Abstract
Phenolics have a role in defenses against herbivores, but the defensive functions of specific groups of phenolics are still poorly understood. For example, ellagitannins (a type of hydrolyzable tannin) are predicted to decrease insect herbivore performance, but the effect of different types of ellagitannins on generalist and specialist herbivores has rarely been assessed. Here, we test the effects of the dominant oligomeric ellagitannins of Oenothera biennis and other Onagraceae on herbivore performance. We fed artificial diets containing between 1 and 100 mg/g of polyphenol fractions comprised of varying amounts and compositions of dimeric oenothein B, the trimeric oenothein A and larger oligomers, to one generalist (Spodoptera exigua) and one specialist (Schinia florida) insect herbivore species. We compared the effects of these ellagitannin fractions on herbivore performance to the effects of artificial diet containing total phenolic extracts from O. biennis, which contained these ellagitannins as well as many additional phenolic metabolites including flavonoid glycosides and caffeic acid derivatives. Both the ellagitannin fractions and O. biennis phenolic extracts had strong negative effects on S. exigua and S. florida performance, with stronger effects on the generalist herbivore. Differences between the effects of the various ellagitannin fractions were small and depended on insect life stage. The defensive effects of these ellagitannins were large, with lethal concentrations as low as 0.1% of the diet. These results highlight the important defensive function of ellagitannins against specialist and generalist herbivores and the need to characterize the effects of these understudied phenolics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant secondary metabolites play a large role in defending plants against herbivores. Yet, despite the importance of many secondary metabolites in defense (Denno and McClure 1983; Fritz and Simms 1992; Johnson 2011; Rosenthal and Berenbaum 1991; Wallace 1976; Walters 2011), it is often difficult to demonstrate the defensive function of secondary metabolites. For example, a meta-analysis of 72 quantitative genetics studies failed to find a consistent effect of genetic variation in the concentration of secondary metabolites on herbivore performance (Carmona et al. 2011). This study identified important limitations of previous work examining the defensive function of secondary metabolites, such as the difficulty of establishing the effects of secondary metabolites that have diverse classes of compounds (e.g. phenolics, alkaloids, glucosinolates) with many biological functions. Similarly, the toxicity of chemical defenses may be due to additional factors that are difficult to quantify in genetically-based tests, such as synergism between compounds and compound diversity. Focusing on the effects and modes of action of a few, structurally similar compounds could yield more accurate tests of the defensive function of secondary metabolites (Carmona et al. 2011; Salminen and Karonen 2011). We take such an approach to understand the possible defensive functions of macrocyclic ellagitannin oligomers, an abundant type of hydrolyzable tannin found in many members of the Onagraceae as well as other plants species (Agrawal et al. 2012; Johnson et al. 2009).
Tannins are among the most widespread group of secondary compounds in higher plants. Tannins are broadly divided into condensed tannins (proanthocyanidins) and hydrolyzable tannins, which include both simple gallic acid derivatives, gallotannins, and ellagitannins (Niemetz and Gross 2005; Quideau and Feldman 1996; Salminen and Karonen 2011). Many of these compounds are recognized for their role in plant defenses against insect herbivores and pathogens (Harborne and Williams 2000; Salminen and Karonen 2011). The defensive function of tannins may be associated with the ability to bind proteins in the guts of herbivores, thereby limiting nutrient uptake (Feeny 1976). For insect herbivores, especially lepidopterans with alkaline guts, tannins more likely cause oxidative stress in the gut (Appel 1993; Salminen and Karonen 2011), including oxidative damage to nutrients (Felton and Summers 1995). Additionally, ellagitannins might act as anti-feedants by making plants unpalatable. Here we focus on the defensive role of ellagitannins, which are the dominant group of tannins found in many plant families (Johnson et al. 2014; Moilanen et al. 2015; Salminen et al. 2001, 2002, 2004; Yarnes et al. 2006).
Recent work has shown that ellagitannins may be the most bioactive class of tannins, although different ellagitannin compounds exhibit six-fold variation in both oxidative activity (Moilanen and Salminen 2008) and protein precipitation capacity (Karonen et al. 2015). The effects of ellagitannins on insect herbivores has been confirmed by feeding herbivores low to medium-level concentrations (15 mg/g) of tannin mixtures containing ellagitannins (pedunculagin and pentagalloyl glucose) (Barbehenn et al. 2009), and by placing high concentrations of individual ellagitannins (50 mg/g of vescalagin) in artificial diets fed to generalist and specialist herbivores (Roslin and Salminen 2008). These experiments showed the potential importance of ellagitannins in defense, but they did not compare varying levels of the major compounds present in plant chemical defense profiles.
