Abstract
Queens of many social insect species are known to maintain reproductive monopoly by pheromonal signalling of fecundity. Queens of the primitively eusocial wasp Ropalidia marginata appear to do so using secretions from their Dufour’s glands, whose hydrocarbon composition is correlated with fertility. Solitary nest foundresses of R. marginata are without nestmates; hence expressing a queen signal can be redundant, since there is no one to receive the signal. But if queen pheromone is an honest signal inextricably linked with fertility, it should correlate with fertility and be expressed irrespective of the presence or absence of receivers of the signal, by virtue of being a byproduct of the state of fertility. Hence we compared the Dufour’s gland hydrocarbons and ovaries of solitary foundresses with queens and workers of post-emergence nests. Our results suggest that queen pheromone composition in R. marginata is a byproduct of fertility and hence can honestly signal fertility. This provides important new evidence for the honest signalling hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many social insects, especially highly eusocial species, queens are known to maintain reproductive monopoly by means of chemical signals or pheromones (Winston and Slessor, 1992; Katzav-Gozansky et al., 2002; D’Ettorre et al., 2004; Monnin, 2006). There is growing evidence for chemical signalling of reproductive status in primitively eusocial species as well (Bonavita-Cougourdan et al., 1991; Peeters et al., 1999; Liebig et al., 2000; Sledge et al., 2001; Dapporto et al., 2005; Sramkova et al., 2008). Queen pheromones have been proposed as honest signals of fertility by which a queen signals her fertility status to her workers (Keller and Nonacs, 1993; Heinze and D’Ettorre, 2009; van Zweden, 2010). Thus workers perceive the fertility of their queen through the queen pheromone and are selected to refrain from reproduction. When the fertility of a queen declines, she can be overthrown or even killed by her workers (Engels and Imperatriz-Fonseca, 1990). This implies that pheromone production should be correlated with the state of fertility of an individual.
Solitary foundresses in Ropalidia marginata, individuals who found small nests and raise brood alone, provide an interesting system to study the honesty of queen signalling. Since solitary foundresses do not have any nestmates, expressing a queen signal is redundant, since there is no one to receive the signal. But, if the pheromone is an honest signal arising as an inevitable byproduct of the state of fertility, i.e. inextricably linked to the fertility of an individual, expression of pheromone and pheromone composition should necessarily be correlated with the degree of fertility of the individual (Keller and Nonacs, 1993; Heinze and D’Ettorre, 2009; van Zweden, 2010). Such a signal (“index” or “constraint”) should be produced by any fertile individual, irrespective of the presence or absence or receivers of the signal (nestmates), by virtue of being physiologically linked to fertility (Maynard Smith and Harper, 1995; Searcy and Nowicki, 2005). A fertile individual cannot help but produce the signal due to this physiological constraint.
The R. marginata queen is remarkably docile and non-interactive, and thereby unlike queens of other primitively eusocial wasps, she cannot use dominance behaviour or other behavioural interactions to maintain reproductive monopoly (Gadagkar, 2001; Bhadra et al., 2007). The Dufour’s gland in R. marginata has been shown to be at least one source of the queen pheromone by which queens appear to maintain reproductive monopoly (Bhadra et al., 2010; Mitra et al., 2011), presumably by applying the Dufour’s gland contents on the nest surface (Bhadra et al., 2007). The Dufour’s gland hydrocarbon composition has also been shown to be correlated with ovarian activation status in queens (Mitra and Gadagkar, 2011). Here we compared the Dufour’s gland hydrocarbon composition and ovaries of solitary foundresses with those of queens and workers of normal post-emergence nests in an attempt to provide an independent line of evidence for the honesty of the queen signal. Our objective was to investigate whether the queen pheromone composition depends primarily on the state of ovarian activation, irrespective of the presence or absence of nestmates (receivers of the queen signal). We found that solitary foundresses were intermediate between queens and workers with respect to both their Dufour’s gland hydrocarbon composition and their ovaries, suggesting that queen pheromone composition in this species can be a byproduct of the state of fertility and hence may honestly signal fertility. This study specifically looks at fertility signalling by social insect queens in a naturally occurring solitary condition during the nest initiation stage and provides new evidence supporting the honest signal hypothesis.
Materials and methods
Eight solitary foundresses were used in this study. All of them had founded small nests naturally in and around the vespiary at the Centre for Ecological Sciences, Indian Institute of Science, Bangalore (13°00′N and 77°32′E), India, and had laid some eggs. We compared these solitary foundresses with 12 queens and 36 workers from post-emergence colonies (colony sizes ranging from 7 to 20 individuals) collected from various localities in Bangalore. R. marginata has perennial colonies which can be founded and abandoned throughout the year, and the queen is the sole egg layer in a colony (Gadagkar, 2001).
