Abstract
The cotton mealybug, Phenacoccus solenopsis, the distribution of which was formerly limited to Nearctic and Neotropical regions, recently invaded many countries in various regions including Asia, Africa, and the Pacific. More recently, P. solenopsis was newly recorded in Japan and is currently an emerging pest of agricultural crops. In this study, we determined the structure of a sex pheromone of P. solenopsis in order to develop an effective lure for monitoring this pest. From volatiles emitted by virgin adult females, we isolated a compound attractive to males. By means of coupled gas chromatography–mass spectrometry and nuclear magnetic resonance spectroscopy, we identified this as (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl 3-methylbut-2-enoate. This compound was synthesized and shown to be attractive to male P. solenopsis. Analysis by gas chromatography using an enantioselective stationary phase and polarimetry analyses of the natural pheromone and synthetic enantiomers showed the natural compound to be the (R)-(−)-enantiomer. This compound is an ester of maconelliol, which has an unusual cyclobutane structure found in sex pheromones of other mealybug species, and senecioic acid, also found in the pheromones of other mealybug species. However, this is the first example of the ester of maconelliol and senecioic acid as a natural product.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mealybugs (Hemiptera: Pseudococcidae) are small sap-sucking insects that are covered with powdery wax. They exist worldwide and are parasites of various plants, including many agricultural crops (Ben-Dov 1994). Some mealybugs recently have expanded their distributions, probably as a result of increasing global commerce in live plant materials (Gullan et al. 2003) and/or climate change (Gutierrez et al. 2008; Jara et al. 2013). Exotic pests can cause serious damage in the area they invade, and the cotton mealybug, Phenacoccus solenopsis Tinsley, is one such example. Distribution of this species previously was limited to the Nearctic and Neotropical regions, but, since 2005, it has been known to have invaded cotton-growing areas of Pakistan and India (Hodgson et al. 2008). The infestation by P. solenopsis, which was described by the name of Phenacoccus gossypiphilous Abbas, Arif & Saeed in a nomen nudum (Abbas et al. 2005), was recorded in cotton fields of the Punjab and Sindh provinces of Pakistan. This outbreak occurred on both Bt and non-Bt cotton, leading to the use of large amounts of pesticides against this pest — more than 120 million US dollars’ worth in two months in 2007 (Hodgson et al. 2008). The pest is known to have subsequently invaded many countries in various regions including Asia, Africa, and the Pacific (García Morales et al. 2016). More recently, this species has been recorded in Japan (García Morales et al. 2016; Tanaka and Tabata 2014; Tanaka and Uesato 2012) and has been reported to attack agricultural crops including tomato and eggplant (Inada 2016). Detection and monitoring of this potential agricultural pest is thus urgently required for worldwide quarantine. Traps baited with pheromones would be a useful monitoring tool that would be less time-consuming than other methods (Gut et al. 2004).
Sex pheromones have been isolated and identified in 15 mealybug species (Tabata and Ichiki 2015). Most of the isolated compounds contain irregular monoterpene moieties. In monoterpenes, the two isoprene units are generally coupled by a 1–4′ linkage (“head-to-tail” connection; Breitmaier 2006), whereas mealybug monoterpene moieties contain unusual non-head-to-tail connections, such as the 1–2′ linkage that is found in lavandulol (5-methyl-2-isopropenyl-4-hexenol), a characteristic component of lavender fragrance (e.g., Tabata et al. 2015). Although there are common motifs, the mealybug pheromone components previously examined differ among species, and each species produces a characteristic compound (Millar et al. 2005; Zou and Millar 2015). Thus, the sex pheromone systems of mealybugs are clearly species-specific and provide a good opportunity not only to develop a pest management tool but also to study the structural diversity of the chemicals used in communication.
In the present study, we used chromatography to isolate the sex pheromone compound of P. solenopsis from volatiles emitted by virgin female. Coupled gas chromatography–mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectrometry were used to determine the chemical structure. The pheromone candidate was synthesized enantioselectively, and its attractiveness to males was confirmed. Moreover, the absolute configuration of the natural pheromone was determined by comparisons to synthetic enantiomers in GC analyses with an enantioselective stationary phase.
