Abstract
Plant responses to wounding and herbivore attack are orchestrated by complex signaling pathways that link the production of chemical and physical signals at the wound site to activation of gene expression and other cellular processes. The systemic nature of many wound-induced responses provides an attractive opportunity to study intercellular signaling pathways that operate over long distances within the plant. Genetic dissection of the wound-response pathway in tomato indicates that (1) systemin and its precursor protein, prosystemin, are upstream components of an intercellular signaling cascade that requires the biosynthesis and action of jasmonic acid (JA); and (2) physiological processes regulated by this pathway confer host resistance to a broad spectrum of plant invaders. Grafting experiments conducted with mutants defective in systemic wound signaling indicate that systemin functions at or near the wound site to trigger the production of JA, which in turn acts non-cell autonomously to promote systemic defense responses. The location of JA biosynthetic enzymes within the companion cell-sieve element complex of vascular bundles, together with the accumulation of JA in vascular tissues, support a role for jasmonates as phloem-mobile signals. The recent discovery of enzymes involved in the metabolism of JA to volatile methyl-JA and bioactive JA-amino acid conjugates has potential implications for the mechanism by which JA promotes wound signaling. Species-specific differences in the mechanism of wound signaling appear to reflect the way in which the wound-induced jasmonate pathway is regulated by other signals including systemin, cell wall-derived oligosaccharides, ethylene, and insect-derived elicitors. Adding to the complexity of the wound-induced jasmonate cascade are wound-signaling pathways that operate independently of JA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Higher plants have evolved a diverse repertoire of self-protection mechanisms to cope with the threat of herbivores that chew, suck, or otherwise destroy plant tissues. One common strategy employed by many plants is the wound-induced expression of phytochemicals involved in herbivore deterrence, wound healing, and other defense-related processes. The rapid synthesis and deployment of these chemical arsenals is regulated by complex signal transduction pathways that link the production of specific signals at the site of tissue damage to changes in gene expression and, ultimately, changes in plant metabolism that negatively affect herbivore growth and reproduction. The widespread occurrence of induced resistance to herbivores (Karban and Baldwin 1997) suggests the existence of common mechanisms to generate, transport, and perceive wound signals into physiologically relevant responses that enhance plant resistance to biotic stress.
An important aspect of many wound-induced plant defense responses is their occurrence in undamaged leaves located distal to the site of attack. Wound-inducible proteinase inhibitors (PIs), which function as anti-nutritive agents by blocking digestive proteases in the herbivore gut, represent one of the best examples of this phenomenon. In the well-characterized tomato (Lycopersicon esculentum) system, PI-encoding genes are expressed systemically within about 2 hours after mechanical wounding or herbivory (Ryan 2000; Howe and others 2000). More than 30 years ago, Green and Ryan (1972) proposed that signals generated at the site of insect attack travel through the plant and systemically activate defensive responses that confer protection against subsequent attacks. The systemic nature of this and other induced plant defense responses is analogous to the vertebrate immune response in which endocrine signals are delivered to target tissues via the circulatory system (Bergey and others 1996). Although several chemical and physical signals have been implicated in the regulation of wound-induced systemic plant defenses (Malone 1996; Herde and others 1999; Ryan 2000; León and others 2001), very little is known about how these signals interact with one another to effect cell-to-cell communication over long distances.
The discovery of jasmonic acid (JA) and its methyl ester, methyl-JA (MeJA) as potent signals for the expression of PIs provided the first evidence that members of the jasmonate family of oxylipins (referred to here collectively as JAs) play an important role in induced defense against insects (Farmer and Ryan 1990; Farmer and others 1992; Farmer and Ryan 1992; Howe and others 1996). Since that discovery, detailed studies of wound signaling in several plant species have shaped the current paradigm that JAs are “master” signals for a multitude of induced defense responses. Researchers today are using a wealth of experimental approaches, ranging from molecular genetics to chemical ecology (Kessler and others 2004), to understand more completely the role of JAs in plant defense. Much of our knowledge about this signaling pathway has come from studies with solanaceous species (tomato, tobacco and potato) and Arabidopsis. However, increasing evidence indicates that JAs are important wound signals in species throughout the plant kingdom, including monocots and trees (Schafleitner and Wilhelm 2002; Martin and others 2003; Rakwal and Agrawal 2003; Engelberth and others 2004; Hudgins and others 2004). Several recent papers have reviewed the topic of wound signaling, its relationship to plant defense, and interactions that occur between the JA and other defense signaling pathways (Walling 2000; Ryan 2000; León and others 2001; Kessler and Baldwin 2002; Gatehouse 2002; Turner and others 2002; Kunkel and Brooks 2002; Wasternack and Hause 2002; Rojo and others 2003; Farmer and others 2003). Here, I describe the latest information on the role of JAs as signals in the wound response. Emphasis is placed on studies in the well-characterized tomato system, which has been exploited as a model for genetic dissection of wound signaling. JA-independent wound responses are also discussed for the purpose of highlighting the complexity of wound signaling and the potential importance of interactions between JA-dependent and -independent pathways.
WOUND-INDUCIBLE PIS: A PARADIGM FOR JA-REGULATED PLANT DEFENSE
Wound-inducible PIs in tomato and other solanaceous species have been widely used as a model system to study the mechanism of wound signaling. These studies have established the paradigm that extracellular signals (so-called primary wound signals) generated in response to wounding signal the intracellular production of JAs, which in turn activate the expression of defensive genes (Farmer and Ryan 1992; Ryan 2000). The identification and characterization of signals involved in the wound-induced PI expression was facilitated by a facile bioassay in which chemical elicitors are supplied to tomato seedlings through the cut stem, and PI accumulation in leaf tissue is then measured. Extensive use of this assay resulted in the discovery of several PI-inducing signals including cell-wall-derived oligogalacturonides (OGAs) and systemin (Ryan 1992). The production of OGAs in response to wounding appears to involve the action of a polygalacturonase (PG) whose expression in tomato leaves is wound-inducible (Bergey and others 1999). The relative immobility of OGAs in the plant vascular system suggests that these compounds function mainly as local wound signals. However, because PG activity is also induced systemically in response to wounding, OGAs may also play a role in the systemic response (Ryan 2000). Aldington and co-workers (1991) proposed that the bioactivity of OGAs results from direct physical effects on the plasma membrane. However, the involvement of a specific receptor for OGAs cannot be excluded (Navazio and others 2002).
Systemin is approximately 10,000-fold more active than OGAs in inducing PI expression in the tomato seedling bioassay (Ryan 1992) and, as discussed below, is implicated as a signal in the systemic wound response. Systemin is derived from the C-terminal end of a precursor protein (prosystemin) whose amino acid sequence shows little similarity to other proteins in the database. Several lines of genetic evidence indicate that prosystemin performs an essential role in wound-induced defense responses. For example, transgenic tomato plants expressing an antisense prosystemin (Prosys) cDNA are deficient in wound-induced systemic expression of PIs and exhibit increased susceptibility to herbivores (McGurl and others 1992; Orozco-Cárdenas and others 1993). Conversely, overexpression of prosystemin from a 35S-Prosys transgene constitutively activates PI expression in the absence of wounding, thereby conferring enhanced resistance to herbivores (McGurl and others 1994; Li and others 2002b). A recent major breakthrough in our understanding of wound signaling was the identification of the systemin receptor (SR160; Scheer and Ryan 2002). This 160-kDa plasma membrane-bound protein is a member of the leucine-rich repeat (LRR) receptor-like kinase family of proteins. Of considerable interest to the field of plant signaling was the discovery that SR160 is identical to the tomato brassinosteroid (BR) receptor, tBRI1 (Wang and He 2003). Additional research into the mechanism by which SR160/tBRI1 perceives two different ligands (that is, a peptide and a steroid), and then signals appropriate downstream responses, is clearly warranted.
Transcriptional activation of PI and other defense-related genes in response to wounding, systemin, and OGAs depends on the biosynthesis and subsequent action of JA that is synthesized via the octadecanoid pathway (Farmer and Ryan 1992; Ryan 2000; Doares and others 1995; Li and others 2002a). The signal transduction events that couple the perception of OGAs and systemin at the plasma membrane to the activation of JA synthesis in the chloroplast remain to be elucidated. JA synthesized in response to wounding, systemin, and OGA acts in concert with ethylene (O’Donnell and others 1996) and hydrogen peroxide (Orozco-Cárdenas and others 2001; Sagi and others 2004) to positively regulate the expression of downstream target genes. Readers are referred to other articles in this special issue for a detailed description of JA biosynthesis and JA signaling, respectively.
GENETIC ANALYSIS OF WOUND SIGNALING IN TOMATO
The use of mutants offers a powerful approach to elucidate the molecular components of the wound-signaling pathway and their role in induced resistance. To date, forward genetic screens for wound-response mutants have been conducted only in tomato. Several features of this agriculturally important plant make it attractive as a model system for genetic analysis of plant-insect interactions. First, cultivated tomato is a natural host to over 100 arthropod herbivores from various feeding guilds that attack roots, leaves, or fruit (Kennedy 2002). Second, biochemical and physiological studies have produced a wealth of information relevant to the mechanism of wound signaling in tomato. Third, several well-characterized defensive proteins such as PIs and polyphenol oxidase (PPO) provide robust markers for identifying mutants. Fourth, genetic analysis of wound signaling in this system can provide insight into the role of peptide signals (that is, systemin) in induced defense. Finally, extensive molecular and genetic tools also are available to facilitate the identification of genes that have been defined by mutational analysis (Van der Hoeven and others 2002).