Field experiments have shown considerable variation in the concentrations of different macrocyclic ellagitannin oligomers within and between species of the Onagraceae (Anstett et al. 2015; Baert et al. 2017; Johnson et al. 2009; Parker et al. 2012). For example, there is large heritable variation within Oenothera biennis for the concentrations of the ellagitannin compounds oenothein A (trimer of tellimagrandin I) and its precursor oenothein B (dimer of tellimagrandin I) (Anstett et al. 2015; Johnson et al. 2009; Parker et al. 2012). Larger oligomers (with up to eleven subunits) also occur within O. biennis (Karonen et al. 2010; Salminen et al. 2011) and in other species of the Onagraceae such as Epilobium angustifolium (Baert et al. 2015, 2017). The variation in the concentrations of macrocyclic oligomers has been shown to impact insect herbivore performance (Agrawal et al. 2012; Anstett et al. 2015). In a study that took advantage of natural genetic variation in specific ellagitannins, higher levels of oenothein A were negatively correlated with damage by specialist flower and fruit herbivores across 137 genotypes of O. biennis, while its precursor oenothein B was positively correlated with increased herbivory (Anstett et al. 2015). Oenothera biennis also expressed higher levels of oenothein A in response to selection by specialist seed predators (Agrawal et al. 2012). While a negative effect of oenothein A on herbivores was implicated by these studies, these results were correlative and a direct experimental confirmation of the defensive function of these ellagitannins is still needed.
Here we use a diet manipulation approach to directly test the effect of the most abundant ellagitannins in O. biennis on generalist and specialist insect herbivore species. We compare the defensive role of macrocyclic ellagitannin oligomers to the effects of the complete composition of phenolics from O. biennis, including flavonoids, caffeic acid derivatives and ellagitannins. We compare the effect of ellagitannins to total phenolic extracts by experimentally varying the concentrations of various phenolic compounds within artificial diets fed to one generalist insect herbivore species (Spodoptera exigua), and one specialist insect herbivore species (Schinia florida). Using a series of these feeding trial experiments, we ask: (1) Do macrocyclic oligomeric ellagitannins decrease the performance of generalist and specialist insect herbivores? (2) How do fractions with varying amounts of oenothein B, oenothein A, and larger oenothein oligomers, differ in their effects on herbivores? (3) How do the antiherbivore effects of these ellagitannins compare to the effects of the total mixture of phenolic compounds present in O. biennis? Our results provide direct experimental evidence that macrocyclic ellagitannin oligomers have potent activity against generalist and specialist insect herbivores of O. biennis.
Methods and Materials
Study System
Oenothera biennis L. (common evening primrose, Onagreaceae) is a biennial herbaceous plant that grows in open habitats throughout eastern North America. This species is consumed by a diverse community of insect herbivores (Johnson and Agrawal 2005, 2007), and contains a diversity of defensive chemicals including a high concentration of ellagitannins (>5% dry weight) (Agrawal et al. 2012; Anstett et al. 2015; Johnson et al. 2009). Oenothera biennis also exhibits a genetically based trade-off between its two most abundant ellagitannins, oenothein B and oenothein A (Anstett et al. 2015; Johnson et al. 2009). Oenothein B and A are typically the dominant ellagitannins in Oenothera spp, with oenothein B comprising two subunits of tellimagrandin I and oenothein A comprising three subunits (Fig. 1). These compounds contain a large number of phenolic rings with oxidatively active groups (such as the hexahydroxydiphenoyl groups) that can cause oxidative stress and damage to nutrients in the alkaline guts of insects (Appel 1993; Barbehenn et al. 2006; Salminen and Karonen 2011; Salminen et al. 2011).
We used larvae of the herbivorous moth Spodoptera exigua Hübner (Noctuidae: Lepidoptera) as a model to study the effects of phenolics and ellagitannins on a generalist caterpillar. Spodoptera exigua is frequently used in laboratory experiments because of its extremely generalized diet, which includes >37 plant families (Normark and Johnson 2011). Spodoptera exigua eggs were acquired from Benzon Research (Carlisle, PA, USA), and were reared on a soy-flour and wheat germ-based diet (Beet Armyworm Diet, Sutherland Products, Mayodan, NC, USA). The insects were maintained in a colony with >100 mating individuals for 2–3 generations before being utilized in experiments.
