Abstract
Plant secondary metabolites can have opposing effects on adapted specialist and non-adapted, generalist herbivores. In this study, we used Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae) as a generalist, non-adapted model herbivore to test the possible effects of Crotalaria pallida (Fabaceae: Papilionoideae) defenses on herbivore performance. Neonate H. virescens larvae were able to consume C. pallida leaves and fruits and grow for a few instars, but none of them survived to pupation. We added isolated pyrrolizidine alkaloids (PAs) to an artificial diet at different concentrations, and PA concentration significantly affected the number of larvae that achieved pupation. Larval survival was not reduced at a PA concentration similar to the concentration on green seeds of C. pallida, but it was significantly reduced at PA concentration 5 and 100 times higher. These results suggest that PAs in isolation are not the defense responsible for the mortality in fresh C. pallida plants, indicating the importance of other possible defenses. The negative effect of PAs on fitness of the non-adapted, generalist herbivore is in agreement with few previous studies, but it is in clear contrast to a previous study on the effects of PAs on the adapted specialist herbivore Utetheisa ornatrix (L.) that were able to sequester PAs with no fitness costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary metabolites are one of the most pervasive defense mechanisms against herbivores in plants (Schoonhoven et al 2005). Many studies have shown the negative effects of these substances on survival and fecundity of herbivores (Awmack & Leather 2002). However, herbivorous insects have evolved mechanisms to tolerate specific plant defense chemicals. Moreover, in some cases, plant defenses can be used as a cue by specialist herbivores to find their host plants or be used as phagostimulants (Bernays & Chapman 1994). Many specialist herbivorous insects can also sequester these defensive chemicals and use them as protection against predators or for attraction of mates (Bowers 1992, Nishida 2002, Macel 2011, Trigo 2011). For example, the moth Utetheisa ornatrix (L.) (Lepidoptera: Erebidae) is able to sequester specific defensive chemicals, pyrrolizidine alkaloids (PAs), from its host plant and use it for defense and as a male mating pheromone (Eisner & Meinwald 1995). In a series of experiments in which PAs were added to an artificial diet at different concentrations, fitness components of this moth were affected only slightly or not at all by PAs, suggesting that sequestration in this species does not incur in fitness cost (Cogni et al 2012). In addition, larvae showed preference for feeding on diets with higher concentrations of PAs in choice experiments (Hoina et al 2012). These results indicate that for some specific plant defenses, specialist herbivores can be positively affected by sequestration without any negative effect or fitness costs.
On the other hand, these plant defensive chemicals may have a negative effect on non-adapted, generalist herbivores; for example, the generalist arctiines Estigmene acrea (Drury) and Grammia geneura (Strecker) are adapted to PAs, sequestering them (Harmtann et al 2004); however, PAs have deterrent and toxic effects on a variety of non-adapted, generalist insect herbivores (van Dam et al 1995, Macel et al 2005, Narberhaus et al 2005). These findings suggest that adapted specialist herbivores can act as agents of natural selection that lower the levels of defensive chemicals in host plant populations, while non-adapted generalists may increase these levels (van der Meijden 1996). For example, introduced populations of Senecio jacobaea (Asteraceae) that lacked a specialist herbivore evolved increased levels of PAs and, consequently, increased resistance to generalist herbivores (Joshi & Vrieling 2005). Similarly, generalist damage was negatively correlated to glucosinolate sinigrin concentrations in Brassica nigra (Brassicaceae), but specialist damage was positively correlated (Lankau 2007). The increase in sinigrin concentration was favored when specialists were removed, disfavored when generalists were removed, and neutral when both generalists and specialists were present (Lankau 2007).