With these considerations in mind, Lightner and others (1993) screened an ethylmethane sulfonate (EMS)-mutagenized population for plants that fail to accumulate PIs in response to mechanical wounding. This effort yielded two non-allelic mutants (JL1 and JL5) that are defective in wound-induced PI expression. The JL5 mutant (renamed def1) was shown to be deficient in wound- and systemin-induced JA accumulation and compromised in defense against insect attack (Howe and others 1996). Treatment of def1 plants with exogenous JAs induces the expression of PI and other defense-related genes, and restores resistance to herbivores (Li and others 2002b). Available data suggest that DEF1 does not correspond to any known JA biosynthetic gene in tomato. Rather, this locus may be involved in regulating the activity of one or more JA biosynthetic enzymes (Li and others 2002a; Stenzel and others 2003). A defect in JA biosynthesis in the JL1 mutant line is indicated by a severe deficiency in wound-induced JA accumulation and in the ability of the mutant to respond to exogenous JA (Lightner and others 1993; Lee GI, Jayanty S, and Howe GA, unpublished data).
For a second strategy to identify wound-response mutants of tomato, we employed the 35S-Prosys transgenic plants that constitutively accumulate PIs and PPO (Howe and Ryan 1999). An EMS-mutagenized population was screened for plants that are deficient in PI and PPO expression in the absence of wounding. Several spr (suppressed in prosystemin-mediated responses) mutants identified were defective in wound-induced systemic PI expression. Genetic complementation tests showed that this collection of mutants included new alleles of def1 and two novel complementation groups designated SPR1 and SPR2. A map-based cloning approach was used to identify the SPR2 gene (Li and others 2003). It encodes a plastidic omega-3 fatty acid desaturase that is required for the conversion of linoleic acid to linolenic acid, which is the precursor of most bioactive JAs. Consistent with this finding, spr2 plants produce less than 10% of wild-type levels of JA in response to wounding and consequently are compromised in defense against insects (Li and others 2003). The phenotype of spr1 plants indicates that SPR1 plays a role in an early step in systemin signaling (see below).
Genetic screens for jasmonic acid-insensitive (jai) mutants led to the identification of mutants disrupted in the function of the tomato homolog of the Arabidopsis Coronatine Insensitive1 (COI1) gene (Li and others 2001; Li and others 2004). Studies in Arabidopsis have shown that COI1 encodes an F-box protein that participates in the formation of an E3 ligase complex (Xie and others 1998; Xu and others 2002; Devoto and others 2002). COI1 is thought to mediate ubiquitin-dependent proteolysis of one or more target proteins that regulate the expression of JA-responsive genes (Turner and others 2002). Gene expression profiling studies indicate that COI1 is essential for expression of JA-responsive genes, as well as the wound-induced expression of genes involved in anti-herbivore defense (Titarenko and others 1997; Reymond and others 2000; Li and others 2004). Indeed, virtually all known jasmonate-mediated defense responses in Arabidopsis and tomato require COI1. The central role of COI1 in the regulation of JA-dependent defense responses in tomato and Arabidopsis, together with the existence of COI1 orthologs in monocots (Li and others 2004), indicates that this component of the wound-signaling pathway is likely conserved in all higher plants.
The current collection of tomato wound-response mutants can be classified as being defective in either JA biosynthesis (def1, spr2, JL1), JA responsiveness (jai1), or responsiveness to systemin (spr1). Thus, a major conclusion of genetic analysis of wound signaling in tomato is that the jasmonate cascade occupies a central role in wound-induced defense, including systemic responses. The ability of def1, spr1, spr2, and jai1 to suppress 35S-Prosys-mediated responses (Howe and Ryan 1999; Li and other 2001; Li and others 2004) further demonstrates that wounding and systemin induce PI expression through a common signaling pathway involving JA, and that systemin and JA are essential components of the systemic wound response. These conclusions confirm and extend the original signaling model proposed by Farmer and Ryan (1992). Transgene-mediated anti-sense suppression of JA biosynthesis has provided additional genetic evidence for a role of JA in wound-induced local and systemic responses in this system (Stenzel and others 2003). Although abscisic acid (ABA) is not considered to be a primary wound signal in tomato (Birkenmeirer and Ryan 1998), it is noteworthy that ABA mutants of tomato and potato are deficient in PI expression in response to wounding and elicitation by systemin (Hildmann and others 1992; Peña-Cortés and others 1996; Carrera and Prat 1998). Loss of ABA function appears to disrupt wound signaling by blocking JA biosynthesis (Peña-Cortés and others 1996), which further supports the central role of JA in wound signaling.
Genetic analysis if wound signaling in tomato has shown that the JA pathway also plays a critical role in determining the outcome of plant-pest interactions. Specifically, the increased susceptibility of wound-response mutants to both herbivores (Howe and others 1996; Li and others 2002b; Thaler and others 2002; Li and others 2003; Li and others 2004) and pathogens (Thaler and others 2004) demonstrates that genes required for JA biosynthesis and JA signaling are essential for plant protection against biotic stress. In addition to providing plant protection, it is also evident that the wound-induced JA pathway exerts profound effects on herbivores that feed on tomato plants. For example, bioassays performed with Tetranychus urticae (two-spotted spider mite) indicate that the JA pathway significantly affects both the fecundity and host preference of the herbivore (Li and others 2004). These findings are consistent with recent field studies showing that JA-induced defenses influence herbivore community composition (Kessler and others 2004).
ROLE OF JA IN SYSTEMIC WOUND SIGNALING
An important question regarding the role of JAs in wound signaling is whether JA action is restricted to cells in which it is produced or whether JAs function non-cell autonomously to promote responses in distal target tissues. Several lines of evidence support the latter scenario, in which JAs act as intercellular signals. First, local application of JAs to a single leaf or leaflet can elicit the expression of wound-response genes in distal untreated tissues. This phenomenon was first demonstrated for PI expression in tomato plants (Farmer and others 1992). Systemic PI expression is also elicited by localized application of the Pseudomonas syringae phytotoxin coronatine (COR), which effectively mimics the action of JAs. COR-induced systemic PI expression requires COI1 but not the capacity of the plant to synthesize JA (Zhao and others 2003). This observation suggests that COR is transported from the site of application to distal leaves, where it activates gene expression in a COI1-dependent manner. Systemic activation of PI gene expression in response to COR-producing strains of P. syringae supports this hypothesis (Pautot and others 1991). Additional evidence for a role of JA in long-distance signaling comes from 14C-JA application experiments in tobacco. Radiolabeled JA applied to a single leaf was readily translocated to roots, which synthesize nicotine in response to leaf wounding (Zhang and Baldwin 1997). Both the timing and quantity of 14C-JA transport were similar to that of wound-induced changes in endogenous JA levels in roots. The movement of labeled JA from the site of application to roots and young immature leaves, but not mature leaves, indicates that transport of exogenous JA occurs via the phloem.
A necessary criterion for the long-distance signal is that wounding or other biotic stress should increase the abundance of the signal in undamaged leaves. Indeed, studies in tomato, Arabidopsis, and tobacco have shown that wounding induces a modest but significant systemic increase in JA accumulation (Herde and others 1996; Wang and others 2000; von Dahl and Baldwin 2004). The ability to detect systemic increases in JA appears to depend on the quantity and quality of leaf damage inflicted. von Dahl and Baldwin (2004) reported that application of caterpillar regurgitant to wounded tobacco plants caused a systemic increase in JA levels, whereas mechanical wounding alone did not. This observation may explain other studies showing that mechanical wounding results in very slight increases or no significant increase in systemic JA levels (Rojo and others 1999a; Strassner and others 2002). The level of systemic JA observed in the tobacco study (von Dahl and Baldwin 2004) was about 5% of that in damaged leaves, with maximum systemic accumulation occurring about 1.5 hours after treatment. Although JA-regulated systemic responses were not measured in these experiments, the timing of the systemic increase in JA is generally consistent with the onset of wound-induced systemic responses. In potato plants induced for systemic acquired resistance (SAR) by infection with P. syringae pv. maculicola, levels of OPDA, a bioactive precursor of JA (Weber 2002), increased in both infected and non-infected leaves (Landgraf and others 2001). Systemic increases in OPDA were not accompanied by corresponding increases in JA, suggesting that OPDA might play a role in SAR. It remains to be determined whether wound- and pathogen-induced systemic accumulation of JAs results from translocation of these compounds from the site of tissue damage or from de novo synthesis in undamaged leaves.
Grafting experiments conducted with wound-signaling mutants provide an approach to determining whether a specific gene product is involved in the production of the systemic (that is, graft-transmissible) signal in the wounded leaf or the recognition of that signal in undamaged responding leaves (Li and others 2002a) (Figure 1). Analysis of systemic wound signaling in grafts between wild-type and jai1 tomato plants showed that JA responsiveness is not strictly required for the production of the systemic signal in damaged leaves, but rather is necessary for the recognition or processing of that signal in systemic leaves. Conversely, grafts between wild-type and JA-deficient spr2 plants indicated that JA synthesis is required to produce the systemic signal in wounded leaves, but is not required in systemic undamaged leaves (Fig.ure1B). Similar results were obtained with the JA-deficient def1 mutant (Li and others 2002a). The most straightforward interpretation of these results is that JA or a related compound derived from the octadecanoid pathway acts as a transmissible wound signal (Figure 2). Based on the spatially distinct roles of JA biosynthesis and JA perception in long-distance wound signaling, it can be predicted that systemic signaling should occur in a grafted plant lacking both JA responsiveness (for example, jai1) in wounded rootstock leaves and JA biosynthesis (for example, spr2) in undamaged scion leaves. Conversely, systemic signaling in the reciprocal jai1/spr2 graft combination should be effectively blocked because of defects in both signal production and signal recognition. Indeed, these predictions were confirmed by experimentation (Li and others 2002a) (Figure 1C). Results from grafting experiments conducted with JA biosynthesis and JA response mutants of Arabidopsis appear to be consistent with the hypothesis that JAs are an important component of the systemic wound signal in this system as well (Hawkes and Turner 2004).