Schinia florida Guenée (Noctuidae: Lepidoptera) larvae have a specialized diet that includes the flowers and fruits of O. biennis and close relatives (Sargent 1969). High levels of ellagitannins and in particular oenothein A are correlated with decreased damage to O. biennis (Agrawal et al. 2012; Anstett et al. 2015), making this moth a useful model system to test the direct effects of ellagitannins on specialist insect herbivores. Second and third instar Schinia florida were collected from the field in August 2016 in Mississauga and King City, ON, Canada, and used directly in feeding experiments. Our attempts to have earlier instars feed on artificial diet were unsuccessful and previous attempts to rear colonies of S. florida have been unsuccessful (A. Agrawal Pers. Comm.). In our experiments, S. florida did feed on Beet Army Worm diet without any apparent aversion starting at the 2nd instar, and these larvae were able to successfully complete development until pupation.
Experimental Design
To test for the effect of phenolics and the differences in the defensive function of ellagitannin compounds, we selected two genotypes of O. biennis known to have high levels of oenothein B and low concentrations of oenothein A, and two genotypes with high concentrations of oenothein A and low concentrations of oenothein B (Table S1). These genotypes also contained a diversity of ~70 other phenolic compounds, which included flavonoid glycosides and caffeic acid derivatives (Salminen unpublished data). Seeds were germinated on moistened filter paper within sealed petri dishes exposed to a full spectrum of sunlight. Forty seedlings of each genotype were grown for 6 months in a growth chamber (Conviron CMP6050, Winnipeg, Canada). The chamber was set to a 16:8 h day:night cycle with a 25 °C day: 20 °C night temperature regime, a ramp rate of 1 °C/h, and 500 μmol/m2/s of light intensity during the day. Fully expanded, non-senescing leaves were collected and stored in a − 80 °C freezer before extraction to obtain the total phenolic extract. Previous work on Oenothera biennis found that the constitutive concentration of total phenolics grown in a controlled environment varied between 45 and 160 mg/g (Anstett et al. 2016) depending on the genotype, which falls within the range of the concentration of total phenolics observed from plants growing in the field (Anstett et al. 2015).
To obtain sufficient quantities of the purified ellagitannins, we purified individual fractions of oenothein oligomers from the confamilial plant species Epilobium angustifolium (Onagraceae) as outlined in Baert et al. (2015), excluding other types of phenolics and many classes of ellagitannins to specifically obtain oenothein B, oenothein A, and higher oligomers. Importantly, macrocyclic tellimagrandin I –based ellagitannins are identical and interchangeable between E. angustifolium and O. biennis (Baert et al. 2015, 2016; Karonen et al. 2010; Salminen et al. 2011). Briefly, these fractions were obtained by maceration of E. angustifolium flower tissue in an 80% aqueous acetone solution for 48 h followed by filtering and lyophilizing. This procedure was carried out many times yielding hundreds of grams of extract. A fractionation step was then carried out by putting a mixed slurry containing flower extract through four elutions of water (fraction I), methanol/water (1:1, v:v, fraction II), methanol (fraction III), and finally, acetone/water (4:1, v:v, fraction IV), using Sephadex LH-20 gel in a Büchner funnel. The organic solvents were then evaporated and the remaining solutions were lyophilized again. The final fractions used in the experiments contained only ellagitannins and no other phenolics. Fraction II was enriched for oenothein B, whereas fraction IV was enriched for oenothein A plus larger oligomers of tellimagrandin I (Table S2).
Insect diets were made to contain varying concentrations of either ellagitannin fraction II, ellagitannin fraction IV, or the total phenolic extracts. The ellagitannin fraction diets contained 0, 1.0, 2.5, 5.0, 10.0, 50.0, and 100.0 mg/g (dry weight) of either fraction II or IV (i.e., 0 to 10% of ellagitannins per unit mass of diet). The total phenolic extract diets contained concentration of 0, 1.0, 2.5, 5.0 or 10.0 mg/g (dry weight) of total phenolics (i.e., 0 to 1% of phenolics per unit mass of diet). Higher phenolic concentrations were not included because concentrations >10 mg/g resulted in 100% mortality for the generalist caterpillar. Total phenolics extracts, as well as ellagitannin fractions, were applied to the food mixture suspended in ddH2O (Milli-Q Reference System, Millipore). Approximately 4 mL of food was made for each insect every 5 days by mixing insect food with boiling water at a ratio of 0.7 g to 3 mL. After heating and stirring for 2 min, 1 mL of water containing the appropriate amount of total phenolic extract or ellagitannin was added. Controls only received water. This mixture was heated and stirred for 1.5 min and then transferred to small plastic cups. These cups were left to cool and set prior to commencing the feeding assay. We analyzed the diets containing phenolics and ellagitannins using ultraperformance liquid chromatography connected to diode array and mass spectrometry detectors (UPLC-DAD-MS/MS), which confirmed that our diet preparation did not affect the diversity and concentration of phenolics in the food (data not shown).