Methods and Materials
Analytical Instruments
Analyses by coupled gas chromatography-mass spectrometry (GC-MS) analyses were performed with an HP 5890 gas chromatograph (Hewlett-Packard, Avondale, PA, USA) equipped with an SX-102 A mass spectrometer (JEOL, Tokyo, Japan). The temperatures of the GC interface and the ion source were 210 °C and 220 °C, respectively. A nonpolar column (DB-1; 0.25 mm internal diam × 30 m length, 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA) was used. Helium was the carrier gas at a constant flow rate of 1.0 ml/min. The column oven temperature was maintained at 60 °C for 1 min, raised to 220 °C at 8 °C/min, and held at the final temperature for 5 min. Mass spectra were obtained in the electron-impact mode at 70 eV. For certain runs, n-alkanes were added as references for calculation of retention indices (Kováts indices, KI).
An enantioselective cyclodextrin column (beta-DEX 120; 0.25 mm internal diameter × 30 m length, 0.25 μm film thickness; Restek, Bellefonte, PA, USA) also was used, and the GC oven temperature was programmed to 100 °C for 5 min and then raised to 180 °C at 5 °C/min.
Nuclear magnetic resonance (NMR) spectra were obtained at 30 °C with a JNM-A600 spectrometer (1H: 600.05 MHz, 13C: 150.80 MHz; JEOL). Micro-bottom NMR tubes (SP-501; 1.7 mm outside diam; Shigemi Co., Tokyo, Japan) were used with 35-μl of deuterated benzene (min. 99.96 %; Sigma-Aldrich, St. Louis, MO, USA) as the solvent. Optical rotation was measured with a P-1020 polarimeter (JASCO, Tokyo, Japan).
Collection of Volatiles from Insects
Mealybugs were collected from sunflowers (Helianthus annuus) growing on Ôhama beach (24.3°N, 124.2°E; Ishigaki City, Okinawa Prefecture, Japan) on 16 July 2015. The insects were reared on germinated broad bean seeds and maintained in a rearing room (16:8 h L:D; 23 °C; 50 % RH). Males and females were manually separated immediately after the males made cocoons, and females were transferred to a new container (10 cm diam × 4 cm height) with diet.
Volatiles from virgin female mealybugs were collected by placing germinated broad bean seeds with approximately 200 virgin females into a 0.2-L glass jar. Ambient air cleaned through activated charcoal (15 g) was pulled over the virgin females in the jar and then passed through an adsorbent (1.0 g of HayeSep Q; 60/80 mesh; Alltech, Deerfield, IL, USA) at a flow rate of 0.1 L/min using a vacuum pump. Every 3–4 d, the volatiles were extracted from the adsorbent with 15 ml of hexane, the extract was concentrated with an evaporator at room temperature, and the residue was kept at −20 °C. The collection of volatiles continued for 6 wk., and four rounds of the collection were performed to obtain approximately 33,600 female day equivalents (FDE) in total.
Voucher specimens are stored at the National Agriculture and Food Research Organization (Tsukuba, Japan). Partial mitochondrial DNA sequences of these were determined by a previously described method (Tabata et al. 2016) and deposited in the GenBank/DDBJ/EMBL database (accession number LC171400).
Preparative Liquid Chromatography
Open-column chromatography was carried out on silica gel (0.2 g; Wakogel C-200; Wako Pure Chemicals, Osaka, Japan) by a method for separating lipids by class. The column was successively eluted with 2 ml each of 0, 5, 15, and 50 % diethyl ether in hexane. High-performance liquid chromatography (HPLC) was performed with an HP 1050 instrument (Hewlett-Packard) with a silica gel column (Inertsil SIL; 4.6 mm internal diam × 250 mm length, 5 μm particle size; GL Sciences Inc., Tokyo, Japan). The elution solvent was 5 % diethyl ether in hexane at a flow rate of 1 ml/min, and the eluate was monitored with a UV detector (λ = 210 nm).