Schematic illustration of grafting experiments used to investigate the role of JA in wound-induced systemic expression of defensive proteinase inhibitors (PIs) in tomato. Scions and rootstocks of the indicated genotype were joined at the graft junction (horizontal bar). For experiments shown in A, B, C, and E, rootstock leaves were wounded (hatched mark) and PI gene expression in the damaged leaves and undamaged scion leaves was measured 8 hours later. Leaves exhibiting low (or no) PI expression are not shaded whereas leaves showing high PI expression are shaded. For the experiments depicted in panel D, no wounds were inflicted because the 35S-Prosys (PS) transgenic line constitutively produces a systemic signal. WT, wild type; PS, 35S-Prosys.
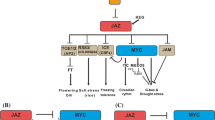
Model of wound signaling based on genetic studies in tomato. Wound-induced signals including systemin, OGAs, and putative unidentified compounds (?) activate the octadecanoid pathway for JA biosynthesis in response to mechanical wounding or herbivory. Production of JA mediates local and systemic activation of Early and Late response genes (solid arrows). Mutations that block various steps in the wound-signaling pathway are indicated (gray bars). Grafting experiments conducted with these mutants support the hypothesis that JA or a derivative thereof (JA-x) functions as a systemic signal. Wounding also activates JA-independent signaling pathways (hatched lines) that regulate local and systemic expression of Early genes, as well as other genes (for example, WIPK) whose expression is completely independent of JA and systemin. AOCas, antisense suppression of allene oxide cyclase.
Results obtained from grafting experiments with JA biosynthetic mutants (for example, spr2) are not consistent with the hypothesis that systemin generated in wounded leaves is translocated to distal tissues where it induces JA biosynthesis and subsequent PI expression. First, the inability of wounded spr2 leaves to generate the transmissible signal shows that JA biosynthesis is required for the production of the long-distance signal. Second, the ability of spr2 scions to express PIs in response to wounding of wild-type leaves indicates that de novo JA synthesis in target tissues is not required for systemic signaling. Because spr2 plants are insensitive to systemin (Howe and Ryan 1999; Li and others 2003), the transmissible signal responsible for PI expression in spr2 scions is most likely a trienoic fatty acid-derived compound (for example, JA) rather than systemin. A model of wound signaling that is consistent with the grafting studies and other available genetic data is shown in Figure 2.
INTERACTION BETWEEN JA, SYSTEMIN, AND OTHER WOUND SIGNALS
A wealth of biochemical and genetic evidence demonstrates that systemin induces the expression of defensive genes by triggering JA synthesis (Farmer and Ryan 1992; Doares and others 1995; Howe and others 1996; Ryan, 2000; Li and others 2003; Stenzel and others 2003). This role for systemin in wound signaling can be reconciled with the grafting studies (Li and others 2002a) if (pro)systemin acts at or near the wound site (that is, in rootstock tissues) to increase wound-induced JA to a level that is necessary for the systemic response. Support for this idea was obtained from grafting experiments conducted with 35S-Prosys transgenic plants. Initial studies showed that these plants constitutively produce a systemic signal that activates PI expression in wild-type scion leaves (Figure 1D) (McGurl and others 1994). More recent studies (Li and others 2002a) showed that recognition of the 35S-Prosys-derived signal in scion leaves is blocked by jai1 but not by mutations such as spr2 that disrupt JA biosynthesis (Figure 1D). These observations are consistent with a scenario in which 35S-Prosys constitutively activates the synthesis of JA, which is then mobilized to scion leaves, where it initiates JA signaling in target cells. Activation of PI expression in spr2 scions shows that the long-distance signal emanating from 35S-Prosys rootstocks cannot be systemin, but rather must be a signal that activates PI expression in the absence of de novo JA synthesis in scion leaves.
A role for systemin in the wound-induced localized production of JA in tomato leaves is also in agreement with results obtained from the analysis of the spr1 mutant that is defective in systemin action (Howe and Ryan 1999; Lee and Howe 2003). spr1 plants express PI genes in response to polysaccharide (OGA and chitosan) and octadecanoid (linolenic acid and JA) elicitors, but are insensitive to applied systemin and prosystemin. This phenotype indicates that SPR1 is involved in a signaling step that couples systemin perception to activation of the octadecanoid pathway. Accordingly, spr1 plants provide a useful tool for understanding the role of systemin in the systemic wound response. An important feature of spr1 is that it abrogates wound-induced systemic PI expression much more than local PI expression (Lee and Howe 2003). This phenotype is similar to that of prosystemin antisense plants (Orozco-Cárdenas and others 1993), and supports the idea that systemin functions primarily in the systemic pathway. The spr1-1 mutation abolishes JA accumulation in response to exogenous systemin. In contrast, the mutation only partially reduces wound-induced JA accumulation (Lee and Howe 2003). These observations indicate that the wound-induced JA burst in tomato leaves involves primary signals in addition to systemin, and that the systemin-mediated component of the JA burst plays a role in promoting the systemic response. Consistent with this interpretation, grafting experiments showed that SPR1 function (that is, systemin perception) is involved mainly in the generation of the systemic signal in damaged leaves rather than recognition of this signal in distal undamaged leaves (Figure 1E).
A role for systemin in amplifying the wound-induced JA burst is analogous to that of other signaling molecules that stimulate JA synthesis at the site of wounding. This would include not only plant-derived signals such as OGAs, but also insect-derived fatty acid-amino acid conjugates (FACs) that trigger systemic defense responses by eliciting local JA production (Halitschke and others 2001; Truitt and Paré 2004). Thus, it can be proposed that plants use a diversity of mechanisms to activate the jasmonate pathway and, ultimately, the expression of downstream defensive processes (Figure 3). The ability of green leafy volatiles (GLVs), which are produced in response to wounding or herbivory, to stimulate JA synthesis in maize may represent another mechanism to amplify the wound-induced jasmonate cascade (Engelberth and others 2004).
Multiple wound-induced signals regulate the jasmonate pathway for plant defense. Insect (FACs) and plant (all others)-derived signals produced in response to wounding regulate JA synthesis or subsequent JA signaling events in either a positive (+) or negative (-) manner (thick arrows). Depending on the plant species, some signals (for example, ethylene) may exert opposing effects (+/−) on the JA pathway. Thin arrows denote evidence indicating that JA can modulate the production or action of the various wound signals. No attempt was made to distinguish primary wound signals from others signals whose effects on the JA pathway may be more indirect. All known JA-mediated defense responses require COI1. ABA, abscisic acid; NO, nitric oxide; FACs, insect-derived fatty acid-amino acid conjugates; OGAs, oligogalacturonides.
The diversity of signals capable of regulating wound-induced JA accumulation suggests that different species may use different mechanisms to control JA-dependent wound responses. This hypothesis is consistent with studies indicating that some wound signals (for example, OGAs) perform different roles in tomato and Arabidopsis (Rojo and others 2003). Similarly, because prosystemin-encoding genes have been identified only in certain solanaceous species (Constabel and others 1998; Ryan and Pearce 2003), systemin may represent a species-specific adaptation to enhance wound-induced systemic defense responses. Adding to this complexity is an increasing number of other wound-induced signals that have been shown to interact either positively or negatively with the JA pathway, including ABA, ethylene, nitric oxide, GLVs, and hydrogen peroxide (Figure 3) (Walling 2000; Ryan 2000; León and others 2001; Engelberth and others 2004; Wendehenne and others 2004). Salicylic acid (SA), which typically accumulates in response to pathogen attack but not wounding, also modulates the JA signaling pathway in important ways (Kunkel and Brooks 2002). Thus, it is becoming increasing clear that the JA pathway for induced defense is part of a larger regulatory circuit that is activated in response to tissue damage (Figure 3). It is important to emphasize that interaction as between the JA pathway and other wound-induced signals are likely to be highly dependent on the quality and quantity of wounding.
CELL-TYPE-SPECIFIC LOCATION OF JA BIOSYNTHESIS
Important insight into the potential role of JAs in intercellular wound signaling has come from the realization that JA biosynthesis occurs in cells of the vascular bundle (Ryan 2000; Stenzel and others 2003). One of the first indications of this spatial pattern of JA production came from the observation that an AOS promoter-GUS fusion reporter gene is expressed in major veins of petioles and wounded leaves of Arabidopsis (Kubigsteltig and others 1999). Subsequent detailed characterization of the temporal and spatial expression pattern of allene oxide cyclase (AOC) has confirmed and extended these results. Immunocytochemical analysis, for example, showed that AOC is exclusively located in vascular bundles of petioles, petiolules, and the midrib of leaves of tomato (Hause and others 2000). More recent studies have demonstrated that AOC accumulates in companion cell and sieve elements (SE) of the vascular bundle (Hause and others 2003). Lipoxygenase (LOX) and AOS, which precede AOC in the octadecanoid pathway, were also detected in SEs. A recent proteomics study identified LOX as a constituent of curcurbit isolated vascular bundles (Walz and others 2004). These localization studies are supported by the occurrence of JA in isolated vascular bundles from Plantago major (Hause and others 2003) and by the preferential accumulation of JA and OPDA in the midrib of tomato leaves (Stenzel and others 2003). Of additional relevance to the hypothesis that JAs acts as phloem-borne signals are several studies showing that the plant vascular architecture plays an important role in regulating the intensity of the systemic wound response (Davis and others 1991; Orians and others 2000; Schittko and Baldwin 2003).