Two feeding experiments were conducted to study the effects of phenolics and ellagitannins on S. exigua. One experiment assessed the effects of total phenolic extracts on herbivore performance, and the other assessed the effects of ellagitannin fractions on herbivore performance. In each experiment, fresh food was prepared for every live caterpillar on days 0, 5, 10 and 15. Measurements were made across these same time intervals to allow for an equivalent comparison and high replication among both herbivore species and across the different treatments. For each replicate, one freshly hatched caterpillar was transferred to each food container on day 0 and the caterpillar was subsequently allowed to feed and develop. To assess the effects of phenolics on the generalist caterpillar’s performance, we had five replicate caterpillars per phenolic concentration (1.0, 2.5, 5.0, and 10.0 mg/g), for each of the four O. biennis plant genotypes. Additionally, we had 32 control generalist caterpillars (for the 0 mg/g levels of total phenolics) (see Table S3 for degrees of freedom in each analysis). The second experiment investigated the effects of ellagitannin fractions. For each fraction we had 10 replicate caterpillars per concentration (1.0, 2.5, 5.0, 10.0, 50.0, 100.0 mg/g) and 15 generalist caterpillars received the control diets (0 mg/g) (see Table S4 for degrees of freedom in each analysis).
Similar experiments were performed on the specialist S. florida caterpillars. Food was prepared every 5 days, and we prepared additional food on day 20 to accommodate the longer developmental time of the specialist caterpillars. For the feeding assays that used total phenolic extracts, we had three replicate caterpillars for each of two genotype treatments (see Table S5 for degrees of freedom in each analysis). For the feeding assay that used ellagitannin fractions in the diet, we had five replicate caterpillars for each concentration (1, 5, 10, and 50 mg/g) of each ellagitannin fraction, plus five control caterpillars (0 mg/g) (see Table S6 for degrees of freedom in each analysis). This lower replication compared to the experiments involving S. exigua was due to the practical constraints of obtaining wild caught S. florida caterpillars for experiments.
In all experiments, we recorded live caterpillar mass and survival throughout each experiment. Starting on day 5, caterpillars were weighed every 5 days to determine the effects of the treatments on caterpillar growth. Specialist caterpillars were also weighed on day zero because they varied in initial size; generalist caterpillars were placed on dishes immediately after hatching from eggs and were too small to weigh. Caterpillar survival was tracked for every caterpillar until pupation or death.
Statistical Analysis
We used analysis of variance and analysis of covariance to test how the concentration of phenolics, O. biennis genotype, and ellagitannin fraction affected the performance of the generalist and specialist herbivores. Caterpillar mass was log (x + 1) transformed to improve the fit of the residuals from statistical models to the assumptions of normality and homogeneity of variance. Similarly, the concentration of phenolics and ellagitannin fractions were also log (x + 1) transformed. Initial mass was used as a covariate for all analyses involving the specialist caterpillar. First, we carried out comparisons between controls and mid-level phenolic concentrations (5 and 10 mg/g treatments), and between controls and mid-level oenothein concentrations (5 and 10 mg/g). These data were analyzed using the Anova command in the Car package of R (R Core Development Team 2018). The objective of this analysis was to give a general assessment of the effects of medium to high concentrations of the phenolics on herbivore performance. Next, we tested how insect mass at each individual time period and mortality were affected by treatment group (genotype or fraction) and concentration of phenolics. These analyses were also carried out using the Anova command in the Car package of R.
Results
Generalist Herbivore Performance, Total Phenolic Extracts
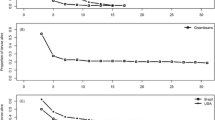
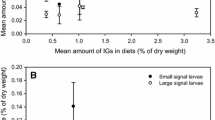
Spodoptera exigua caterpillars fed control diets (0 mg/g) strongly outperformed caterpillars that consumed diets containing any amount of total phenolics (Fig. 2a, Table S3). Control caterpillars had substantially higher mass (P < 0.001, Fig. S1A), and higher survival (P < 0.001, Fig. 3a), than those fed 5 to 10 mg/g of phenolics (Table S3). Even the lowest concentrations of total phenolic extracts (1 mg/g, 0.1% total phenolics) caused a dramatic decrease in insect mass, compared to the control diet (Fig. 2a). Increased concentrations of phenolics did not lead to further reductions in caterpillar performance (Fig. 2a, Table S3), indicating that total phenolics had a clear negative effect on caterpillar performance. For example, while caterpillar survival was nearly 100% on control diets, no caterpillars survived to pupation when feeding on diet containing any concentration of total phenolics (Fig. 3a). Plant genotypes varied in their effects on the mass of caterpillars (Fig. S2A, Table S3). Genotype and total phenolic concentration also interacted to affect caterpillar mass (Table S3). Caterpillars had lower mass with increased total phenolic concentrations for all genotypes except genotype 751 (high oenothein B), which was associated with increased performance of caterpillars at higher total phenolic levels (Fig. S2B).