In this study, we tested the effects of Crotalaria pallida (Fabaceae: Papilionoideae) defenses on the generalist, non-specialized model herbivore Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae). We used this herbivore as a model generalist to contrast with our previous results on the specialist U. ornatrix (Cogni & Futuyma 2009, Cogni et al 2012, Hoina et al 2012, Franco & Cogni 2013). Heliothis virescens is an economically important polyphagous pest that can develop in more than 100 plant species belonging to more than 36 families (Blanco et al 2008, Karpinski et al 2014). This species was chosen as a model because it can feed on many plant species and can consume leaves and fruits. Crotalaria pallida is an annual plant with current pantropical distribution that obscures its native origin (Polhill 1982). The constitutive presence of pyrrolizidine alkaloids is considered the major resistance trait in Crotalaria (Wink & Mohamed 2003, Hartmann 1999, Trigo 2011). Here we tested if a model generalist herbivore is able to consume and survive on the chemically protected C. pallida, and how the main plant defense, pyrrolizidine alkaloids, affects herbivore survival by asking the following: (1) Does H. virescens larva consume C. pallida? (2) Is H. virescens larva able to complete development on C. pallida? (3) How does different pyrrolizidine alkaloid concentrations on the diet affect H. virescens survival?
Material and Methods
Larval performance on fresh plants
Heliothis virescens were purchased from Bio-Serv (Frenchtown, NJ). Crotalaria pallida seeds were collected from two populations: Campinas, state of São Paulo, Southeastern Brazil, and Archbold Biological Station in Central Florida, USA (Cogni & Futuyma 2009). The composition of PAs of plants from these two populations was quantitatively and qualitatively similar (Cogni et al 2011). The quantitative analysis was performed by colorimetric method and the qualitative analysis by GC/MS (Trigo et al 1993, 2003, Cogni et al 2011). We grew plants from seeds in the greenhouse at the Life Science Building at Stony Brook University in Stony Brook, NY, under natural sunlight. Plants were watered daily and fertilized every other week as in Cogni et al (2011). We fed larvae on fresh leaves for the first 4 days; after that, larvae were fed with unripe seeds. This was done to simulate the wild condition for the most common herbivore of Crotalaria plants, U. ornatrix. Neonates of U. ornatrix first consume leaf material for some days before getting inside the pod to prey on unripe seeds (Ferro et al 2006, Eisner 2003). We put neonate larvae individually in 1.5-mL microcentrifuge tubes with a leaf disc for 48 h. Leaf discs were made from fresh leaves and were 1 cm in diameter. After 4 days of feeding on leaves, larvae were transferred to individual petri dishes (5-cm diameter) with a moistened filter paper and green pods. Pods were opened with a razor blade to completely expose seeds to larvae. Every other day, larvae were transferred to a clean dish and new pods were provided. The amount of pod given to each larva was 1/3 pod on days 5 and 7, 1/2 on day 9, 1 on days 11 and 13, and 2 (every other day) after day 13. We checked for larval survivorship on alternate days. All experiments were carried out in an incubator at 29°C and 12:12 photoperiod. We used 117 individual neonate larvae for the Brazilian plants and 98 for the plants from USA.
In addition to the main experiment with H. virescens eating on C. pallida plants, we carried out two controls concurrently. First, as a control for the ability of our H. viscerens stock to grow on fresh plant material in the laboratory, we used green beans, a non-toxic host plant. Pods of organic green beans, Phaseolus vulgaris (Fabaceae: Papilionoideae), were purchased weekly from a local grocery store and kept refrigerated. The pods of green beans were also provided opened to the larva with the unripe seeds exposed. The same procedures described above for the C. pallida plants were used. The amount of green bean pods provided each day was similar in size to the amount of C. pallida pods used on the first experiment. One hundred twenty-eight individual neonate larvae were used. Second, as a control for the suitability of our greenhouse grown C. pallida plants for herbivore development, we used the Crotalaria specialist herbivore U. ornatrix. Adults of U. ornatrix were collected at the same locations as the C. pallida seeds. Utetheisa ornatrix from Brazil was tested with the plants from Brazil; U. ornatrix from USA was tested with the plants from USA. The exactly same procedures described above for the H. virescens were used for U. ornatrix. We used 110 individual neonate larvae for the Brazilian plants and 110 for the plants from USA.