Localization of JA biosynthetic enzymes in the CC-SE complex is noteworthy in light of work showing that the Prosys gene of tomato is expressed in vascular bundles of minor and midrib veins of leaves, petiolules, petioles, and stems (Jacinto and others 1997). The spatial co-localization of JA biosynthetic enzymes and prosystemin to vascular bundles is consistent with their role in the production of the systemic wound signal (Figure 4) (Ryan 2000; Li and others 2002a; Stenzel and others 2003). Recent immunocytochemical and in situ hybridization studies showed that prosystemin is specifically localized to the vascular phloem parenchyma cells and that expression in these tissues increases in response to wounding and jasmonate treatment (Narváez-Vásquez and Ryan 2004). The expression of JA biosynthetic enzymes and prosystemin in companion and phloem parenchyma cells, respectively, suggests that activation of JA biosynthesis by systemin involves systemin-mediated signaling between these two cell types (Figure 4). If this is the case, one would predict that the systemin receptor (SR160/tBRI1) is expressed in companion cells. The highly restricted spatial expression pattern of prosystemin in phloem tissue is also interesting in light of the fact that SR160/tBRI1 is a receptor for both systemin and BRs (Wang and He 2004). Because BRs do not undergo long-distance transport (Symons and Reid 2004), it is possible that SR160/tBRI1 mediates systemin and BR signaling in different cell types, thus precluding competition of the two ligands for the same receptor under physiological conditions. This scenario is consistent with the notion that the BR receptor evolved initially and then was later recruited by plants in the solanaceous family to facilitate systemic wound signaling (Narváez-Vásquez and Ryan 2002).
Schematic diagram depicting the location of prosystemin and JA biosynthetic enzymes in vascular bundles of tomato leaves. JA produced in the companion cell-sieve element complex may be transported long distances in the phloem. Hatched arrows indicate points of potential positive feedback by JA on systemin synthesis and action (see text for details). ODP, octadecanoid pathway enzymes (LOX, AOS, AOC) for JA biosynthesis; OPDA, 12-oxo-phytodienoic acid precursor of JA.
It has been proposed that systemin and JA comprise components of a positive feedback loop that functions to promote the long-distance wound response (Li and others 2002a; Ryan and Moura 2002; Lee and Howe 2003; Stenzel and others 2003; Stratmann 2003). On one side of this loop is the ability of systemin to activate JA biosynthesis and upregulate the expression of genes encoding JA biosynthetic enzymes. On the other side of the feedback loop is evidence indicating that JA positively regulates systemin action by increasing the expression of Prosys and the abundance of the systemin receptor (Jacinto and others 1997; Scheer and Ryan 1999). Several observations indicate that this feedback circuit is likely not essential for the production or propagation of the systemic signal, but rather is involved in amplifying systemic signaling in response to sustained wounding (that is, herbivory). For example, the relatively slow timing of JA-induced Prosys expression and systemin-binding activity is not consistent with a causal role for these events in propagation of the systemic signal (McGurl and others 1992; Scheer and Ryan 1999). Moreover, grafting experiments show that jai1 plants, which are defective in all known JA-mediated responses, including JA-induced expression of Prosys, are capable of producing a graft-transmissible signal for PI expression (Li and others 2002; Li and others 2004).
ROLE OF JA METABOLISM IN WOUND SIGNALING
Newly synthesized JA is subject to a variety of enzymatic transformations that profoundly affect the range of signaling activities of the molecule. The balance between de novo formation of JA and its further metabolism is likely to play a critical role in JA-dependent wound signaling. It is well established that JA and several of its derivatives, including MeJA and JA-amino acid conjugates, are biologically active when applied to plant tissues (Wasternack and Hause 2002). However, with the growing realization that exogenous JAs are readily metabolized in plant tissues (for example, Swiatek and others 2004), it is unclear whether the exogenous compounds interact directly with the jasmonate perception apparatus or whether they are first metabolized to a bioactive form that initiates JA signaling. A significant advance in our understanding of this question has come from the identification of genes encoding various JA-metabolizing enzymes. Characterization of the Arabidopsis jar1 mutant that is defective in some JA-mediated processes led to the discovery that JAR1 encodes an ATP-dependent adenylate-forming enzyme that catalyzes conjugation of JA to Ile (Staswick and others 2002; Staswick and Tiryaki 2004). The JA-insensitive phenotype of jar1 plants thus indicates that JA-Ile is necessary for at least some JA-signaled responses. It will be interesting to determine whether the JAR1-mediated conjugation step plays a role in the perception, transport, or further metabolism of the JA signal.
JA methyl transferase (JMT), which converts JA to MeJA, also may play an important role in JA-dependent wound signaling (Seo and others 2001). Overexpression of JMT in Arabidopsis resulted in increased levels of MeJA, constitutive expression of JA-responsive genes, and enhanced resistance to a fungal pathogen. Based on these results, it was proposed that MeJA functions as an intercellular signal transducer for defense responses (Seo and others 2001). Characterization of loss-of-function JMT mutants is needed to substantiate this idea. To the contrary, wound-induced increases in the systemic pool of MeJA in N. attenuata do not result from translocation of MeJA from the wound site (von Dahl and Baldwin 2004). This study also reported that the wound-induced JA burst is not associated with the release of significant quantities of volatile MeJA into the headspace. This finding supports the earlier conclusion that systemic PI expression in tomato is mediated by a signal traveling within the plant rather than MeJA diffusing through the atmosphere (Farmer and others 1992). Although some plants, such as Artemisia, constitutively release large quantities of volatile MeJA (Farmer and Ryan 1990), there is little direct evidence that this release constitutes a mechanism for activating systemic defense responses.
JA-dependent wound signaling may be influenced by other metabolic transformations of jasmonates, including de-esterification of MeJA to JA. A tomato cDNA encoding an esterase capable of catalyzing this reaction was recently identified (Stuhlfelder and others 2004). This MeJA esterase (MJE) belongs to the α/β fold hydrolase superfamily of proteins that includes the SA-binding protein-2, which may function as a SA receptor (Kumar and Klessig 2003). Given the existence of several highly related esterases in this family, it will be important to determine the in vivo specificity of MJE for MeJA. It is interesting to note that JAR1 accepts JA but not MeJA as a substrate (Staswick and others 2002). Thus, MeJA-induced responses mediated by JAR1 appear to involve initial hydrolysis of MeJA to JA, followed by formation of JA-Ile conjugates (Staswick and Tiryaki 2004). Genetic manipulation of the relative abundance and spatial distribution of MeJA, JA, and JA-Ile should provide additional insight into the role of JA metabolism in wound signaling.
JA-INDEPENDENT WOUND SIGNALING
In response to tissue damage, plant cells activate the expression of different classes of wound-response genes that differ in their amplitude, time course (that is, onset and duration), and spatial pattern of expression (Titarenko and others 1997; Ryan 2000; Howe and others 2000; Schittko and others 2001; Lee and Howe 2003). Studies with Arabidopsis and tomato have shown that this phenomenon reflects, at least in part, the existence of multiple wound response-pathways that differ in their requirement for JA. Two criteria are useful for assessing the role of JA in wound-induced gene expression. The first is whether exogenous JA differentially regulates the gene’s expression in the absence of wounding. The second and more stringent test is whether wound-induced expression of the gene is disrupted in mutants defective in JA biosynthesis or JA perception. The strict dependence of JA-mediated changes in gene expression on COI1 makes studies in the Arabidopsis coI1 and tomato jai1 mutants ideal for this purpose (Titarenko and others 1997; Reymond and others 2000; Li and others 2004). Initial insight into distinct JA-dependent and -independent signaling pathways came from studies of wound-induced gene expression in various mutants of Arabidopis (Titarenko and others 1997; McConn and others 1997; Nishiuchi and others 1997). Based on these and more recent studies in tomato (see below), three general classes of genes that differ in their requirement for JA can be defined: i) genes whose wound-induced expression is completely dependent on JA; ii) genes whose wound-induced expression occurs independently of JA; and iii) genes whose wound-induced expression is mediated by a combination of JA-dependent and JA-independent signaling pathways.
Some attempts have been made to correlate the JA responsiveness of certain genes with their wound-induced temporal and spatial pattern of expression. Titarenko and others (1997) reported an association between the JA responsiveness and wound-induced systemic expression of genes in Arabidopsis. Subsequent studies, however, indicated that JA promotes both local and systemic responses, and that local responses are suppressed by OGA-mediated ethylene production (León and others 1998; Rojo and others 1999a). Identification of an increasing number of genes exhibiting wound-induced systemic expression in the absence of JA clearly demonstrates the existence of long-distance signaling pathways that operate independently of JA (O’Donnell and others 1998; LeBrasseur and others 2002; Yamada and others 2004; Chang and others 2004; Hiraga and others 2000).
A role for JA in the regulation of specific classes of wound-response genes has also emerged from studies in tomato. In this system, at least three classes of genes that differ with respect to their timing of wound-induced expression and regulation by systemin and JA have been described (Ryan 2000; Howe and others 2000; Strassner and others 2002; Lee and Howe 2003). Transcripts of so-called late response genes, which encode PIs and other defense-related proteins, begin to accumulate locally and systemically about 2 hours after wounding and reach maximum levels 8 to 12 h after wounding. Genes exhibiting more rapid and transient expression comprise a second class of early wound-response genes. This group of genes encodes components of the JA-mediated wound-response pathway, including prosystemin and JA biosynthetic enzymes, and exhibits stronger local expression than systemic expression in response to wounding (Strassner and others 2002). Comparative analysis of gene expression in wild-type and jai1 plants indicates that both the basal and wound-induced expression of late genes is strictly dependent on the JA cascade (Li and others 2002a; Li and others 2004; L. Li and G. Howe, unpublished results). In contrast, the basal expression of early genes, together with a significant portion of their wound-induced expression, is independent of JA (Howe and others 2000; Li and others 2004; L. Li and G. Howe, unpublished results). A third class of wound-response genes in tomato is regulated independently of JA and systemin. Whereas some of these are expressed much stronger locally than systemically (O’Donnell and others 1998; Gross and others 2004), others such as a wound-inducible MAPK (WIPK) show robust expression both locally and systemically (Lee and Howe, 2003). The relationship between JA-dependent and independent wound-signaling pathways in tomato is depicted in Figure 2. This model is generally consistent with the description of JA-dependent and-independent wound responses in Arabidopsis, and thus suggests that the existence of multiple wound-signaling pathways is a common feature of higher plants.