Time-series of the mass of generalist Spodoptera exigua fed diet containing a total phenolics or b oenothein, and the specialist Schinia florida fed diet containing c total phenolics or d oenothein. Mass is given on a log scale for S. exigua and S. florida to aid in visualization. Lines represent the best fit line from linear regression. Individual data points have been removed to reduce clutter and make the overall trends clearer. Data are only present up to day 10 for the control, because all caterpillars in this treatment pupated before day 15
Survival results for bioassay experiments with generalist Spodoptera exigua when fed diet containing a total phenolics or b oenothein, and the specialist Schinia florida fed diet containing c total phenolics or d oenothein. Each point indicates the mean survival value. Bars indicate standard error. For d results are split into two fractions with varying amounts of oenothein A, oenothein B, and higher oligomers (see Table S2). Survival is from the entire experiment is displayed. Total phenolics and ellagitannin fractions are given in dry weight units
Generalist Herbivore Performance, Ellagitannin Fractions
Diets containing ellagitannin fraction II or ellagitannin fraction IV reduced the performance of the generalist caterpillar compared to control diets (Fig. 2b, Table S4). Control caterpillars had higher mass (P < 0.001) and survival (P < 0.001, Fig. S1B) than caterpillars fed diet containing moderate concentrations of oenothein ellagitannins (5 and 10 mg/g) (Fig. S1B, Table S4). When considering all concentrations at day 5, caterpillars exposed to fraction II (i.e. enriched for oenothein B) had 20% higher mass than caterpillars exposed to fraction IV (i.e. enriched for oenothein A and higher oligomers) (P = 0.02; Fig. S2C). At day 10, caterpillars exposed to fraction IV had 40% higher mass than caterpillars exposed to fraction II, although this effect was only marginally significant (P = 0.05). Higher concentrations of either fraction of oenothein ellagitannins led to lower caterpillar mass (Fig. 2b). The effect of ellagitannin fraction and the fraction X concentration interaction were not significant (Table S4). Caterpillars exposed to higher concentrations of either ellagitannin fraction had lower survival (P = 0.001, Fig. 3b), with no caterpillars surviving above concentrations of 2.5 mg/g (0.025% ellagitannin fractions). There was no significant effect of ellagitannin fraction or fraction X concentration interaction on the survival of generalist caterpillars (Table S4).
Specialist Herbivore Performance, Total Phenolic Extracts
The effects of total phenolics was tested on performance of the specialist caterpillar Schinia florida. Overall, variation in the concentration of total phenolics did not strongly affect caterpillar mass (Fig. 2c). Caterpillar mass did not significantly differ between caterpillars fed control diet versus those fed 5–10 mg/g of phenolics (Table S5). However, survival of caterpillars was 80% on control diets versus 17% on diets containing 5 to 10 mg/g of total phenolics (P = 0.01) (Fig. 3c). Phenolic concentration and plant genotype did not affect caterpillar mass or survival when it was treated as a continuous variable, indicating the negative effect of phenolics on the survival of the specialist caterpillar was caused by the clear toxic effects of total phenolic extracts (Table S5). Initial caterpillar mass was a significant predictor for some variables (Table S5).
Specialist Herbivore Performance, Ellagitannin Fractions
The specialist S. florida was also negatively impacted by the ellagitannin fractions (Fig. 2d, Table S6). Caterpillars feeding on the control diet had higher masses (Table S6) and higher survival (P < 0.001), when compared to caterpillars feeding on diets with 5 to 10 mg/g (i.e. moderate concentrations) of ellagitannin fractions (Fig. S1D). The concentration of oenothein ellagitannins had a significant effect on caterpillar mass on day 5, with caterpillars having 45% higher mass in the fraction II treatment when compared to the fraction IV treatment (P = 0.049; Fig. 2d). By day 10, caterpillars fed fraction II had only marginally higher mass (24% higher) than those fed fraction IV (P = 0.07). There was also a marginally non-significant interaction between fraction and concentration on day 10 (P = 0.08), which was caused by proportionally higher caterpillar mass at higher oenothein B concentrations. There were no significant fraction effects on specialist caterpillar mass on day 15, which is likely due to reduced statistical power caused by lower replication; there were concentration effects on caterpillar mass on day 20 (P = 0.03, Fig. 2d). Finally, for caterpillar survival there was also a significant interaction between concentration and ellagitannin fraction (P = 0.04; Fig. 3d). Specifically, caterpillars exhibited higher survival when feeding on diets containing 1 and 5 mg/g of fraction IV compared to caterpillars feeding on fraction II, whereas caterpillars exhibited higher survival on the fraction II treatment compared to the fraction IV treatment on day 20 (Fig. 3d). All variables were significantly predicted by initial mass (Table S6).