Larval performance on artificial diet supplemented with PAs
We measured H. virescens larval survival on an artificial diet based on Phaseolus beans (Signoretti et al 2008) to which we added different concentrations of PAs. PAs were extracted from leaves and flowers of Senecio brasiliensis (Asteraceae) as in Trigo et al (1993), and after crystallization with acetone, the PA extract was 100% pure (Hoina et al 2012). We used S. brasiliensis as the PA source because the yield (~4 mg/g) of these alkaloids is higher than in C. pallida seeds. The extracted PAs consisted of a mixture of senecionine-type PAs including approximately 4% of senecionine, 69% of intergerrimine, and 27% of retrorsine. These are the same category of PAs (senecionine-type) found in unripe seeds of C. pallida (usaramine ~85% and intergerrimine ~15%) (Ferro et al 2006). These PAs were identified and quantified as described earlier for the fresh plant experiment. These PAs just vary at C-12 (senecionine and integerrimine have an OH, and retrorsine and usaramine a CH2OH) and C-15 (senecionine and retorsine are Z isomers, and integerrimine and usaramine E isomers). Other Crotalaria species, such as Crotalaria incana and Crotalaria micans, with integerrimine as the main PA (Flores et al 2009), are also used as host plant by U. ornatrix in the Neotropics (Cogni 2010, Sourakov 2015, J. R. Trigo, personal communication). Although structurally related PAs may differ in their effects to different generalist herbivores (Macel et al 2005), we have data showing no difference between the mixture of senecionine-type PAs or monocrotaline (a structurally divergent PA) in the performance of the specialist U. ornatrix and the generalist H. virescens (R Cogni and JR Trigo, personal communication). In C. pallida, unripe seeds have an average of 0.24 μg/mg of PAs, and leaves have only 0.054 μg/mg (Ferro et al 2006). Five treatments were used (concentrations represent weigh of PAs by weight of dry diet): 0% (0x) PAs added, 4.8 × 10−3% (0.2×), 2.4 × 10−2% (1×), 1.2 × 10−1% (5×), and 2.4% (100×). Sample size for each treatment was, respectively, 147, 154, 164, 162, and 162. All experiments were carried out in an incubator at 29°C and 12:12 photoperiod. Just after hatching, larvae were transferred individually to 2-mL tubes containing 0.6 mL of diet. After 3 weeks, we used a 10-mL tube with 3-mL of diet. Every week, larvae were transferred to a new tube with fresh diet. Mortality was checked every other day. Larval survival on the PA concentration treatments was compared by the test to compare more than two proportions (Zar 1999, p. 562), followed by a comparison of each proportion to the proportion of survived larvae on the control, with a procedure analogous to the Dunnett test, using q-distribution (Zar 1999, p. 565).
Results
Larval performance on fresh plants
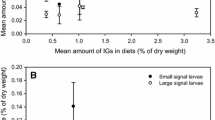
Neonate H. virescens larvae were able to consume C. pallida leaves and fruits. Many larvae were able to grow when eating C. pallida (Fig 1a), but none of the larvae survived to pupation (Fig 1a). Heliothis virescens larvae mortality trends were similar for the two C. pallida populations (Fig 1a). On the other hand, on the control group in which H. virescens was fed on green beans, 19% of the neonate larvae achieved pupation (Fig 1b). The Crotalaria specialist herbivore U. ornatrix was able to develop on the C. pallida plants used in this experiment. Twenty percent of U. ornatrix larvae survived to pupation on the C. pallida from Brazil and 35% on the C. pallida from USA (Fig 1c).
Larval performance on artificial diet supplemented with PAs
Heliothis virescens larvae were able to consume the artificial diet with the PAs added and survive to pupation (Fig 2). Larval survival was affected by PA concentration in the diet (0% PAs = 12.2% survival, 4.8 × 10−3% PAs = 12.3%, 2.4 × 10−2% PAs = 11.0%, 1.2 × 10−1% PAs = 4.9%, 2.4% PAs = 1.2%; χ2 = 18.42, df = 4, p < 0.001). Larval survival was not reduced at a PA concentration similar to the concentration (2.4 × 10−2%) on green seeds of C. pallida (q = 0.297, p > 0.05). Survival was just significantly reduced at PA concentration 100 times higher than the concentration found on green C. pallida seeds (q = 5.123, p < 0.05).
