapine bee (Hymenoptera: Apidae): results of a translocation experiment. Oikos 94: 337–343
Cronin A.L., Bridge C. and Field J. 2011. Climatic correlates of temporal demographic variation in the tropical hover wasp Liostenogaster flavolineata. Insect. Soc. 58: 23–29
Eickwort G.C. and Eickwort K.R. 1971. Aspects of the biology of Costa Rican halictine bees, II. Dialictus umbripennis and adaptations of its caste structure to different climates. J. Kansas Entomol. Soc. 44: 343–373
Eickwort G.C., Eickwort J.M., Gordon J. and Eickwort M.A. 1996. Solitary behavior in a high altitude population of the social sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Behav. Ecol. Sociobiol. 38: 227–223
Field J., Paxton R.J., Soro A. and Bridge C. 2010. Cryptic plasticity underlies a major evolutionary transition. Curr. Biol. 20: 2028–2031
Field J., Paxton R.J., Soro A., Craze P. and Bridge C. 2012. Body size, demography and foraging in a socially plastic sweat bee: a common garden experiment. Behav. Ecol. Sociobiol. 66: 743–756
Frankie G.W., Haber, W.A., Vinson S.B., Bawa K.S., Ranchi P.S. and Zamora-Villalobos N.A. 2004. Flowering phenology and pollination systems diversity in the seasonal dry forest. In: Biodiversity Conservation in Costa Rica: Learning the Lessons in a Seasonal Dry Forest (Frankie G.W., Mata-Jiménez A. and Vinson S.B., Eds), University of California Press, Berkeley. pp 17–29
Janzen D.H. 1967. Synchronization of sexual reproduction of trees within the dry season in Central America. Evolution 21: 620–637
Kalacska M., Bohlman S., Sanchez-Azofeifa G.A., Castro-Esau K. and Caelli T. 2007. Hyperspectral discrimination of tropical dry forest lianas and trees: Comparative data reduction approaches at the leaf and canopy levels. Remote Sens. Environ. 109: 406–415
Kapheim K.M., Bernal S.P., Smith A.R., Nonacs P. and Wcislo W.T. 2011. Support for maternal manipulation of developmental nutrition in a facultatively eusocial bee, Megalopta genalis (Halictidae). Behav. Ecol. Sociobiol. 65: 1179–1190
Kapheim K.M., Smith A.R., Ihle K.E., Amdam G.V., Nonacs P. and Wcislo W.T. 2012. Physiological variation as a mechanism for developmental caste-biasing in a facultatively eusocial sweat bee. Proc. R. Soc. Lond. B 279: 1437–1446
Kapheim K.M., Smith A.R., Nonacs P., Wcislo W.T. and Wayne R.K. (in press). Foundress polyphenism and the origins of eusociality in a facultatively eusocial sweat bee, Megalopta genalis (Halictidae). Behav. Ecol. Sociobiol. Published online 29 November 2012. doi:10.1007/s00265-012-1453-x
Michener C.D. 1961. Social polymorphism in Hymenoptera. In: Insect Polymorphism, Symposia of the Royal Entomological Society of London No. 1 (Kennedy J.S., Ed), Bartholomew Press, Surrey. pp 43–56
Michener C.D. 1974. The Social Behavior of the Bees. Belknap Press, Cambridge MA
Michener C.D. 1990. Reproduction and castes in social halictine bees. In: Social Insects, an Evolutionary Approach to Castes and Reproduction (Engels W., Ed), Springer-Verlag, Berlin. pp 77–121
Mueller U.G. and Wolf-Mueller B. 1993. A method for estimating the age of bees: age-dependent wing wear and coloration in the wool-carder bee Anthidium manicatum. J. Insect Behav. 6: 529–537
Pyke C.R., Condit R., Aguilar S. and Lao S. 2001. Floristic composition across a climatic gradient in a neotropical lowland forest. J. Veg. Sci. 12: 553–566
Plateaux-Quénu C., Plateaux L. and Packer L. 2000. Population-typical behaviours are retained when eusocial and non-eusocial forms of Evylaeus albipes (F.) (Hymenoptera, Halictidae) are reared simultaneously in the laboratory. Insect. Soc. 47: 263–270