Preparative Gas Chromatography
Preparative GC was carried out with an Agilent 6890 N gas chromatograph (Agilent Technologies Inc., Wilmington, DE, USA) equipped with a flame ionization detector kept at 220 °C and a preparative fraction collection system (Gerstel GmbH & Co. KG, Mulheim an der Ruhr, Germany) cooled by a dry ice–acetone bath. A polar column (TC-FFAP; 0.53 mm internal diam × 15 m length, 1 μm film thickness; GL Sciences Inc.) was installed, and the gas chromatograph oven temperature was held at 50 °C for 1 min and then increased to 200 °C at 8 °C/min. A fully programmable injector (OPTIC 3; ATAS GL International, Eindhoven, The Netherlands) maintained at 40 °C for 10 s and then raised to 200 °C at 5 °C/s was used for large-volume injection; 10 μl of the sample were injected in splitless mode for 1 min. Helium was used as the carrier gas at 5 ml/min.
Bioassays
The attractiveness to males of each chromatographic fraction was assayed as described previously (Arai 2000). Adult males (10 individuals) were released into a glass dish (9 cm diam, 2 cm height). A sample (0.1 FDE) dissolved in 5 μl of hexane was loaded onto a piece of filter paper (Toyo Roshi Co. Ltd., Tokyo, Japan) and placed in the dish. After 5 min, the number of males mounted on the sample as a percentage of the number of males released was used as an index of attractiveness. Three replicates were performed for each sample in the glass dish assay.
Attractiveness of the synthetic pheromones was further confirmed by a trap bioassay in a greenhouse (3.0 × 4.8 × 2.8 m) located at NARO (Tsukuba, Ibaraki Prefecture, Japan), where mealybugs naturally occurred on potted potato plants. Males were captured by a white delta trap with a sticky board (12 × 22 cm) baited with 0.05 mg of the synthetic pheromone impregnated into a red rubber septum (8 mm outside diam × 19 mm height; Wheaton, Millville, NJ, USA). A trap baited with a blank (solvent only) septum was prepared as a control. One set of traps for each sample was placed 0.6 m above the ground at approximately 1.5 m intervals, and the assay was performed seven times with randomly exchange of the trap locations from 11 to 17 June 2016. Data were analyzed by using generalized linear mixed models with a Poisson error distribution to assess the effects of treatments and random effects to account for overdispersion of scores among the tests. The calculations were performed by the function ‘glmer’ of the lme4 package in the R statistical software package (version 3.2.5; R Development Core Team 2016). Multiple comparisons were performed with Bonferroni corrections.
Micro-Chemical Reactions
The compound proposed as pheromone (100 ng) was dissolved in 1 ml of 0.5 M KOH in ethanol solution for ethanolysis. The reaction mixture was held overnight at room temperature, poured into 1 ml of H2O, and extracted with hexane (3 × 1 ml). The combined extracts were concentrated to approximately 30 μl with an evaporator at room temperature, and an aliquot (2 μl) was used for GC-MS analysis. The residue was dissolved in 1 ml of CH2Cl2 and was re-esterified with 10 μl of 0.1 mM solution of 3-methyl-2-butenoic acid (senecioic acid; Tokyo Chemical Industry Co., Tokyo, Japan) in the presence of 10 μl each of 0.1 mM solution of 4-dimethylaminopyridine (DMAP; Tokyo Chemical Industry Co.) and N,N′-diisopropylcarbodiimide (DIC; Wako Pure Chemicals). The reaction mixture was stirred overnight at 40 °C, poured into 1 ml of H2O, and extracted with CH2Cl2 (3 × 1 ml). The combined extracts were dried over Na2SO4 and concentrated to approximately 30 μl with an evaporator at room temperature, and an aliquot (2 μl) was used for GC-MS analysis. For hydrogenation, the proposed pheromone (100 ng) was dissolved in 50 μl of ethanol, and the solution was stirred in the presence of platinum black catalyst in a H2 atmosphere. After 10 min, the solution was centrifuged, and an aliquot (2 μl) of the supernatant was used for GC-MS analysis.