Discussion
We showed a clear negative effect of PAs on fitness of a non-adapted generalist herbivore. This result is in agreement with few other studies showing negative effects of PAs on non-adapted generalists. For example, PAs of Cynoglossum offinale (Boraginaceae) have deterrent effects on a variety of generalist herbivores (van Dam et al 1995). Macel et al (2005) also showed that PAs have negative effects on fitness of a variety of generalist herbivores and that structurally related PAs differed in their effects to different herbivores such as thrips, aphids, and locust. In another example, PAs presented toxic effects on growth and survival of the eri silk moth, Samia cynthia (Drury) (Lepidoptera: Saturniidae), another generalist herbivore (Narberhaus et al 2005). These results are in clear contrast with our previous study on the effects of PAs on the adapted specialist herbivore U. ornatrix that indicates that sequestration of PAs by the specialist (Eisner & Meinwald 1995, Conner & Weller 2004, Conner 2009) does not incur in fitness costs (Cogni et al 2012). The lack of costs by the specialist has important implications for evolution of plant–herbivore interactions. It suggests, for example, that selection by specialist herbivores may decrease the levels of certain chemical defenses in plant populations. In the present study, we showed that this is not the case for non-adapted herbivores, suggesting that adapted specialists and non-adapted generalists may apply opposing selection on the levels of chemical defenses, potentially maintaining genetic variation in plant populations (van der Meijden 1996, Joshi & Vrieling 2005, Lankau 2007).
Interestingly, in the experiment with controlled diet with isolated PAs, H. virescens survival was only negatively affected at PA concentrations 5 and 100 times higher than the concentration in the seeds of C. pallida. However, H. virescens could not survive when fed with fresh plant seeds. These results suggest that PAs are not the sole mechanism for herbivore defense in fresh plants, indicating that the possible existence of other defensive traits on the seeds may be responsible for the inability of H. virescens to complete development on C. pallida. Possible candidate defenses include isoflavonoids, non-protein amino acids, and proteinase inhibitors (Pilbeam & Bell 1979, Pilbeam et al 1979, Rego et al 2002, Wink & Mohamed 2003, Pando et al 2004). Another possibility is that these compounds do not affect the herbivore only in isolation but that herbivore’s response is affected by interactions among two or more plant metabolites (Steppuhn & Baldwin 2007, Agrawal 2011). Finally, a third hypothesis is that artificial diets are generally rather nutritious which could counterbalance the negative effects of PAs (see Slansky 1993).
The two main conclusions of this study open a series of questions for future investigations in the system. Our first main conclusion is that a non-adapted generalist herbivore is negatively affected by PAs, contrasting with previous results on the ability of the adapted specialist herbivore to sequester PAs with no fitness costs. In the neotropics, U. ornatrix is the main natural enemy of Crotalaria plants. In some localities, the pod-borer Etiella zinckenella (Treitschke) (Lepidoptera: Pyralidae) can also be found as an important herbivore (Cogni et al 2011). By preying on seeds, U. ornatrix can have a significant impact on the fitness of Crotalaria plants; up to 20% of C. pallida fruits in the field may be damaged by U. ornatrix (Ferro et al 2006, Cogni et al 2011, Pereira & Trigo 2013). It would be interesting to compare in the field the frequency of the adapted herbivore with a non-adapted such as the pyralid moth E. zinckenella, as well as PA concentrations in different plant populations. Field experiments can also be developed to measure the possible opposing selection that the adapted and the non-adapted herbivores may impose to C. pallida. Our second main conclusion is that PAs in isolation may not be the defense responsible for the mortality of the generalist herbivore on fresh C. pallida seeds. It would be interesting to test how other adapted and non-adapted generalist herbivores are affected by PAs at the concentration found on the plant. Future research can also try to isolate other possible chemical defenses on seeds of C. pallida. This would also be interesting because previous research suggested that patterns of local adaptation of the specialist herbivore U. ornatrix to its host plant C. pallida are not explained by variation in PAs or nutritional quality of the plants, but by possible variation on an unknown chemical defense (Cogni & Futuyma 2009, Cogni et al 2011).