Purcell J. 2011. Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol. Rev. 86: 475–491
Quezada-Euán J.J.G., López-Velasco A., Pérez-Balam J., Moo-Valle H., Velazquez-Madrazo A. and Paxton R.J. 2011. Body size differs in workers produced across time and is associated with variation in the quantity and composition of larval food in Nannotrigona perilampoides (Hymenoptera, Meliponini). Insect. Soc. 58: 31–38
R Development Core Team. 2011. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. URL http://www.R-project.org/. Accessed August 2011
Rehan S.M., Richards M.H. and Schwarz M.P. 2009. Evidence of social nesting in the Ceratina of Borneo (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 82: 194–209
Richards M.H. 2004. Annual and social variation in forging effort of the obligately eusocial sweat bee, Halictus ligatus (Hymenoptera: Halictidae). J. Kansas Entomol. Soc. 77: 484–502
Richards M.H. and Packer L. 1995. Annual variation in survival and reproduction of the primitively eusocial sweat bee Halictus ligatus (Hymenoptera: Halictidae). Can. J. Zool. 73: 933–941
Richards M.H. and Packer L. 1996. The socioecology of body size variation in the primitively eusocial sweat bee, Halictus ligatus (Hymenoptera: Halictidae). Oikos 77: 68–76
Richards M.H., Packer L. and Seger J. 1995. Unexpected patterns of parentage and relatedness in a primitively eusocial bee. Nature 373: 239–241
Roubik D.W. 1989. Ecology and Natural History of Tropical Bees. Cambridge University Press, Cambridge
Roulston T.H. and Cane J.H. 2000. The effect of diet breadth and nesting ecology on body size variation in bees (Apiformes). J. Kansas Entomol. Soc. 73: 129–142
Roulston T.H. and Cane J.H. 2002. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16: 49–65
Sakagami S.F. and Munukata M. 1972. Distribution and bionomics of a transpalaearctic eusocial halictine bee, Lasioglossum (Evylaeus) calceatum, in northern Japan, with reference to its solitary life cycle at high altitude. J. Fac. Sci. Hokkaido Univ. VI Zool. 18: 411–439
Santos L.M., Tierney S.M. and Wcislo W.T. 2010. Nest descriptions of Megalopta aegis (Vachal) and M. guimaraesi Santos & Silveira (Hymenoptera, Halictidae) from the Brazilian Cerrado. Rev. Bras. Entomol. 54: 332–334
Schwarz M.P., Richards M.H. and Danforth B.N. 2007. Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Ann. Rev. Entomol. 52: 127–150
Smith A.R., Kapheim K.M., O’Donnell S. and Wcislo W.T. 2009. Social competition but not subfertility leads to a division of labour in the facultatively social sweat bee Megalopta genalis (Hymenoptera: Halictidae). Anim. Behav. 78: 1043–1050
Smith A.R., Lopez I., Moreno E., Roubik D.W. and Wcislo W.T. 2012. Pollen use and foraging distance of Megalopta sweat bees in relation to resource availability in a tropical forest. Ecol. Entomol. 37: 309–317
Smith A.R., Wcislo W.T. and O’Donnell S. 2008. Body size shapes caste expression, and cleptoparasitism reduces body size in the facultatively eusocial bee Megalopta (Hymenoptera: Halictidae). J. Insect Behav. 21: 394–406
Soucy S.L. 2002. Nesting biology and socially polymorphic behavior of the sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Ann. Entomol. Soc. Am. 95: 57–65
Soucy S.L. and Danforth B.N. 2002. Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Evolution 56: 330–341
Tierney S.M., Gonzales-Ojeda T. and Wcislo W.T. 2008a. Biology of a nocturnal bee, Megalopta atra (Hymenoptera: Halictidae; Augochlorini) from the Panamanian highlands. J. Nat. Hist. 42: 1841–1847