Synthesis of Maconelliyl Senecioate
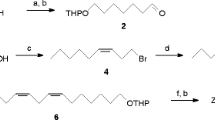
(R)- and (S)-(2,2-Dimethyl-3-isopropylidenecyclobutyl)methanol (maconelliol) were prepared from (+)- and (−)-α-pinene (>99 % ee; Sigma-Aldrich), respectively, by previously described methods (Zhang and Nie 2005; Zhang et al. 2004b). To a solution of each enantiomer of the alcohol (7.7 mg, 50 μmol) in CH2Cl2, senecioic acid (7.5 mg, 75 μmol), DIC (12 μl, 75 μmol), and DMAP (9.2 mg, 75 μmol) were added. After the reaction mixture was stirred at 40 °C for 24 h, the solvent was removed with an evaporator. The residue was poured into 2 ml of H2O and extracted with hexane (3 × 2 ml). The combined extract was washed with 4 ml of brine, dried over Na2SO4, concentrated, and purified by using silica gel chromatography with 5 % diethyl ether in hexane as an eluent to give maconelliyl senecioate (9.6–10.8 mg, 81–92 % yield); GC-MS (EI 70 eV): Rt 13.87 min (on DB-1), m/z 55 (9 %), 67 (4 %), 83 (33 %), 93 (15 %), 105 (10 %), 121 (100 %), 136 (37 %), 236 (0.3 %, M+); 1H–NMR δH: 1.14 [3H, s, (CH 3)(CH3)C<], 1.25 [3H, s, (CH3)(CH 3)C<], 1.40 [3H, s, (CH 3)(CH3)C = C<], 1.43 [3H, s, (CH 3)(CH3)C = CH], 1.51 [3H, s, (CH 3)(CH3)C = C<], 2.12 [1H, br, CHCHHC=], 2.13 [3H, s, (CH3)(CH 3)C = CH-], 2.23 [1H, m, CHCH2OCO], 2.51 [1H, br, CHCHHC=], 4.23 [2H, dd, J = 1.8, 8.4 Hz, CH 2OCO], 5.75 [1H, s, CH = C(CH3)2]; 13C–NMR δC: 18.6, 19.6, 20.0, 21.1, 26.9, 28.0, 28.6, 39.8, 44.4, 64.7, 116.7, 122.4, 137.7, 155.9, 166.3; (R)-maconelliyl senecioate: [α]22.0 D = −14.6 (observed, 99.8 % ee, c 0.181, hexane); (S)-maconelliyl senecioate: [α]22.0 D = +11.9 (observed, 71.8 % ee, c 0.130, hexane).
Results
Isolation of Pheromone
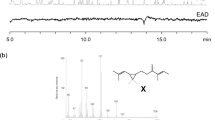
The crude collection of volatiles emitted by virgin female P. solenopsis (Fig. 1a) was fractionated into four fractions (0, 5, 15, and 50 % diethyl ether in hexane) by means of open-column chromatography on silica gel. Only the 5 % diethyl ether/hexane fraction exhibited attractiveness to male mealybugs (86.7 ± 13.3 %, mean ± SEM of males attracted). Esters are predicted to elute in this fraction, and it was separated further into seven fractions (Lc. 1–7) by means of HPLC (Fig. 1b). Males showed the strongest attraction toward Lc. 3 (93.3 ± 6.7 %). Lc. 3 was subjected to preparative GC and separated into three fractions (Gc. 1–3; Fig. 1c). Only Gc. 2, which consisted of a single compound, showed significant attractiveness (100 %), and this compound was assumed to be a component of sex pheromone. The amount of pheromone was approximately 0.2 ng per FDE.