Gas chromatographic analysis of Dufour’s gland
We analysed the Dufour’s glands by gas chromatography following Mitra et al. (2011). Identification of compounds had been done earlier by mass spectrometric analyses (Bhadra et al., 2010). We matched the retention times and pattern of peaks with those obtained in earlier analyses; for statistical analyses, we considered only those peaks that had been identified earlier.
For univariate analysis, we converted the area under each peak in an individual to percentage by dividing with area under all peaks in that individual. Kruskal–Wallis test was done followed by Mann–Whitney U tests to compare solitary foundresses, queens and workers with respect to percent area under peaks 1–4 pooled together (Heneicosane, 11-methylheneicosane, Tricosane and 11-methyltricosane, respectively), and for percent area under all remaining peaks pooled together, following Mitra and Gadagkar (2011).
For multivariate analysis, we used peaks present in at least 70% of all samples. As a result, peak # 20a (Hentriacontane) got eliminated from multivariate analysis. The area under each peak was square root transformed followed by log ratio transformation (Bhadra et al., 2010; Mitra et al., 2011) and the transformed areas subjected to discriminant analysis to see if solitary foundresses cluster with queens or with workers based on their Dufour’s gland hydrocarbon composition.
Ovarian measurements
After dissecting the Dufour’s gland, we dissected the ovaries of each wasp and measured them, calculated an ovarian index from the measurements, and considered the score of each individual wasp on the ovarian index as a proxy for fertility following Mitra and Gadagkar (2011). Comparisons of ovarian indices of solitary foundresses, queens and workers were done by Kruskal–Wallis test followed by Mann–Whitney U tests.
Statistical analyses used the software package StatistiXL 1.7.
Results
Gas chromatographic analysis showed that Dufour’s gland profiles of solitary foundresses were qualitatively similar to queens and workers (Online Resource 1). About 34 peaks were found in total. All 34 peaks had been identified earlier (Bhadra et al., 2010; Mitra et al., 2011) and were used here in statistical analyses.
Comparisons using compounds that differ significantly between queens and workers
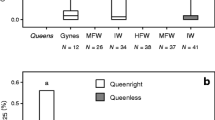
Queens have been shown to have consistently lower percent area than workers for peaks 1, 2, 3 and 4, (Heneicosane, 11-methylheneicosane, Tricosane and 11-methyltricosane, respectively) and have consistently higher percent area than workers for the remaining peaks (Mitra and Gadagkar, 2011). The percent area under these peaks have also been found to be correlated with ovarian index of queens, implying that such differences may be important in defining the queen signal (Mitra and Gadagkar, 2011). For percent area under peaks 1, 2, 3 and 4, Kruskal–Wallis test showed that variation between groups was higher than variation within groups (F 2,52 = 29.664, P < 0.0001). Solitary foundresses were intermediate—lower than workers but higher than queens (Mann–Whitney U test, U = 210, P = 0.022 for comparison with workers and U = 92, P < 0.001 for comparison with queens, respectively). Queens were lower than workers, as found previously (U = 429, P < 0.001) (Fig. 1a). Similarly, for percent area under the remaining peaks, Kruskal–Wallis test showed that variation between groups was higher than variation within groups (F 2,52 = 29.664, P < 0.0001). Solitary foundresses were higher than workers and lower than queens (U = 210, P = 0.022 for comparison with workers and U = 92, P < 0.001 for comparison with queens, respectively). Queens were higher than workers, as found previously (U = 429, P < 0.001) (Fig. 1b).
a Percent area under peaks 1, 2, 3 and 4, b percent area under the remaining peaks, and c ovarian index of workers (w), solitary foundresses (sf) and queens (q) of Ropalidia marginata. Different letters denote significant difference between distributions for each variable (n = 36 w, 12 q, 8 sf, Mann–Whitney U test: P < 0.05)
Comparisons using all compounds simultaneously
Solitary foundresses could be differentiated from queens and workers by discriminant analysis using the transformed areas under all peaks (Fig. 2a). Both discriminant functions 1 and 2 were significant and all individuals were classified correctly (function 1: Wilk’s λ = 0.014, P < 0.001; function 2: Wilk’s λ = 0.236, P = 0.010; classification analysis: 100% correct classification). Peak # 20a (Hentriacontane) was left out of Euclidean distance between points on the discriminant functions plot (Fig. 2a) showed that variation in distances between groups were higher than variation in distances among groups (Kruskal–Wallis test: F 2,812 = 347.466, P < 0.0001). Distances between solitary foundresses and queens were not different from distances between solitary foundresses and workers (Mann–Whitney U = 14,915, P = 0.247). Distances between queens and workers were higher than both distances between solitary foundresses and queens (U = 36,726, P < 0.001) as well as distances between solitary foundresses and workers (U = 111,308, P < 0.001) (Fig. 2b).