Discussion
Our results lead to several important conclusions about the role of hydrolyzable tannins as potential defenses against insect herbivores, which directly address our research questions. First, we found direct evidence that macrocyclic ellagitannin oligomers have large negative effects on the performance of generalist and specialist insect herbivores (Question 1). Second, differences between fractions rich in smaller (dimer) or larger (trimer) oligomers were small and dependent on insect life stage (Question 2). Finally, total phenolics had a larger negative impact on the generalist caterpillar, whereas the ellagitannin fractions generally had a larger negative impact on the specialist caterpillar (Question 3). Here we discuss these results and compare them to previous work on ellagitannins and herbivore specialization.
Effects of Ellagitannins on Herbivore Performance
The evidence for ellagitannins having a direct negative effect on insect herbivore performance is now considerable. It was previously shown that total ellagitannins and the individual ellagitannin compound vescalagin decreased herbivore performance (Barbehenn et al. 2009; Roslin and Salminen 2008). As well, total ellagitannins and the ellagitannin oenothein A have been associated with decreased herbivory using correlative approaches (Agrawal et al. 2012; Anstett et al. 2015; Johnson et al. 2009; McArt et al. 2013). Here we experimentally show that mixtures of oenothein B, oenothein A, and larger oligomers decrease herbivore performance of a generalist and a specialist herbivorous moth species. The effects of these ellagitannins were large, so that even low concentrations (0.25–0.5% of diet dry weight) resulted in large negative and often lethal effects on the generalist and specialist caterpillars (Figs. 2 and 3). Our results provide some of the strongest direct evidence that ellagitannins are an important anti-herbivore defense, and justify their use as a model for understanding the defensive effects of compounds that potentially act on herbivourous insects through oxidative damage, anti-feedant activity or less likely protein precipitation (Appel 1993; Barbehenn et al. 2008; Salminen and Karonen 2011).
Effects of Chemical Defenses on Generalist Versus Specialist Herbivores
While oenothein A, oenothein B and higher oenothein oligomers are important defenses against herbivores, they exist within the context of a wider range of chemical defenses. Although these are potent compounds, it is possible that other compounds, can act in conjunction with ellagitannins to generate higher negative impacts on certain herbivores (Kim et al. 2018). This is true for the generalist S. exigua, which had much lower survival when feeding on a diet containing just 1 mg/g of total phenolics (0% survival) compared to a diet with an equivalent concentration of the ellagitannin fractions (74% survival: the results from both fractions) (Fig. 3a and b). However, other herbivores may be particularly impacted by just one group of compounds. This was the case for the specialist S. florida, which had lower survival when feeding on a diet with 10 mg/g of the ellagitannin fractions (0% survival: both fractions combined), compared to a diet containing 10 mg/g total phenolic treatment (33% survival; all genotypes combined) (Fig. 3c and d). However, total phenolics were more harmful at lower concentrations (Fig. 3c and d). Therefore, while the generalist insect may be more impacted by a wider variety of chemical defenses, individual compounds may be more effective against the specialist caterpillar S. florida, especially if these compounds are at moderate to high concentrations. We interpret these comparisons between S. exigua and S. florida with an abundance of caution because the methods used for each insect species differed in important ways which may have influenced our results. Moreover, only one generalist and one specialist insect were tested. We caution that it is still unclear if these results are due to differences in diet breadth or differences between the two species unrelated to host specialization.