A wealth of genetic evidence demonstrates that JA-dependent wound responses play an essential role in plant protection against herbivores and some microbial pathogens (Howe and others 1996; McConn and others 1997; Vijayan and others 1998; Rojo and others 1999b; Kessler and Baldwin 2002; Thaler and others 2004). The physiological significance of JA-independent wound responses, however, remains to be established. Some clues have started to emerge from studies of various JA-independent wound-responsive genes. One such wound-responsive peroxidase-encoding gene, for example, was suggested to play a role in wound healing of vascular tissues in tobacco (Sasaki and others 2002). Work in Arabidopsis indicates that interactions between JA-dependent and -independent signaling pathways may optimize the temporal and spatial expression of distinct sets of wound-response genes (Rojo and others 1999a; León and others 1998; León and others 2001). In tomato, JA-independent basal expression of early genes that encode JA biosynthetic enzymes may provide a mechanism to ensure that unstressed (for exmple, herbivore-free) plants retain the capacity to generate an effective JA burst in response to sudden herbivore attack (Li and others 2004). This hypothesis is consistent with studies showing that some early gene products are constitutively expressed to low levels in unwounded tomato leaves (Jacinto and others 1997; Stenzel and others 2003), and that wound-induced JA synthesis does not depend on induced expression of JA biosynthetic genes (Miersch and Wasternack 2000; Ziegler and others 2001). Wound-induced JA-independent expression of early genes could also represent a priming mechanism that allows plants to amplify JA-mediated defense responses in the face of prolonged insect attack. This idea is consistent with studies showing that a prior wounding event stimulates PI expression in response to a secondary wound inflicted several hours after the initial wound (Graham and others 1986).
In contrast to our rapidly expanding knowledge of JA-dependent wound signaling, very little is known about the signal transduction pathways controlling JA-independent wound responses. The rapid (for example, within minutes) and systemic nature of many JA-independent responses is consistent with the involvement of a rapidly propagated hydraulic or electrical signal that is generated in response to certain types of tissue damage (Malone 1996; Herde and others 1999). For example, wound-induced systemic activation of mitogen-activated protein kinase (MAPK) activity, and increased accumulation of the corresponding MAPK mRNA, occurs within minutes of wounding (Seo and others 1995). Steam girdling experiments showed that rapid activation of MAPK activity is mediated by signals propagated through the xylem, which is consistent with a rapidly propagated physical signal (Stratmann and Ryan 1997). Studies in tobacco provide evidence that wound-induced activation of MAPK signaling plays a role in regulating JA biosynthesis (Seo and others 1995; Seo and others 1999). These and other recent studies in rice (Rakwal and Agrawal 2003) suggest the existence of regulatory interactions between JA-dependent and -independent wound-signaling pathways.
CONCLUSIONS AND FUTURE DIRECTIONS
Significant recent advances in our understanding of wound signaling in plants have come from the identification and characterization of genes that are required for wound-induced defense responses, and the demonstration that JAs perform a crucial role in both systemic wound signaling and induced resistance to herbivores. Despite these developments, several gaps in our understanding of the role of JAs in wound signaling still exist. For example, virtually nothing is known about the nature of the early signaling events that couple tissue damage to the production of primary wound signals, such as systemin and OGAs, that act at the level of the plasma membrane. Recent studies (Ellis and others 2002; Vorwerk and others 2004) provide tantalizing genetic evidence that the cell wall may be a rich source of signaling molecules that trigger activation of defense signaling pathways, including the jasmonate pathway. Efforts to determine how genetic alterations in cell wall architecture and composition affect wound-induced defense signaling may address this question. In addition to studies of plant-derived wound signals, it will be important to advance our understanding of how insect-derived elicitors modulate the JA cascade upon their introduction into wound sites (Kessler and Baldwin 2002; Korth 2003). Advances in this direction may come from the identification and characterization of plant proteins that interact with insect elicitors (Truitt and others 2004). Given the identification of the systemin receptor as a receptor kinase (Scheer and Ryan 2002), it is conceivable that other members of this large family of cell surface receptors are involved in the recognition of insect-derived signals. Identification of receptors that transduce the wound signal across the plasma membrane may provide a starting point for investigating intracellular signaling processes that presumably converge on the plastid where JA biosynthesis is initiated. Cloning of genes defined by existing JA-signaling mutants (Berger 2002; Weber 2002) will undoubtedly yield new insights into the molecular basis of wound signaling, as will the isolation of new mutants from novel genetic screens. The availability of robust markers (for example, see LeBrasseur and others 2002) for JA-independent wound responses should facilitate genetic screens designed to elucidate the mechanisms and physiological function of these wound- response pathways.
Increasing evidence supports the hypothesis that JAs function as long-distance signals for plant defense responses. Further development of this idea will require a better understanding of the cellular mechanisms involved in the transport and perception of JAs. Detailed analysis of phloem exudates may be useful for identifying specific oxylipins that are mobilized in the long-distance transport pathway in response to wounding or other biotic stress. The recent discovery of a putative lipid transfer protein involved in the production of the systemic signal for SAR (Maldonado and others 2002) provides additional rationale for investigating the lipid content of phloem exudates. Finally, it is becoming increasing apparent that various local and systemic outputs of the wound-induced JA pathway are influenced by a plethora of other signaling molecules, many of which are produced together with JA in response to wounding (Figure 3). Additional work is needed to clarify the molecular mechanisms by which these multiple signaling pathways interact with the JA pathway, and the role that these interactions play in conferring phenotypic plasticity to plants in a changing environment.
References
S Aldington GJ McDougall SC Fry (1991) ArticleTitleStructure-activity relationships of biologically active oligosaccharides Plant Cell Environ 14 625–636 Occurrence Handle1:CAS:528:DyaK3MXmslemsbY%3D
S Berger (2002) ArticleTitleJasmonate-related mutants of Arabidopsis as tools for studying stress signaling Planta 214 497–504 Occurrence Handle1:CAS:528:DC%2BD38XhtFWit7k%3D Occurrence Handle11925032
DR Bergey GA Howe CA Ryan (1996) ArticleTitlePolypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals Proc Natl Acad Sci USA 93 12053–12058 Occurrence Handle10.1073/pnas.93.22.12053 Occurrence Handle1:CAS:528:DyaK28Xms1Cqt7Y%3D Occurrence Handle8901530
DR Bergey ML Orozco-Cárdenas DS Moura Particlede CA Ryan (1999) ArticleTitleA wound- and systemin-inducible polygalacturonase in tomato leaves Proc Natl Acad Sci USA 96 1756–1760 Occurrence Handle1:CAS:528:DyaK1MXhsFSqtrw%3D Occurrence Handle9990097
GF Birkenmeirer CA Ryan (1998) ArticleTitleWound signaling in tomato plants. Evidence that aba is not a primary signal for defense gene activation Plant Physiol 117 687–693 Occurrence Handle9625722
E Carrera S Prat (1998) ArticleTitleExpression of the Arabidopsis abil-1 mutant allele inhibits proteinase inhibitor wound-induction in tomato Plant J 15 765–771 Occurrence Handle1:CAS:528:DyaK1cXntFCisL4%3D Occurrence Handle9807815
CC Chang L Ball MJ Fryer NR Baker S Karpinski PM Mullineaux (2004) ArticleTitleInduction of ASCORBATE PEROXIDASE 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated-with changes in photosynthesis Plant J 38 499–511 Occurrence Handle1:CAS:528:DC%2BD2cXksleqtLY%3D Occurrence Handle15086807
CP Constabel L Yip CA Ryan (1998) ArticleTitleProsystemin from potato, black nightshade, and bell pepper: primary structure and biological activity of predicted systemin polypeptides Plant Mol Biol 36 55–62 Occurrence Handle1:CAS:528:DyaK1cXht1aktrc%3D Occurrence Handle9484462
JM Davis MP Gordon BA Smit (1991) ArticleTitleAssimilate movement dictates remote sites of wound-induced gene expression in poplar leaves Proc Natl Acad Sci USA 88 2393–2396 Occurrence Handle1:CAS:528:DyaK3MXhvVagtL0%3D Occurrence Handle11607168
A Devoto M Nieto-Rostro D Xie C Ellis R Harmston E Patrick J Davis L Sherratt M Coleman JG Turner (2002) ArticleTitleCOI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis Plant J 32 457–466 Occurrence Handle1:CAS:528:DC%2BD38Xps1Krurs%3D Occurrence Handle12445118
SH Doares T Syrovets EW Weiler CA Ryan (1995) ArticleTitleOligogalacturonides and chitosan activate plant defense genes through the octadecanoid pathway Proc Natl Acad Sci USA 92 4095–4098 Occurrence Handle1:CAS:528:DyaK2MXls1altro%3D Occurrence Handle11607534
C Ellis I Karafyllidis C Wasternack JG Turner (2002) ArticleTitleThe Arabidopsis mutant cevl links cell wall signaling to jasmonate and ethylene responses Plant Cell 14 1557–1566 Occurrence Handle1:CAS:528:DC%2BD38XlvV2rsbo%3D Occurrence Handle12119374
J Engelberth HT Alborn EA Schmelz JH Tumlinson (2004) ArticleTitleAirborne signals prime plants against insect herbivore attack Proc Natl Acad Sci USA 101 1781–1785
EE Farmer CA Ryan (1990) ArticleTitleInterplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves Proc Natl Acad Sci USA 87 7713–7718 Occurrence Handle1:CAS:528:DyaK3MXjs1Sm Occurrence Handle11607107
EE Farmer CA Ryan (1992) ArticleTitleOctadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors Plant Cell 4 129–134 Occurrence Handle1:CAS:528:DyaK38Xkt1ensrs%3D Occurrence Handle12297644
EE Farmer RR Johnson CA Ryan (1992) ArticleTitleRegulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid Plant Physiol 98 995–1002 Occurrence Handle1:CAS:528:DyaK38Xitlajtbw%3D
EE Farmer E Almeras V Krishnamurthy (2003) ArticleTitleJasmonates and related oxylipins in plant responses to pathogenesis and herbivory Curr Opin Plant Biol 6 372–378 Occurrence Handle10.1016/S1369-5266(03)00045-1 Occurrence Handle1:CAS:528:DC%2BD3sXlsValtbo%3D Occurrence Handle12873533
JA Gatehouse (2002) ArticleTitlePlant resistance towards insect herbivores: a dynamic interaction New Phytol 156 145–169 Occurrence Handle1:CAS:528:DC%2BD38XovFGru74%3D
JS Graham G Hall G Pearce CA Ryan (1986) ArticleTitleRegulation of synthesis of proteinase inhibitors I and II mRNAs in leaves of wounded tomato plants Planta 169 399–405 Occurrence Handle1:CAS:528:DyaL2sXhsleju7w%3D
TR Green CA Ryan (1972) ArticleTitleWound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects Science 175 776–777 Occurrence Handle1:CAS:528:DyaE38XhtFSnsrc%3D
N Gross C Wasternack M Köck (2004) ArticleTitleWound-induced RNaseLE expression is jasmonate and systemin independent and occurs only locally in tomato (Lycopersicon esculentum cv Lukullus). Phytochemistry 65 1343–1350 Occurrence Handle1:CAS:528:DC%2BD2cXlsVaku7o%3D Occurrence Handle15231407
R Halitschke U Schittko G Pohnert W Boland IT Baldwin (2001) ArticleTitleMolecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses Plant Physiol 125 711–717 Occurrence Handle1:CAS:528:DC%2BD3MXhs1KlsLo%3D Occurrence Handle11161028
V Hawkes J Turner (2004) ArticleTitleHow are signalling pathways involving jasmonate and calcium linked to the wound response in Arabidopsis International Conference on Arabidopsis Research . .