Total ion chromatogram (TIC) from GC-MS analysis of collection of volatiles from virgin female Phenacoccus solenopsis (a); preparative HPLC chromatogram of 5 % diethyl ether/hexane fraction from silica gel open-column chromatography of the collection of volatiles (b); preparative GC chromatogram of HPLC fraction Lc. 3 (c); the candidate pheromone compound was isolated from GC fraction Gc. 2
Chemical Analyses
In GC-MS analyses, the isolated compound appeared at KI = 1598 on a DB-1 column. High-resolution electron-impact MS data (m/z 236.17845) showed that the molecular formula was C15H24O2 (calculated 236.17763) and indicated that four units of unsaturation (double-bonds or rings) were present. Characteristic mass fragments were observed at m/z 136 [M+ − 100 (C4H7COOH)], 121 [136–15 (CH3)], and 93 [136–43 ((CH3)2CH)] (Fig. 2a). Platinum-catalyzed heterogeneous hydrogenation generated a pair of diastereoisomers in approximately 3:1 ratio. These products had indistinguishable mass spectra with the molecular ion at m/z 240 (236 + 4), indicating that the original compound had two olefinic double bonds (Fig. 2b). The fact that the hydrogenation generated a pair of diastereomers suggests that one of the double bonds was either in a ring or attached to a ring containing an asymmetric carbon. Platinum-catalyzed heterogeneous hydrogenation of olefins proceeds via syn addition of H2, and one face of a double bond in or attached to a ring is usually favored over the other. Moreover, a unique fragment ion at m/z 170 [240–70 (C5H10)] was observed in the mass spectrum after hydrogenation. The same fragmentation pattern also was reported in the hydrogenation product of (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl 2-methylbutanoate (maconelliyl 2-methylbutanoate), and this fragmentation was interpreted as 2 + 2 retro-cleavage of a cyclobutane structure (Zhang et al. 2004a). Thus, the present candidate pheromone of P. solenopsis was suggested to have two olefinic double bonds and one C4 ring.
After basic ethanolysis, an alcohol with a molecular ion at m/z 154 [136 + 18 (H2O)] (Fig. 2c) and a transesterification product of an acid with a molecular ion at m/z 128 (Fig. 2d) were generated. The mass spectrum of the alcohol was almost identical to that reported for maconelliol by Zhang et al. (2004a). The GC-retention time (4.42 min) and the mass spectrum (Fig. 2d) of the esterified acid were identical to those of ethyl senecioate (Tokyo Chemical Industry Co.). Re-esterification of the ethanolysis product with senecioic acid restored the retention time and the mass spectrum pattern of the original compound. These results indicate that the compound was a senecioate of an alcohol similar to maconelliol.
Approximately 4 μg of the isolated candidate pheromone dissolved in 35 μl of C6D6 were placed in a micro-bottom NMR tube, and 1H–NMR spectra and 1H–1H correlation spectra were measured. 1H–NMR analysis showed a characteristic spectrum indicating one olefinic proton, six sets of methyl protons, two sets of methylene protons, and one methine proton (Fig. 3). Four of the methyl groups (singlets at 1.40, 1.43, 1.51, and 2.13 ppm) were indicated to be on double bonds and to form two isopropylidene groups, one of which was assumed to be part of a senecioyl group. The signal downfield at 2.13 ppm probably was affected by a spatially-close carbonyl oxygen and was characteristic of a senecioyl group. The other two methyl groups (singlets at 1.14 and 1.25 ppm) were consistent with being on a quaternary carbon. One olefinic proton (singlet at 5.75 ppm) was considered to be attached to a double bond in a senecioyl group. One set of methylene protons was observed at a characteristic chemical shift of 4.23 ppm, indicating that it was attached to a senecioyl group. This signal was coupled with the methine proton signal (2.23 ppm). The methine proton also was coupled with the other set of methylene protons. Because no other protons were observed, these methine and methylene were considered to be part of a C4 ring with quaternary carbons. Accordingly, the pheromone was deduced to be either (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl senecioate or (3,3-dimethyl-2-isopropylidenecyclobutyl)methyl senecioate. We considered the former to be more plausible, because long-range homoallylic coupling (CH–C = C–CH) was observed for the methylene protons in the four-membered ring; that is, we interpreted the broad signals of these two protons (2.12 and 2.51 ppm) as indicating long-range homoallylic coupling between them and the terminal methyl groups on an isopropylidene group. This idea was supported further by GC-MS data of the hydrogenated product (Fig. 2b); a characteristic fragment ion was observed at m/z 170 [240–70 (C5H10)], which was presumably generated by 2 + 2 retro-cleavage and was assumed to be m/z 184 [240–56 (C4H8)] if the compound was 3,3-dimethyl.
Synthesis, Absolute Configuration, and Activity
The pheromone candidate, (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl senecioate (maconelliyl senecioate), was synthesized via condensation of maconelliol and senecioic acid in the presence of DMAP and DIC. The chemical data including GC retention time, mass spectrum, and 1H–NMR spectrum of the synthetic candidate were identical to those of the natural pheromone.