Limitations
There are four limitations to this study that require consideration when interpreting our results. First, S. florida was collected from only a single geographic region (Ontario, Canada). Oenothera biennis is known to have relatively low levels of chemical defenses in this region, and particularly low concentrations of oenothein A (Anstett et al. 2015), making it possible that the S. florida collected may be poorly adapted to oenothein A and increased phenolics in general. This issue needs to be further explored by characterizing the genetic diversity of S. florida resistance to phenolics and oenothein ellagitannins across multiple regions. Second, S. florida individuals were placed into the experiment as second and third instar larvae, rather than as recently hatched first instar larvae. If it had been possible to use neonate S. florida caterpillars, we may have seen higher mortality. We do not think these limitations are a major concern because earlier mortality would likely increase the effects we observed. Third, the oxidative effects of ellagitannins may be increased in the presence of other metabolites and polyphenol oxidases. While this experiment used enriched fractions of ellagitannins placed into the diet, previous work performed in vitro confirmed strong oxidative activity for these purified compound classes (Barbehenn et al. 2006). Additionally, the results of our study agree with previous conclusions from field experiments about the effects of ellagitannins on herbivores, which found that oenothein A was associated with decreased insect herbivore damage (Agrawal et al. 2012; Anstett et al. 2015). Finally, the use of an artificial diet formulated for S. exigua could have caused nutritional stress for S. florida and potentially increased susceptibility to phenolics. This possibility is unlikely to have altered our conclusions because: i) all S. florida caterpillars experienced the same base diet; ii) caterpillars were able to complete development on the control diet; and iii) caterpillars showed clear responses to variation in the concentration of ellagitannins. Overall, our results and conclusions are robust to these caveats.
Conclusions
Our findings provide clear and compelling support for the defensive function of macrocyclic ellagitannins against generalist and specialist insect herbivores. This study does not find strong evidence that variation in the composition of ellagitannins strongly influences defenses against herbivores as previously claimed (Agrawal et al. 2012; Anstett et al. 2015). Future studies should investigate the physiological mechanisms underlying the defensive function of these compounds in insect guts to further characterize the purported effects of oxidative stress, anti-feedancy, and possibly protein precipitation on insect survival and fecundity. Additionally, characterization of the genes involved in the biosynthesis of oenothein B, oenothein A, and high oligomer ellagitannins would present a major advance in the biochemistry, genetics and evolution of ellagitannin chemistry, and its potential applications.
References
Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen J-P (2012) Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338:113–116
Anstett DN, Ahern JR, Glinos J, Nawar N, Salminen J-P, Johnson MTJ (2015) Can genetically based clines in plant defense explain greater herbivory at higher latitudes? Ecol Lett 18:1376–1386
Anstett DN, Chen W, Johnson MTJ (2016) Latitudinal gradients in induced and constitutive resistance against herbivores. J Chem Ecol 42:772–781
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552
Baert N, Karonen M, Salminen J-P (2015) Isolation, characterisation and quantification of the main oligomeric macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode array detection and electrospray tandem mass spectrometry. J Chromatogr A 1419:26–36
Baert N, Pellikaan WF, Karonen M, Salminen J-P (2016) A study of the structure-activity relationship of oligomeric ellagitannins on ruminal fermentation in vitro. J Dairy Sci 99:8041–8052
Baert N, Kim J, Karonen M, Salminen J-P (2017) Inter-population and inter-organ distribution of the main polyphenolic compounds of Epilobium angustifolium. Phytochemistry 132:54–63
Barbehenn RV, Jones CP, Hagerman AE, Karonen M, Salminen J-P (2006) Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: potential impact on caterpillars. J Chem Ecol 32:2253–2267
Barbehenn R, Weir Q, Salminen J-P (2008) Oxidation of ingested phenolics in the tree-feeding caterpillar Orgyia leucostigma depends on foliar chemical composition. J Chem Ecol 34:748–756
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen J-P (2009) Hydrolyzable tannins as “quantitative defenses”: limited impact against Lymantria dispar caterpillars on hybrid poplar. J Insect Physiol 55:297–304
Carmona D, Lajeunesse MJ, Johnson MTJ (2011) Plant traits that predict resistance to herbivores. Funct Ecol 25:358–367
Denno RF, McClure MS (1983) Variable plants and herbivores in natural and managed systems. Academic Press, New York