a Queens (q), solitary foundresses (sf) and workers (w) of Ropalidia marginata plotted on discriminant functions 1 and 2 (n = 12 q, 8 sf, 36 w). Both functions are significant (P < 0.05). Figures in parenthesis beside each function denote the percent variance explained by that function. b Distance on discriminant functions plot (a) between queens and workers (q–w), solitary foundresses and queens (sf–q), and solitary foundresses and workers (sf–w). Different letters denote significant difference between distributions (Mann–Whitney U test, P < 0.05)
Ovarian index
Kruskal–Wallis test showed that variation in ovarian index between groups was higher than variation within groups (F 2,52 = 68.791, P < 0.0001). Ovarian index of solitary foundresses was intermediate between queens and workers—it was higher than that of workers (Mann–Whitney U = 288, P < 0.001) but lower than that of queens (U = 96, P < 0.001). Ovarian index of workers was lower than that of queens as expected (U = 432, P < 0.001) (Fig. 1c).
Discussion
Dufour’s gland profiles of solitary foundresses were not similar to established queens of post-emergence nests, and neither they were similar to workers, but seemed to occupy a position in between queens and workers. Analysis of pair-wise distance between points on the discriminant functions plot showed that queens and workers were relatively well separated from each other, while solitary foundresses were equidistant from queens and workers. Solitary foundresses were in an intermediate position for percent area under peaks 1, 2, 3 and 4, and for percent area under the remaining peaks also. Although all solitary foundresses had laid eggs, they had lower fecundity than queens. Their state of ovarian activation was intermediate between queens and workers, and this could be a consequence of energy consuming tasks like foraging and nest maintenance that they have to perform. Queens of established post-emergence nests never go out to forage and do almost no work for colony maintenance, and this could be a reason as to why their ovaries are better activated than those of solitary foundresses.
Since solitary foundresses occupy an intermediate position with respect to both Dufour’s gland hydrocarbon composition and ovary activation, this suggests that their intermediate position may be attributed to their intermediate fecundity level, lending support to the honest signal hypothesis (Keller and Nonacs, 1993). In spite of there being no immediate need for signalling their fertility status, owing to the absence of receivers of the signal, solitary foundresses are expressing a profile that is different from that of workers and closer to that of queens. This is presumably because their state of ovary activation is also higher than workers and closer to queens. The correlation of Dufour’s gland composition with state of ovarian activation might hint at underlying physiological mechanisms. It could be possible that factors involved in the ovarian activation pathway influence the biosynthesis of Dufour’s gland compounds. These results suggest that Dufour’s gland composition in R. marginata may arise as an inevitable byproduct of the ovarian activation status of an individual and hence may function as an honest signal that portrays the fertility of an individual.
It has been proposed that for any signal to originate and be incorporated into a communication system the signal should be reliable or honest (Zahavi, 1975; Maynard Smith and Harper, 1995; Zahavi and Zahavi, 1997; Searcy and Nowicki, 2005). This helps eliminate cheaters and thus makes it possible for the receiver to rely on it. Unless a signal is honest on average, both signaller and receiver will not benefit from it and hence the signal will not evolve (Johnstone and Grafen, 1993; Kokko, 1997). There may be several ways in which a signal can achieve honesty (Maynard Smith and Harper, 1995; Searcy and Nowicki 2005). Since in R. marginata, the queen signal appears to be inextricably linked to the state of fertility, this suggests that queen pheromone in this species is likely to be a signal that can be classified as “index” or “constraint”, i.e. a signal that is forced to be reliable because of physiological constraints on signal production (Maynard Smith and Harper, 1995; Searcy and Nowicki 2005). Since R. marginata workers appear to detect the presence of their queen by means of the queen’s Dufour’s gland compounds that she applies on the nest surface, and react accordingly (Bhadra et al., 2007; Bhadra et al., 2010; Mitra et al., 2011), this has important implications for the mechanism of maintenance of eusociality in this species.
Previous work has shown that Dufour’s gland hydrocarbon composition in R. marginata is correlated with ovarian activation status of queens, suggesting that Dufour’s gland hydrocarbons may act as honest signals of fertility (Mitra and Gadagkar, 2011). This study adds to the evidence for the link between fertility of individuals and chemicals likely to be involved in fertility signalling (Sledge et al., 2001; Monnin, 2006; Izzo et al., 2010; Mitra and Gadagkar, 2011), and reinforces the idea that a signal that arises as a byproduct of fertility is honest and hence can be incorporated in a communication system for fertility signalling.