The pheromone compound has a chiral center at the second carbon of the alcohol moiety (maconelliol). We therefore determined the absolute configuration by comparative analyses of the hydrolysis product of the natural pheromone and synthetic (R)- and (S)-maconelliol by GC on an enantioselective column. The GC retention time of the natural maconelliol (15.48 min) was identical to that of (R)-maconelliol rather than that of (S)-maconelliol (15.63 min). The pheromone therefore was concluded to be (R)-(−)-maconelliyl senecioate. The hydrolysis and re-esterification reactions used in this study did not result in any epimerization, as indicated by repeated analyses by enantioselective GC.
Finally, the biological activities of synthetic (R)- and (S)-maconelliyl senecioate were examined by a trap experiment in a greenhouse occupied by mealybugs. (R)-Maconelliyl senecioate (99.8 % ee) elicited strong and significant attractiveness to males; more than 30 males per day (mean value) were attracted and captured by (R)-enantiomer-baited traps. (S)-Maconelliyl senecioate (71.8 % ee) showed significant but relatively weak activity; the number of males attracted to the (S)-enantiomer was less than half the number that were attracted to the (R)-enantiomer (Fig. 4).
Attractiveness of (R)- and (S)-enantiomers of the synthetic pheromone to males in a greenhouse. Each of the enantiomers (0.05 mg) was released from a rubber septum. Significant differences are indicated with lowercase letters (multiple comparisons using generalized linear mixed models followed by the Bonferroni method, N = 7, P < 0.05)
Discussion
We isolated the sex pheromone from volatiles emitted by virgin adult females of the cotton mealybug, P. solenopsis, and determined it to be (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl 3-methybut-2-enoate (maconelliyl senecioate). To the best of our knowledge, the ester of maconelliol and senecioic acid has not been described previously as a natural product. Moreover, we confirmed the pheromone to be (R)-(−)-maconelliyl senecioate by comparisons of the natural and synthetic compounds in GC analyses by using a chiral stationary phase column. The synthetic (R)-enantiomer (99.8 % ee) showed strong attractiveness to male mealybugs. This study will, therefore, open the way for the development of a useful attractant for monitoring the emergence or invasion of P. solenopsis.
Traps baited with the other synthetic enantiomer, (S)-maconelliyl senecioate, attracted a substantial number of males. This suggests that (S)-enantiomer would not negatively affect male attraction, although whether the pure (S)-(+)-enantiomer has attractiveness remains unclear because the (S)-enantiomer prepared in this study was not enantiomerically pure (71.8 % ee). Partial epimerization was considered to have occurred during exothermic reactions for our preparation of (S)-maconelliol. Similar epimerization is reported in an original synthesis of maconelliol (Zhang et al. 2004b). However, this suggests it is not necessary to remove the opposite enantiomer potentially generated during synthesis of (R)-maconelliol for its development as a pest management tool, which is thus economically favorable.
Nineteen pheromone compounds from 16 mealybug species have now been reported. All of the compounds are esters of alcohols and carboxylic acids, and the alcohol moieties are generally monoterpenes, with one exception - isoprenol in Crisicoccus matsumotoi (Tabata et al. 2012). Four of the alcohols include cyclobutane structures (Fig. 5). Planococcus citri (Bierl-Leonhard et al. 1981) and Pseudococcus cryptus (Arai et al. 2003) use (2,2-dimethyl-3-isopropenylcyclobutyl)methyl esters, and Maconellicoccus hirsutus (Zhang et al. 2004a) and P. solenopsis use (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl (maconelliyl) esters. Both of these moieties have the same unusual non-head-to-tail (1–2′) linkage of isoprene units that is found in lavandulol (Sugie et al. 2008; Zhang et al. 2004a). Moreover, the second carbon of the alcohol moiety in each of these four pheromones is in the (R)-configuration. Thus, these four mealybugs may share a mechanism for biosynthesis of unusual cyclobutane structures via a unique connection of isoprene units, a mechanism that differs from the general monoterpene biosynthesis mechanism found in other organisms. Other than mealybugs, monoterpenes related to lavandulol with non-head-to-tail linkages are found in a few plants including Lavandula angustifolia (Lamiaceae) (Da Porto et al. 2009), Seseli indicum (Apiaceae) (Logani et al. 1967), and Artemisia spp. (Asteraceae) (Gunawardena et al. 2002).