Tierney S.M., Gonzales-Ojeda T. and Wcislo W.T. 2008b. Nesting biology and social behavior of two Xenochlora bees (Hymenoptera: Halictidae: Augochlorini) from Perú. J. Kansas Entomol. Soc. 81: 61–72
Tierney S.M. and Schwarz M.P. 2009. Reproductive hierarchies in the African allodapine bee Allodapula dichroa (Apidae: Xylocopinae) and ancestral forms of sociality. Biol. J. Linn. Soc. 97: 520–530
Wcislo W.T. 1996. Commentary on solitary behavior in social bees. Behav. Ecol. Sociobiol. 38: 235–236
Wcislo W.T. 1997. Behavioral environments of sweat bees (Halictinae) in relation to variability in social organization. In: The Evolution of Social Behavior in Insects and Arachnids (Crespi B.J. and Choe J.C., Eds), Cambridge University Press, Cambridge. pp 316–332
Wcislo W.T. 2000. Environmental hierarchy, behavioral contexts, and social evolution in insects. In: Ecologia e Comportamento de Insetos (Martins R.P., Lewinsohn T.M. and Barbeitos M.S., Eds). Oecolog. Brasil. (supplement) 8: 49–84
Wcislo W.T., Arneson L., Roesch K., Gonzalez V.H., Smith A.R. and Fernández-Marín H. 2004. The evolution of nocturnal behaviour in sweat bees, Megalopta genalis and M. ecuadoria: an escape from competitors and enemies? Biol. J. Linn. Soc. 83: 377–387
Wcislo W.T. and Danforth B.N. 1997. Secondarily solitary: the evolutionary loss of social behavior. Trends Ecol. Evol. 12: 468–474
Wcislo W.T. and Gonzalez V.H. 2006. Social and ecological contexts of trophallaxis in facultatively social sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera, Halictidae). Insect. Soc. 53: 220–225
Wcislo W.T. and Tierney S.M. 2009. Behavioural environments and niche construction: the evolution of dim-light foraging in bees. Biol. Rev. 84: 19–37
West-Eberhard M.J. 1996. Wasp societies as microcosms for the study of development and evolution. In: Natural History and Evolution of Paper-Wasps (Turillazzi S. and West-Eberhard M.J., Eds), Oxford University Press, New York. pp 290–317
Wille A. and Orozco E. 1970. The life cycle and behavior of the social bee Lasioglossum (Dialictus) umbripenne (Hymenoptera: Halictidae). Rev. Biol. Trop. 17: 199–245
Wright S.J. and Calderon O. 1995. Phylogenetic patterns among flowering phenologies. J. Ecol. 83: 937–948
Zayed A. and Packer L. 2002. Genetic differentiation across a behavioural boundary in a primitively eusocial bee, Halictus poeyi Lepeletier (Hymenoptera, Halictidae). Insect. Soc. 49: 282–288
Acknowledgments
We thank P. Galgani and Smithsonian Tropical Research Institute (STRI) support staff for logistical help, O. Acevedo, D. Roubik, staff of the Sierra Llorona Lodge and the administration of the Parque Natural Metropolitano facilitated access to field sites. We gratefully acknowledge R. Condit for the use of STRI’s long-term database and S. Dennis for advice on R. The manuscript was further improved by suggestions from M. Schwarz, B. Turner, M.J. West-Eberhard and two anonymous reviewers. Research was supported by STRI Earl S. Tupper Postdoctoral Fellowship to SMT; a SI Restricted Endowment Grant to WTW, SMT and KMK; a STRI-Butler University Internship to CNF; a STRI Short Term Fellowship to SMR. We are grateful to the Autoridad National del Medioambiente of the Republic of Panama for research permit no. SEX/A-34-09.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tierney, S.M., Fischer, C.N., Rehan, S.M. et al. Frequency of social nesting in the sweat bee Megalopta genalis (Halictidae) does not vary across a rainfall gradient, despite disparity in brood production and body size. Insect. Soc. 60, 163–172 (2013). https://doi.org/10.1007/s00040-012-0280-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-012-0280-4