References
Bhadra A., Iyer P.L., Sumana A., Deshpande S.A., Ghosh S. and Gadagkar R. 2007. How do workers of the primitively eusocial wasp Ropalidia marginata detect the presence of their queens? J. Theor. Biol. 246: 574-582
Bhadra A., Mitra A., Deshpande S.A., Chandrasekhar K., Naik D.G., Hefetz A. and Gadagkar R. 2010. Regulation of reproduction in the primitively eusocial wasp Ropalidia marginata: on the trail of the queen pheromone. J. Chem. Ecol. 36: 424-431
Bonavita-Cougourdan A., Théraulaz G., Bagnères A.G., Roux M., Pratte M., Provost E. and Clément J.L. 1991. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. 100B: 667-680
D’Ettorre P., Heinze J., Schulz C., Francke W. and Ayasse M. 2004. Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 207: 1085-1091
Dapporto L., Sledge M.F. and Turillazzi S. 2005. Dynamics of cuticular chemical profiles of Polistes dominulus workers in orphaned nests (Hymenoptera, Vespidae). J. Insect Physiol. 51: 969-973
Engels W. and Imperatriz-Fonseca V.L. 1990. Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees. In: Social Insects: an Evolutionary Approach to Castes and Reproduction (Engels W., Ed), Springer-Verlag, Berlin, pp 167-230
Gadagkar R. 2001. The Social Biology of Ropalidia marginata - Toward Understanding the Evolution of Eusociality. Harvard University Press, Cambridge, Massachusetts.
Heinze J. and D’Ettorre P. 2009. Honest and dishonest communication in social Hymenoptera. J. Exp. Biol. 212: 1775-1779
Izzo A., Wells M., Huang Z. and Tibbetts E. 2010. Cuticular hydrocarbons correlate with fertility, not dominance, in a paper wasp, Polistes dominulus. Behav. Ecol. Sociobiol. 64: 857-864
Johnstone R.A. and Grafen A. 1993. Dishonesty and the handicap principle. Anim. Behav. 46: 759-764
Katzav-Gozansky T., Soroker V. and Hefetz A. 2002. Honeybees Dufour’s gland - idiosyncrasy of a new queen signal. Apidologie 33: 525-537
Keller L. and Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45: 787-794
Kokko H. 1997. Evolutionary stable strategies of age-dependent sexual advertisement Behav. Ecol. Sociobiol. 41: 99-107
Liebig J., Peeters C., Oldham N.J., Markstädter C. and Hölldobler B. 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl Acad. Sci. USA 97: 4124-4131
Maynard Smith J. and Harper D.G.C. 1995. Animal signals: models and terminology. J. Theor. Biol. 177: 305-311
Mitra A. and Gadagkar R. 2011. Can Dufour’s gland compounds honestly signal fertility in the primitively eusocial wasp Ropalidia marginata? Naturwissenschaften 98: 157-161
Mitra A., Saha P., Chaoulideer M.E., Bhadra A. and Gadagkar R. 2011. Chemical communication in Ropalidia marginata: Dufour’s gland contains queen signal that is perceived across colonies and does not contain colony signal. J. Insect Physiol. 57: 280-284
Monnin T. 2006. Chemical recognition of reproductive status in social insects. Ann. Zool. Fenn. 43: 515-530
Peeters C., Monnin T. and Malosse C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266: 1323-1327
Searcy W.A. and Nowicki S. 2005. The Evolution of Animal Communication Reliability and Deception in Signaling Systems. Princeton University Press, Princeton
Sledge M.F., Boscaro F. and Turillazzi S. 2001. Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav. Ecol. Sociobiol. 49: 401-409
Sramkova A., Schulz C., Twele R., Francke W. and Ayasse M. 2008. Ferility signals in the bumblebee Bombus terrestris (Hymenoptera: Apidae). Naturwissenschaften 95: 515-522
van Zweden J.S. 2010. The evolution of honest queen pheromones in insect societies. Comm. Int. Biol. 3: 50-52
Winston M.L. and Slessor K.N. 1992. The essence of royalty: honey bee queen pheromone. Am. Sci. 80: 374-385.
Zahavi A. 1975. Mate selection - a selection for a handicap. J. Theor. Biol. 53: 205-214
Zahavi A. and Zahavi A. 1997. The Handicap Principle: a Missing Piece of Darwin’s Puzzle. Oxford University Press, New York
Acknowledgments
We thank the Department of Science and Technology, the Department of Biotechnology, the Council for Scientific and Industrial Research and the Ministry of Environment and Forests, Government of India for financial assistance. AM carried out chemical analysis, ovarian dissections and statistical analysis. The paper was co-written by AM and RG, and RG supervised the overall work. All experiments reported here comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitra, A., Gadagkar, R. Queen signal should be honest to be involved in maintenance of eusociality: chemical correlates of fertility in Ropalidia marginata . Insect. Soc. 59, 251–255 (2012). https://doi.org/10.1007/s00040-011-0214-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-011-0214-6