Although the pheromones of P. solenopsis and M. hirsutus include maconelliol as the alcohol moiety in common, their acid moieties are different; the former is senecioate, and the latter is (S)-2-methylbutanoate (Fig. 5). The difference in the acid moiety is expected to act as a key for species recognition between the two species when they occur simultaneously on the same host plants. It is unlikely, however, that these two pheromones have co-evolved along with interspecies interferences, because P. solenopsis seems to be of Nearctic or Neotropical origin (Hodgson et al. 2008), whereas M. hirsutus is considered to be native to Indo-Malayan or Australasian regions (Williams 1996). A senecioate pheromone, (S)-lavandulyl senecioate, is reported in another mealybug species, Planococcus ficus (Hinkens et al. 2001). Further comparative studies of pheromone chemistry in mealybugs would provide interesting insights into the diversification of insect pheromone communication systems.
References
Abbas G, Arif MJ, Saeed S (2005) Systematic status of a new species of the genus Phenacoccus Cockerell (Pseudococcidae), a serious pest of cotton, Gossypium hirsutum L., in Pakistan. Pak Entomol 27:83–84
Arai T (2000) The existence of sex pheromone of Pseudococcus cryptus Hempel (Homoptera: Pseudococcidae) and a simple bioassay. Appl Entomol Zool 35:525–528
Arai T, Sugie H, Hiradate S, Kuwahara S, Itagaki N, Nakahata T (2003) Identification of a sex pheromone component of Pseudococcus cryptus. J Chem Ecol 29:2213–2223
Ben-Dov Y (1994) A systematic catalogue of the mealybugs of the world. Intercept, Andover, 686 p
Bierl-Leonhard BA, Moreno DS, Schwarz M, Fargerlund J, Plimmer JR (1981) Isolation, identification, and synthesis of the sex pheromone of the citrus mealybug, Planococcus citri (Risso). Tetrahedron Lett 22:389–392
Breitmaier E (2006) Terpenes. Wiley-VCH, Weinheim, 223 p
Da Porto C, Decorti D, Kikic I (2009) Flavour compounds of Lavandula angustifolia L. To use in food manufacturing: comparison of three different extraction methods. Food Chem 112:1072–1078
García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, Hardy NB (2016) ScaleNet: A literature-based model of scale insect biology and systematics. http://scalenet.info/ Accessed 27 July 2016
Gullan PJ, Downie DA, Steffan SA (2003) A new pest species of the mealybug genus Ferrisia Fullaway (Hemiptera: Pseudococcidae) from the United States. Ann Entomol Soc Am 96:723–737
Gunawardena K, Rivera SB, Epstein WW (2002) The monoterpenes of Artemisia tridentata ssp. vaseyana, Artemisia cana ssp. viscidula and Artemisia tridentata ssp. spiciformis. Phytochemistry 59:197–203
Gut LJ, Stelinski LL, Thomson DR, Miller JR (2004) Behaviour-modifying chemicals: prospects and constraints in IPM. In: Koul O, Dhaliwal GS, Cuperus GW (eds) Integrated pest management: potential, constraints and challenges. CABI Publishing, Oxford, pp. 73–122
Gutierrez AP, Ponti L, d’Oultremont T, Ellis CK (2008) Climate change effects on poikilotherm tritrophic interactions. Clim Chang 87:167–192
Hinkens DM, McElfresh JS, Millar JG (2001) Identification and synthesis of the sex pheromone of the vine mealybug, Planococcus ficus. Tetrahedron Lett 42:1619–1621
Hodgson C, Abbas G, Arif MJ, Saeed S, Karar H (2008) Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa 1913:1–35
Inada M (2016) Phenacoccus solenopsis Tinsley. Plant Protection Station, Ministry of Agriculture, Forestry and Fisheries Japan http://www.maff.go.jp/pps/j/guidance/pestinfo/pdf/pestinfo_108_6.pdf Accessed 27 July 2016