B Hause I Stenzel O Miersch Maucher-H R Kramell J Ziegler C Wasternack (2000) ArticleTitleTissue-specific oxylipin signature of tomato flowers: allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles Plant J 24 113–126 Occurrence Handle1:CAS:528:DC%2BD3cXnvVeqsrc%3D Occurrence Handle11029709
B Hause G Hause C Kutter O Miersch C Wasternack (2003) ArticleTitleEnzymes of jasmonate biosynthesis occur in tomato sieve elements Plant Cell Physiol 44 643–648 Occurrence Handle1:CAS:528:DC%2BD3sXkvFSjsb4%3D Occurrence Handle12826630
O Herde R Atzorn J Fisahn C Wasternack L Willmitzer H Peña-Cortés (1996) ArticleTitleLocalized wounding by heat initiates the accumulation of proteinase inhibitor II in abscisic acid-deficient plants by triggering jasmonic acid biosynthesis Plant Physiol 112 853–860 Occurrence Handle1:CAS:528:DyaK28XmsFKku7Y%3D Occurrence Handle12226423
O Herde HP Cortes C Wasternack L Willmitzer Fisahn (1999) ArticleTitleElectric signaling and Pin2 gene expression on different abiotic stimuli depend on a distinct threshold level of endogenous abscisic acid in several abscisic acid-deficient tomato mutants Plant Physiol 119 213–218 Occurrence Handle1:CAS:528:DyaK1MXmt1Gktw%3D%3D Occurrence Handle9880363
T Hildmann M Ebneth H Peña-Cortés JJ Sanchez-Serrano L Willmitzer S Prat (1992) ArticleTitleGeneral roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding Plant Cell 4 1157–1170 Occurrence Handle10.1105/tpc.4.9.1157 Occurrence Handle1:CAS:528:DyaK3sXks1yisbc%3D Occurrence Handle1392612
S Hiraga H Ito K Sasaki H Yamakawa (2000) ArticleTitleWound-induced expression of a tobacco peroxidase is not enhanced by ethephon and suppressed by methyl jasmonate and coronatine Plant Cell Physiol 41 165–170 Occurrence Handle1:CAS:528:DC%2BD3cXhtlyhs7s%3D Occurrence Handle10795310
GA Howe CA Ryan (1999) ArticleTitleSuppressors of systemin signaling identify genes in the tomato wound response pathway Genetics 153 1411–1421 Occurrence Handle1:CAS:528:DyaK1MXnslSjtr8%3D Occurrence Handle10545469
GA Howe J Lightner J Browse CA Ryan (1996) ArticleTitleAn octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack Plant Cell 8 2067–2077 Occurrence Handle10.1105/tpc.8.11.2067 Occurrence Handle1:CAS:528:DyaK28XntlSiurY%3D Occurrence Handle8953771
GA Howe GI Lee A Itoh L Li A DeRocher (2000) ArticleTitleCytochrome P450-dependent metabolism of oxylipins: cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase from tomato Plant Physiol 123 711–724 Occurrence Handle1:CAS:528:DC%2BD3cXktlehtr0%3D Occurrence Handle10859201
JW Hudgins VR Franceschi (2004) ArticleTitleMethyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambialzone, for traumatic resin duct formation Plant Physiol 135 2134–2149 Occurrence Handle1:CAS:528:DC%2BD2cXnt1Ggs7g%3D Occurrence Handle15299142
T Jacinto B McGurl V Franceschi J Delano-Freier CA Ryan (1997) ArticleTitleTomato prosystemin promoter confers wound-inducible, vascular bundle-specific expression of the β-glucuronidase gene in transgenic tomato plants Planta 203 406–412 Occurrence Handle1:CAS:528:DyaK2sXnvVCgtLk%3D
R Karban IT. Baldwin (1997) Induced response to herbivory Chicago University Press Chicago
GG Kennedy (2002) ArticleTitleTomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annu Rev Entomol 48 51–72
A Kessler IT Baldwin (2002) ArticleTitlePlant responses to insect herbivory: the emerging molecular analysis Annu Rev Plant Biol 53 299–328 Occurrence Handle1:CAS:528:DC%2BD38XlsVWhur8%3D Occurrence Handle12221978
A Kessler R Halitschke IT Baldwin (2004) ArticleTitleSilencing the jasmonate cascade: Induced plant defenses and insect populations Science 305 665–668 Occurrence Handle1:CAS:528:DC%2BD2cXmtFWqsrw%3D Occurrence Handle15232071
KL Korth (2003) ArticleTitleProfiling the response of plants to herbivorous insects Genome Biol 4 221 Occurrence Handle12844352
I Kubigsteltig D Laudert EW Weiler (1999) ArticleTitleStructure and regulation of the Arabidopsis thaliana allene oxide synthase gene Planta 208 463–471 Occurrence Handle1:CAS:528:DyaK1MXktlSrtrw%3D Occurrence Handle10420644
D Kumar DF Klessig (2003) ArticleTitleHigh-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity Proc Natl Acad Sci USA 100 16101–16106 Occurrence Handle1:CAS:528:DC%2BD2cXhtVGjug%3D%3D Occurrence Handle14673096
BN Kunkel DM Brooks (2002) ArticleTitleCross talk between signaling pathways in pathogen defense Curr Opin Plant Biol 5 325–331 Occurrence Handle10.1016/S1369-5266(02)00275-3 Occurrence Handle1:CAS:528:DC%2BD38Xls1ejtLs%3D Occurrence Handle12179966
P Landgraf I Feussner A Hunger D Scheel S Rosahl (2002) ArticleTitleSystemic accumulation of 12-oxo-phytodienoic acid in SAR-induced potato plants Eurep J Plant Pathol 108 279–283 Occurrence Handle1:CAS:528:DC%2BD38XksFWqsr8%3D
ND LeBrasseur GC Macintosh MA Perez-Amador M Saitoh PJ Green (2002) ArticleTitleLocal and systemic wound-induction of RNase and nuclease activities in Arabidopsis: RNS1 as a marker for a JA-independent systemic signaling pathway Plant J 29 393–403 Occurrence Handle1:CAS:528:DC%2BD38XisVagsro%3D Occurrence Handle11846873
GI Lee GA Howe (2003) ArticleTitleThe tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression Plant J 33 567–576 Occurrence Handle1:CAS:528:DC%2BD3sXhslOgtL0%3D Occurrence Handle12581314
J León E Rojo E Titarenko JJ Sánchez-Serrano (1998) ArticleTitleJasmonic acid-dependent and independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana Mol Gen Genet 258 412–419 Occurrence Handle9648747
J León E Rojo JJ Sánchez-Serrano (2001) ArticleTitleWound signaling in plants J Exp Bot 52 1–9 Occurrence Handle10.1093/jexbot/52.354.1 Occurrence Handle1:CAS:528:DC%2BD3MXhvVOitr4%3D
L Li C Li GA Howe (2001) ArticleTitleGenetic analysis of wound signaling in tomato. Evidence for a dual role of jasmonic acid in defense and female fertility Plant Physiol 127 1414–1417 Occurrence Handle1:CAS:528:DC%2BD38XjtVWhtA%3D%3D Occurrence Handle11743083
L Li C Li GI Lee GA Howe (2002a) ArticleTitleDistinct roles for jasmonic acid synthesis and action in the systemic wound response of tomato Proc Natl Acad Sci USA 99 6416–6421 Occurrence Handle1:CAS:528:DC%2BD38XjslWnt7Y%3D
C Li MM Williams Y-T Loh GI Lee GA Howe (2002b) ArticleTitleResistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway Plant Physiol 130 494–503 Occurrence Handle1:CAS:528:DC%2BD38XntFOrtLY%3D
C Li G Liu C Xu GI Lee P Bauer HQ Ling MW Ganal GA Howe (2003) ArticleTitleThe tomato Suppressor of prosystemin-mediated responses2 (Spr2) gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal Plant Cell 15 1646–1661 Occurrence Handle1:CAS:528:DC%2BD3sXlslWktbY%3D Occurrence Handle12837953
L Li Y Zhao BC McCaig BA Wingerd J Wang ME Whalon E Pichersky GA Howe (2004) ArticleTitleThe tomato homolog of COI1 is required for maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development Plant Cell 16 126–143 Occurrence Handle1:CAS:528:DC%2BD2cXosVeltg%3D%3D Occurrence Handle14688297
J Lightner G Pearce CA Ryan J Browse (1993) ArticleTitleIsolation of signaling mutants of tomato (Lycopersicon esculentum) Mol Gen Genet 241 595–601 Occurrence Handle8264534
AM Maldonado P Doerner RA Dixon CJ Lamb RK Cameron (2002) ArticleTitleA putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis Nature 419 399–403 Occurrence Handle10.1038/nature00962 Occurrence Handle1:CAS:528:DC%2BD38XntlGgsLk%3D Occurrence Handle12353036
M Malone (1996) ArticleTitleRapid, long-distance signal transmission in higher plants Adv Bot Res 22 163–228 Occurrence Handle1:CAS:528:DyaK28XmvFSrs70%3D
DM Martin J Gershenzon J Bohlmann (2003) ArticleTitleInduction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce Plant Physiol 132 1586–1599 Occurrence Handle10.1104/pp.103.021196 Occurrence Handle1:CAS:528:DC%2BD3sXlsFGhu7o%3D Occurrence Handle12857838