References
Agrawal AA (2011) Current trends in the evolutionary ecology of plant defence. Funct Ecol 25:420–432
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman and Hall, New York
Blanco CA, Terán-Vargas AP, Abel CA, Portilla M, Rojas MG, Morales-Ramos JA, Snodgrass GL (2008) Plant host effect on the development of Heliothis virescens F. (Lepidoptera: Noctuidae). Environ Entomol 37:1538–1547
Bowers MD (1992) The evolution of unpalatibility and the cost of chemical defense in insects. In: Roitberg BD, Isman MB (eds) Insect chemical ecology: an evolutionary approach. Chapman and Hall, New York, pp 216–244
Cogni R (2010) Resistance to plant invasion? A native specialist herbivore shows preference for and higher fitness on an introduced host. Biotropica 42:188–193
Cogni R, Futuyma DJ (2009) Local adaptation in an insect plant interaction depends on the spatial scale. Biol J Linn Soc 97:494–502
Cogni R, Trigo JR, Futuyma DJ (2011) Varying herbivore population structure correlates with lack of local adaptation in a geographic variable plant-herbivore interaction. PLoS ONE 6:e29220. doi:10.1371/journal.pone.0029220
Cogni R, Trigo JR, Futuyma DJ (2012) A free lunch? No cost for acquiring defensive plant pyrrolizidine alkaloids in a specialist arctiid moth (Utetheisa ornatrix). Mol Ecol 21:6152–6162
Conner WE (2009) Utetheisa ornatrix, the ornate arctiid. In: Conner WE (ed) Tiger moths and woolly bears-behavior, ecology and evolution of the Arctiidae. Oxford University Press, New York, pp 1–10
Conner WE, Weller SJ (2004) A quest of alkaloids: the curious relationship between tiger moths and plants containing pyrrolizidine alkaloids. In: Cardé RT, Millar JG (eds) Advances in insect chemical ecology. Cambridge University Press, New York, pp 248–282
Eisner T, Meinwald J (1995) The chemistry of sexual selection. Proc Natl Acad Sci U S A 92:50–55
Eisner T (2003) For love of insects. Harvard University Press, Cambridge
Ferro VG, Guimarães PR, Trigo JR (2006) Why do larvae of Utetheisa ornatrix penetrate and feed in pods of Crotalaria species? Larval performance vs. chemical and physical constraints. Entomol Exp Appl 121:23–29
Flores AS, Tozzi AMGA, Trigo JR (2009) Pyrrolizidine alkaloid profiles in Crotalaria species from Brazil: chemotaxonomic significance. Biochem Syst Ecol 37:459–469
Franco MS, Cogni R (2013) Common-garden experiments reveal geographical variation in the interaction among Crotalaria pallida (Leguminosae: Papilionideae), Utetheisa ornatrix L. (Lepidoptera: Arctiidae), and extrafloral nectary visiting ants. Neotropical Entomol 42:223–229
Hartmann T (1999) Chemical ecology of pyrrolizidine alkaloids. Planta 207:483–495
Harmtann T, Theuring C, Beuerle T, Ernst L, Singer MS, Bernays EA (2004) Acquired and partially de novo synthesized pyrrolizidine alkaloids in two polyphagous arctiids and the alkaloid profiles of their larval food-plants. J Chem Ecol 30:229–254
Hoina A, Martins CHZ, Trigo JR, Cogni R (2012) Preference for high concentrations of plant pyrrolizidine alkaloids in the specialist arctiid moth Utetheisa ornatrix depends on previous experience. Arthropod-Plant Interactions 7:169–175
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Karpinski A, Haenniger S, Schöfl G, Heckel DG, Groot AT (2014) Host plant specialization in the generalist moth Heliothis virescens and the role of egg imprinting. Evol Ecol 28:1075–1093
Lankau RA (2007) Specialist and generalist herbivores exert opposing selection on a chemical defense. New Phytol 175:176–184
Macel M (2011) Attract and deter: a dual role for pyrrolizidine alkaloids in plant-insect interactions. Phytochem Rev 10:75–82
Macel M, Bruinsma M, Dijkstra SM, Ooijendijk T, Niemeyer HM, Klinkhamer PGL (2005) Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. J Chem Ecol 31:1493–1508