Jara V, Meza FJ, Zaviezo T, Chorbadjian R (2013) Climate change impacts on invasive potential of pink hibiscus mealybug, Maconellicoccus hirsutus (green), in Chile. Clim Chang 117:305–317
Logani MK, Varshney IP, Pandey RC, Dey S (1967) β-Cyclolavandulal, a new naturally occurring monoterpene type. Tetrahedron Lett 8:2645–2648
Millar JG, Daane KM, McElfresh JS, Moreira JA, Bentley WJ (2005) Chemistry and applications of mealybug sex pheromones. In: Petroski RJ, Tellez MR, Behle RW (eds) Semiochemicals in pest and weed control. American Chemical Society, Washington DC, pp. 11–27
R Development Core Team (2016) R: A language and environment for statistical computing. http://www.R-project.org/ Accessed on 15 April 2016
Sugie H, Teshiba M, Narai Y, Tsutsumi T, Sawamura N, Tabata J, Hiradate S (2008) Identification of a sex pheromone component of the Japanese mealybug, Planococcus kraunhiae (Kuwana). Appl Entomol Zool 43:369–375
Tabata J, Ichiki RT (2015) A new lavandulol-related monoterpene in the sex pheromone of the grey pineapple mealybug, Dysmicoccus neobrevipes. J Chem Ecol 41:194–201
Tabata J, Ichiki RT, Tanaka H, Kageyama D (2016) Sexual versus asexual reproduction: distinct outcomes in relative abundance of parthenogenetic mealybugs following recent colonization. Plos One 11:e0156587
Tabata J, Narai Y, Sawamura N, Hiradate S, Sugie H (2012) A new class of mealybug pheromones: a hemiterpene ester in the sex pheromone of Crisicoccus matsumotoi. Naturwissenschaften 99:567–574
Tabata J, Teshiba M, Shimizu N, Sugie H (2015) Mealybug mating disruption by a sex pheromone derived from lavender essential oil. J Essent Oil Res 27:232–237
Tanaka H, Tabata J (2014) A new record of Phenacoccus solenopsis Tinsley, 1898 from Kyushu district, Japan. Jpn J Entomol Soc 17:119–120
Tanaka H, Uesato T (2012) New records of some potential pest mealybugs (Hemiptera: Coccoidea: Pseudococcidae) in Japan. Appl Entomol Zool 47:413–419
Williams DJ (1996) A brief account of the hibiscus mealybug Maconellicoccus hirsutus (Hemiptera: Pseudococcidae), a pest of agriculture and horticulture, with descriptions of two related species from southern Asia. Bull Entomol Res 86:617–628
Zhang A, Nie J (2005) Enantioselective synthesis of the female sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus. J Agric Food Chem 53:2451–2455
Zhang A, Amalin D, Shirali S, Serrano MS, Franqui RA, Oliver JE, Klun JA, Aldrich JR, Meyerdirk DE, Lapointe SL (2004a) Sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus, contains an unusual cyclobutanoid monoterpene. Proc Natl Acad Sci U S A 101:9601–9606
Zhang A, Nie J, Khrimian A (2004b) Chiral synthesis of maconelliol: a novel cyclobutanoid terpene alcohol from pink hibiscus mealybug, Maconellicoccus hirsutus. Tetrahedron Lett 45:9401–9403
Zou Y, Millar JG (2015) Chemistry of the pheromones of scale and mealybug insects. Nat Prod Rep 32:1067–1113
Acknowledgments
We thank Dr. H. Tanaka (Kyushu University) for providing useful information regarding mealybug collection and taxonomy. NMR spectroscopy analyses were carried out with the support of Dr. S. Hiradate of the Advanced Analysis Center at NARO. J.T. gratefully acknowledges a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (no. 16 K08103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabata, J., Ichiki, R.T. Sex Pheromone of the Cotton Mealybug, Phenacoccus solenopsis, with an Unusual Cyclobutane Structure. J Chem Ecol 42, 1193–1200 (2016). https://doi.org/10.1007/s10886-016-0783-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0783-y