M McConn RA Creelman E Bell JE Mullet J Browse (1997) ArticleTitleJasmonate is essential for insect defense in Arabidopsis Proc Natl Acad Sci USA . 94–5473–5477
B McGurl G Pearce M Orozco-Cárdenas CA Ryan (1992) ArticleTitleStructure, expression, and antisense inhibition of the systemin precursor gene Science 255 1570–1573 Occurrence Handle1:CAS:528:DyaK3sXhvVCltr4%3D Occurrence Handle1549783
B McGurl M Orozco-Cárdenas G Pearce CA Ryan (1994) ArticleTitleOverexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis Proc Natl Acad Sci USA 91 9799–9802 Occurrence Handle1:CAS:528:DyaK2cXmsFWgu7g%3D Occurrence Handle7937894
O Miersch C Wasternack (2000) ArticleTitleOctadecanoid and jasmonate signaling in tomato (Lycopersicon esculentum Mill.) leaves: endogenous jasmonates do not induce jasmonate biosynthesis Biol Chem 381 715–722 Occurrence Handle1:CAS:528:DC%2BD3cXntlGmsbc%3D Occurrence Handle11030429
J Narváez-Vásquez CA Ryan (2002) ArticleTitleThe systemin precursor gene regulates both defensive and developmental genes in Solanum tuberosum Proc Natl Acad Sci USA 99 15818–15821 Occurrence Handle12426402
J Narváez-Vásquez CA Ryan (2004) ArticleTitleThe cellular localization of prosystemin: a functional role for phloem parenchyma in systemic wound signaling Planta 218 360–369 Occurrence Handle14534786
L Navazio R Moscatiello D Bellincampi B Baldan F Meggio M Brini C Bowler P Mariani (2002) ArticleTitleThe role of calcium in oligogalacturonide-activated signalling in soybean cells Planta 215 596–605 Occurrence Handle1:CAS:528:DC%2BD38XlvVKhtbs%3D Occurrence Handle12172842
T Nishiuchi T Hamada H Kodoma K Iba (1997) ArticleTitleWounding changes the spatial expression pattern of the Arabidopsis plastid ω3 fatty acid desaturase gene (FAD7) through different signal transduction pathways Plant Cell 9 1701–1712 Occurrence Handle1:CAS:528:DyaK2sXntFahu7o%3D Occurrence Handle9368411
PJ O’Donnell C Calvert R Atzorn C Wasternack HMO Leyser DJ Bowles (1996) ArticleTitleEthylene as a signal mediating the wound response of tomato plants Science 274 1914–1917 Occurrence Handle10.1126/science.274.5294.1914 Occurrence Handle8943205
PJ O’Donnell MR Truesdale CM Calvert A Dorans MR Roberts DJ Bowles (1998) ArticleTitleA novel tomato gene that rapidly responds to wound- and pathogen-related signals Plant J 14 137–142 Occurrence Handle1:CAS:528:DyaK1cXjtV2isLg%3D Occurrence Handle15494059
CM Orians J Pomerleau R Ricco (2000) ArticleTitleVascular architecture generates fine scale variation in the systemic induction of proteinase inhibitors in tomato J Chem Ecol 26 471–485 Occurrence Handle1:CAS:528:DC%2BD3cXhsVKiu70%3D
M Orozco-Cárdenas B McGurl CA Ryan (1993) ArticleTitleExpression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae Proc Natl Acad Sci USA 90 8273–8276 Occurrence Handle11607423
M Orozco-Cárdenas J Narvaez-Vasquez CA Ryan (2001) ArticleTitleHydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate Plant Cell 13 179–191 Occurrence Handle10.1105/tpc.13.1.179 Occurrence Handle11158538
V Pautot FM Holzer LL Walling (1991) ArticleTitleDifferential expression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding Mol Plant-Microbe Interact 4 284–292 Occurrence Handle1:CAS:528:DyaK38XhvVGhtg%3D%3D Occurrence Handle1932815
H Peña-Cortés S Prat R Atzorn C Wasternack L Willmitzer (1996) ArticleTitleAbscisic acid-deficient plants do not accumulate proteinase inhibitor II following systemin treatment Planta 198 447–451
R Rakwal GK Agrawal (2003) ArticleTitleWound signaling-coordination of the octadecanoid and MAPK pathways Plant Physiol Biochem 41 855–861 Occurrence Handle1:CAS:528:DC%2BD3sXnvVOntbw%3D
P Reymond H Weber M Damond EE Farmer (2000) ArticleTitleDifferential gene expression in response to mechanical wounding and insect feeding in Arabidopsis Plant Cell 12 707–720 Occurrence Handle10.1105/tpc.12.5.707 Occurrence Handle1:CAS:528:DC%2BD3cXjvFyhurk%3D Occurrence Handle10810145
E Rojo J León JJ Sánchez-Serrano (1999a) ArticleTitleCross-talk between wound signaling pathways determines local versus systemic gene expression in Arabidopsis thaliana Plant J 20 135–142 Occurrence Handle1:CAS:528:DyaK1MXotFaitrc%3D
J Royo J Leon G Vancanneyt JP Albar S Rosahl F Ortego P Castanera JJ Sanchez-Serrano (1999b) ArticleTitleAntisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests Proc Natl Acad Sci USA 96 1146–1151 Occurrence Handle1:CAS:528:DyaK1MXpvFansg%3D%3D
E Rojo R Solano JJ Sánchez-Serrano (2003) ArticleTitleInteractions between signaling compounds involved in plant defense J Plant Growth Regul 22 82–98 Occurrence Handle1:CAS:528:DC%2BD3sXosF2kurw%3D
CA Ryan (1992) ArticleTitleThe search for the proteinase inhibitor-inducing factor, PIIF Plant Mol Biol 19 123–133 Occurrence Handle1:CAS:528:DyaK38Xks1Kmsbo%3D Occurrence Handle1600164
CA Ryan (2000) ArticleTitleThe systemin signaling pathway: differential activation of plant defensive genes Biochim Biophys Acta 1477 112–121 Occurrence Handle10.1016/S0167-4838(99)00269-1 Occurrence Handle1:CAS:528:DC%2BD3cXhsFGktLs%3D Occurrence Handle10708853
CA Ryan DS Moura (2002) ArticleTitleSystemic wound signaling in plants: a new perception Proc Natl Acad Sci USA 99 6519–6520 Occurrence Handle1:CAS:528:DC%2BD38XjvFCkt7k%3D Occurrence Handle12011413
CA Ryan G Pearce (2003) ArticleTitleSystemins: a functionally defined family of peptide signals that regulate defensive genes in Solanaceae species Proc Natl Acad Sci USA 100 14577–14580 Occurrence Handle1:CAS:528:DC%2BD3sXpsFaku7o%3D Occurrence Handle12949264
M Sagi O Davydov S Orazova Z Yesbergenova R Ophir JW Stratmann R Fluhr (2004) ArticleTitlePlant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum Plant Cell 16 616–628 Occurrence Handle1:CAS:528:DC%2BD2cXisFGkt7Y%3D Occurrence Handle14973161
K Sasaki S Hiraga H Ito S Seo H Matsui Y Ohashi (2002) ArticleTitleA wound-inducible tobacco peroxidase gene expresses preferentially in the vascular system Plant Cell Physiol 43 108–117 Occurrence Handle1:CAS:528:DC%2BD38XhtVyqt7s%3D Occurrence Handle11828028
R Schafleitner E Wilhelm (2002) ArticleTitleIsolation of wound-responsive genes from chestmut (Catanea sativa) microstems by mRNA display and their differential expression upon wounding and infection with the chestnut blight fungus (Chryphonectria parasitica) Physiol Mol Plant Pathol 61 339–348 Occurrence Handle1:CAS:528:DC%2BD3sXktlKiu7w%3D
JM Scheer CA Ryan (1999) ArticleTitleA 160-kD systemin receptor on the surface of Lycopersicon peruvianum suspension-cultured cells Plant Cell 11 1525–1536 Occurrence Handle10.1105/tpc.11.8.1525 Occurrence Handle1:CAS:528:DyaK1MXmtVWlsr4%3D Occurrence Handle10449585
JM Scheer CA Ryan (2002) ArticleTitleThe systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family Proc Natl Acad Sci USA 99 9585–9590
U Schittko D Hermsmeier IT Baldwin (2001) ArticleTitleMolecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. II Accumulation of plant mRNAs in response to insect-derived cues. Plant Physiol 125 701–710 Occurrence Handle1:CAS:528:DC%2BD3MXhs1KlsL0%3D
U Schittko IT Baldwin (2003) ArticleTitleConstraints to herbivore-induced systemic responses: bidirectional signaling along orthostichies in Nicotiana attenuata J Chem Ecol 29 763–770 Occurrence Handle1:CAS:528:DC%2BD3sXitFarsbg%3D Occurrence Handle12757332
S Seo M Okamoto H Seto K Ishizuka H Sano Y Ohashi (1995) ArticleTitleTobacco MAP kinase: a possible mediator in wound signal transduction pathways Science 270 1988–1992 Occurrence Handle1:CAS:528:DyaK28XltlSj Occurrence Handle8533090
S Seo H Sano Y Ohashi (1999) ArticleTitleJasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase Plant Cell 11 289–298 Occurrence Handle1:CAS:528:DyaK1MXhsVyntbg%3D Occurrence Handle9927645