Narberhaus I, Zintgraf V, Dobler S (2005) Pyrrolizidine alkaloids on three trophic levels—evidence for toxic and deterrent effects on phytophages and predators. Chemoecology 15:121–125
Nishida R (2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47:57–92
Pando LA, Carvalho DD, Toyama MH, Ciero L, Novello J et al (2004) Purification and characterization of a lectin from Crotalaria paulina seeds. Protein J 23:437–444
Pereira MF, Trigo JR (2013) Ants have a negative rather than a positive effect on extrafloral nectaried Crotalaria pallida performance. Acta Oecol 51:49–53
Pilbeam DJ, Bell EA (1979) Free amino acids in Crotalaria seeds. Phytochemistry 18:973–985
Pilbeam DJ, Polhill RM, Bell EA (1979) Free amino acids and alkaloids of South American, Asian and Australian Crotalaria species. Bot J Linn Soc 79:259–266
Polhill RM (1982) Crotalaria in Africa and Madagascar. CRC Press, Rotterdam, 396p
Rego EJL, Carvalho DD, Marangoni S, Oliveira B, Novello JC (2002) Lectins from seeds of Crotalaria pallida (smooth rattlebox). Phytochemistry 60:441–446
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology, 2nd edn. Oxford University Press, Oxford
Signoretti AGC, Nava DE, Bento JMS, Parra JRP (2008) Biology and thermal requirements of Utetheisa ornatrix (L.) (Lepidoptera: Arctiidae) reared on artificial diet. Braz Arch Biol Technol 51:647–653
Slansky F (1993) Nutritional ecology: The fundamental quest for nutrients. In: Stamp NE, Casey TM (eds) Caterpillars: Ecological and evolutionary constraints on foraging. Chapman and Hall. pp 29-91
Sourakov A (2015) You are what you eat: native versus exotic Crotalaria species (Fabaceae) as host plants of the Ornate Bella Moth, Utetheisa ornatrix (Lepidoptera: Erebidae: Arctiinae). J Nat Hist 49:39–40
Steppuhn A, Baldwin IT (2007) Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol Lett 10:499–511
Trigo JR (2011) Effects of pyrrolizidine alkaloids through different trophic levels. Phytochem Rev 10:83–98
Trigo JR, Witte L, Brown KS Jr, Hartmann T, Barata LES (1993) Pyrrolizidine alkaloids in the arctiid moth Hyalurga syma. J Chem Ecol 19:669–679
Trigo JR, Leal IR, Matzenbacher NI, Lewinsohn TM (2003) Chemotaxonomic value of pyrrolizidine alkaloids in southern Brazil Senecio (Senecioneae: Asteraceae). Biochem Syst Ecol 31:1011–1022
van Dam NM, Vuister LWM, Bergshoeff C, de Vos H, van der Meijden E (1995) The “raison d’être” of pyrrolizidine alkaloids in Cynoglossum officinale: deterrent effects against generalist herbivores. J Chem Ecol 21:507–523
van der Meijden E (1996) Plant defense, an evolutionary dilemma: contrasting effects of (specialist and generalist) herbivores and natural enemies. Entomol Exp Appl 80:307–310
Wink M, Mohamed GIA (2003) Evolution of chemical defense traits in the Leguminosae: mapping of distribution patterns of secondary metabolites on a molecular phylogeny inferred from nucleotide sequences of the rbcL gene. Biochem Syst Ecol 31:897–917
Zar JH (1999) Biostatistical Analysis. Prentice Hall, Upper Saddle River, New Jersey, 929p
Acknowledgments
We are grateful to J.R.P.Parra and D. Navas for providing the artificial diet recipe for U. ornatrix. M.F. Pereira and A. Hoina helped in the laboratory. We thank two anonymous reviewers for suggestion on the manuscript. Thanks to FERTL (Functional Ecology Research and Training Laboratory) for equipment use. We also thank IBAMA and USDA for permits to export and import live organisms. Financial support was provided by NSF (DEB 0807418) to RC, and by FAPESP (11/17708-0) and CNPq (306103/2013-3) to JRT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Raúl A Laumann – Embrapa
Rights and permissions
About this article
Cite this article
Cogni, R., Trigo, J.R. Pyrrolizidine Alkaloids Negatively Affect a Generalist Herbivore Feeding on the Chemically Protected Legume Crotalaria pallida . Neotrop Entomol 45, 252–257 (2016). https://doi.org/10.1007/s13744-016-0361-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0361-6