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Manse RL (eds) Biochemical interaction between plants and insects. Plenum Press, New York, pp 1–40
Felton GW, Summers CB (1995) Antioxidant systems in insects. Arch Insect Biochem 29:187–197
Fritz RS, Simms EL (1992) Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. University of Chicago Press, Chicago
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Johnson MTJ (2011) Evolutionary ecology of plant defenses against herbivores. Funct Ecol 25:305–311
Johnson MTJ, Agrawal AA (2005) Plant genotype and environment interact to shape a diverse arthropod community on evening primrose (Oenothera biennis). Ecology 86:874–885
Johnson MTJ, Agrawal AA (2007) Covariation and composition of arthropod species across plant genotypes of evening primrose (Oenothera biennis). Oikos 116:941–956
Johnson MTJ, Agrawal AA, Maron JL, Salminen J-P (2009) Heritability, covariation and natural selection on 24 traits of common evening primrose (Oenothera biennis) from a field experiment. J Evol Biol 22:1295–1307
Johnson MTJ, Ives AR, Ahern J, Salminen J-P (2014) Macroevolution of plant defenses against herbivores in the evening primroses. New Phytol 203:267–279
Karonen M, Parker J, Agrawal AA, Salminen J-P (2010) First evidence of hexameric and heptameric ellagitannins in plants detected by liquid chromatography/electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom 24:3151–3156
Karonen M, Oraviita M, Mueller-Harvey I, Salminen J-P, Green RJ (2015) Binding of an oligomeric ellagitannin series to bovine serum albumin (BSA): analysis by isothermal titration calorimetry (ITC). J Agric Food Chem 63:10647–10654
Kim J, Pälijärvi M, Karonen M, Salminen J-P (2018) Oxidatively active plant phenolics detected by UHPLC-DAD-MS after enzymatic and alkaline oxidation. J Chem Ecol 44:483–496
McArt SH, Halitschke R, Salminen J-P, Thaler JS (2013) Leaf herbivory increases plant fitness via induced resistance to seed predators. Ecology 94:966–975
Moilanen J, Salminen J-P (2008) Ecologically neglected tannins and their biologically relevant activity: chemical structures of plant ellagitannins reveal their in vitro oxidative activity at high pH. Chemoecology 18:73–83
Moilanen J, Koskinen P, Salminen J-P (2015) Distribution and content of ellagitannins in Finnish plant species. Phytochemistry 116:188–197
Niemetz R, Gross GG (2005) Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 66:2001–2011
Normark BB, Johnson NA (2011) Niche explosion. Genetica 139:551–564
Parker J, Salminen J-P, Agrawal AA (2012) Evolutionary potential of root chemical defense: genetic correlations with shoot chemistry and plant growth. J Chem Ecol 38:992–995
Quideau S, Feldman KS (1996) Ellagitannin chemistry. Chem Rev 96:475–504
R Core Development Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rosenthal GA, Berenbaum MR (1991) Herbivores: their interactions with secondary plant metabolites. Volume II: Ecological and evolutionary processes. Academic Press, San Diego
Roslin T, Salminen J-P (2008) Specialization pays off: contrasting effects of two types of tannins on oak specialist and generalist moth species. Oikos 117:1560–1568
Salminen J-P, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338
Salminen J-P, Ossipov V, Haukioja E, Pihlaja K (2001) Seasonal variation in the content of hydrolyzable tannins in leaves of Betula pubescens. Phytochemistry 57:15–22
Salminen J-P, Ossipov V, Pihlaja K (2002) Distribution of hydrolyzable tannins in the foliage of Finnish birch species. Z Naturforsch C 57:248–256
Salminen J-P, Roslin T, Karonen M, Sinkkonen J, Pihlaja K, Pulkkinen P (2004) Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J Chem Ecol 30:1693–1711
Salminen J-P, Karonen M, Sinkkonen J (2011) Chemical ecology of tannins: recent developments in tannin chemistry reveal new structures and structure-activity patterns. Chemistry 17:2806–2816
Sargent TD (1969) Behavioral adaptations of cryptic moths. V. Preliminary studies on an anthophilous species, Schinia florida (Noctuidae). J New York Entomol Soc 77:123–128
Wallace J (1976) Biochemical interaction between plants and insects. Plenum, New York
Walters D (2011) Plant defense: warding off attack by pathogens, herbivores and parasitic plants. Wiley, Oxford
Yarnes CT, Boecklen WJ, Tuominen K, Salminen J-P (2006) Defining phytochemical phenotypes: size and shape analysis of phenolic compounds in oaks (Fagaceae, Quercus) of the Chihuahuan Desert. Can J Bot 84:1233–1248
Acknowledgements
We thank X. Zhao, J. Anstett, E. Kibkalo, J. Kim and T. Lempiäinen. This project was funded by an NSERC CGS-D Vanier, and a NSERC Banting Fellowship to D. Anstett. The project was further funded by an NSERC Discovery Grant, the Canadian Foundation for Innovation, and Ontario’s Early Researcher Award to M. Johnson. J.-P. Salminen was supported by grant no. 258992 from the Academy of Finland.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Anstett, D.N., Cheval, I., D’Souza, C. et al. Ellagitannins from the Onagraceae Decrease the Performance of Generalist and Specialist Herbivores. J Chem Ecol 45, 86–94 (2019). https://doi.org/10.1007/s10886-018-1038-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-1038-x