HS Seo JT Song JJ Cheong YH Lee YW Lee I Hwang JS Lee YD Choi (2001) ArticleTitleJasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses Proc Natl Acad Sci USA 98 4788–4793 Occurrence Handle1:CAS:528:DC%2BD3MXjtVantb0%3D Occurrence Handle11287667
PE Staswick I Tiryaki (2004) ArticleTitleThe oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine Arabidopsis Plant Cell 16 2117–2127 Occurrence Handle1:CAS:528:DC%2BD2cXmvVantr8%3D Occurrence Handle15258265
PE Staswick I Tiryaki ML Rowe (2002) ArticleTitleJasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation Plant Cell 14 1405–1415 Occurrence Handle10.1105/tpc.000885 Occurrence Handle1:CAS:528:DC%2BD38Xlt1alsbo%3D Occurrence Handle12084835
I Stenzel B Hause H Maucher A Pitzschke O Miersch J Ziegler CA Ryan C Wasternack (2003) ArticleTitleAllene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato—amplification in wound signalling Plant J 33 577–589 Occurrence Handle1:CAS:528:DC%2BD3sXhslOgtLo%3D Occurrence Handle12581315
J Strassner F Schaller U Frick GA Howe EW Weiler N Amrhein P Macheroux A Schaller (2002) ArticleTitleCharacterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response Plant J 32 585–601 Occurrence Handle10.1046/j.1365-313X.2002.01449.x Occurrence Handle1:CAS:528:DC%2BD38Xps1Kru7g%3D Occurrence Handle12445129
J Stratmann (2003) ArticleTitleLong distance run in the wound response—jasmonic acid pulls ahead Trends Plant Sci 8 247–250 Occurrence Handle1:CAS:528:DC%2BD3sXksF2htbY%3D Occurrence Handle12818656
JW Stratmann CA Ryan (1997) ArticleTitleMyelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors Proc Natl Acad Sci USA 94 11085–11089 Occurrence Handle1:CAS:528:DyaK2sXmtlClsbs%3D Occurrence Handle9380763
C Stuhlfelder MJ Mueller H Warzecha (2004) ArticleTitleCloning and expression of a tomato cDNA encoding a methyl jasmonate cleaving esterase Eur J Biochem 271 2976–2983 Occurrence Handle1:CAS:528:DC%2BD2cXmtVyrtbw%3D Occurrence Handle15233793
A Swiatek W Dongen ParticleVan EL Esmans H Onckelen ParticleVan (2004) ArticleTitleMetabolic fate of jasmonates in tobacco bright yellow-2 cells Plant Physiol 135 161–172 Occurrence Handle1:CAS:528:DC%2BD2cXkt12ntbg%3D Occurrence Handle15133155
JS Thaler MA Farag PW Paré M Dicke (2002) ArticleTitleJasmonate-deficient plants have reduced direct and indirect defences against herbivores Ecol Lett 5 764–774
JS Thaler B Owen VJ Higgins (2004) ArticleTitleThe role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles Plant Physiol 135 530–538 Occurrence Handle1:CAS:528:DC%2BD2cXkt12msrc%3D Occurrence Handle15133157
E Titarenko E Rojo J León JJ Sánchez-Serrano (1997) ArticleTitleJasmonic acid-dependent and independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana Plant Physiol 11 817–826
CL Truitt PW Pare (2004) ArticleTitleIn situ translocation of volicitin by beet armyworm larvae to maize and systemic immobility of the herbivore elicitor inplanta Planta 218 999–1007 Occurrence Handle1:CAS:528:DC%2BD2cXjt1aisb0%3D Occurrence Handle14685859
CL Truitt HX Wei PW Paré (2004) ArticleTitleA plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin Plant Cell 16 523–532 Occurrence Handle1:CAS:528:DC%2BD2cXhsFKisL0%3D Occurrence Handle14729912
JG Turner C Ellis A Devoto (2002) ArticleTitleThe jasmonate signal pathway Plant Cell . S153–S164
R Van der Hoeven ParticleVan der C Ronning J Giovannoni G Martin S Tanksley (2002) ArticleTitleDeductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing Plant Cell 14 1441–1456 Occurrence Handle12119366
P Vijayan J Shockey CA Levesque RJ Cook J Browse (1998) ArticleTitleA role for jasmonate in pathogen defense of Arabidopsis Proc Natl Acad Sci USA 95 7209–7214 Occurrence Handle10.1073/pnas.95.12.7209 Occurrence Handle1:CAS:528:DyaK1cXjslymt7o%3D Occurrence Handle9618564
CC von Dahl Particlevon IT Baldwin (2004) ArticleTitleMethyl jasmonate and cis-jasmone do not dispose of the herbivore-induced jasmonate burst in Nicotiana attenuata Physiol Plant 120 474–481 Occurrence Handle1:CAS:528:DC%2BD2cXisVKhs7s%3D Occurrence Handle15032845
S Vorwerk S Somerville C Somerville (2004) ArticleTitleThe role of plant cell wall polysaccharide composition in disease resistance Trends Plant Sci 9 203–209 Occurrence Handle1:CAS:528:DC%2BD2cXivVGjsbo%3D Occurrence Handle15063871
LL Walling (2000) ArticleTitleThe myriad plant responses to herbivores J Plant Growth Regul 19 195–216 Occurrence Handle1:CAS:528:DC%2BD3cXnvF2hu7w%3D Occurrence Handle11038228
C Walz P Giavalisco M Schad M Juenger J Klose J Kehr (2004) ArticleTitleProteomics of curcurbit phloem exudate reveals a network of defence proteins Phytochemistry 65 1795–1804
C Wang CA Zien M Afitlhile R Welti DF Hildebrand X Wang (2000) ArticleTitleInvolvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis Plant Cell 12 2237–2246
ZY Wang JX He (2004) ArticleTitleBrassinosteroid signal transduction—choices of signals and receptors Trends Plant Sci 9 91–96 Occurrence Handle1:CAS:528:DC%2BD2cXhtVOrsrw%3D Occurrence Handle15102375
C Wasternack B Hause (2002) ArticleTitleJasmonates and octadecanoids: signals in plant stress responses and development Prog Nucleic Acid Res Mol Biol 72 165–221
H Weber (2002) ArticleTitleFatty acid-derived signals in plants Trends Plant Sci 7 217–224 Occurrence Handle1:CAS:528:DC%2BD38Xjtl2kt7o%3D Occurrence Handle11992827
D Wendehenne J Durner DF Klessig (2004) ArticleTitleNitric oxide: a new player in plant signaling and defence Curr Opin Plant Biol 7 449–455 Occurrence Handle1:CAS:528:DC%2BD2cXlsVWhur8%3D Occurrence Handle15231269
DX Xie BF Feys S James M Nieto-Rostro JG Turner (1998) ArticleTitleCOI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility Science 280 1091–1094 Occurrence Handle10.1126/science.280.5366.1091 Occurrence Handle1:CAS:528:DyaK1cXjt1egsbk%3D Occurrence Handle9582125
LH Xu FQ Liu E Lechner P Genschik WL Crosby H Ma W Peng DF Huang DX Xie (2002) ArticleTitleThe SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis Plant Cell 14 1919–1935 Occurrence Handle1:CAS:528:DC%2BD38XmslCgsr0%3D Occurrence Handle12172031
K Yamada M Nishimura I Hara-Nishimura (2004) ArticleTitleThe slow wound-response of γVPE is regulated by endogenous salicylic acid in Ardbidopsis Planta 218 599–605 Occurrence Handle1:CAS:528:DC%2BD2cXhtFGjt7c%3D Occurrence Handle14600834
Z-P Zhang IT Baldwin (1997) ArticleTitleTransport of [2–14C] jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203 436–441 Occurrence Handle1:CAS:528:DyaK2sXnvVCgtb8%3D
Y Zhao R Thilmony C Bender SY He GA Howe (2003) ArticleTitleThe Hrp type III secretion system and coronatine of Pseudomonas syringae pv. tomato coordinately modify host defense by targeting the jasmonate signaling pathway in tomato Plant J 36 485–499 Occurrence Handle1:CAS:528:DC%2BD2cXkvVKi Occurrence Handle14617079
J Ziegler M Keinänen IT Baldwin (2001) ArticleTitleHerbivore-induced allene oxide synthase transcripts and jasmonic acid in Nicotiana attenuata Phytochemistry 58 729–738 Occurrence Handle1:CAS:528:DC%2BD3MXnslWnsrY%3D Occurrence Handle11672737
Acknowledgements
I am grateful to Dr. Gyu In Lee for critital reading of the manuscript. Research in my laboratory is supported by grants from the National Institutes of Health (R01GM57795), the U.S. Department of Energy (DE-FG02-91ER20021), the Michigan Life Sciences Corridor (085P1000466), and the Michigan Agricultural Experiment Station at MSU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howe, G.A. Jasmonates as Signals in the Wound Response. J Plant Growth Regul 23, 223–237 (2004). https://doi.org/10.1007/s00344-004-0030-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-004-0030-6