Abstract
We investigated foraging decisions by adult females of the common green bottle fly, Lucilia sericata, in accordance with their physiological state. When we gave female flies a choice between visually occluded, fresh canine feces (feeding site) and a CO2-euthanized rat (carrion oviposition site), 3-d-old “protein-starved” females responded equally well to feces and carrion, whereas protein-fed gravid females with mature oocytes responded only to carrion, indicating resource preferences based on a fly’s physiological state. Dimethyl trisulfide (DMTS) is known to attract gravid L. sericata females to carrion. Therefore, we analyzed headspace from canine feces by gas chromatographic-electroantennographic detection (GC-EAD) and GC/mass spectrometry. In bioassays, of the 17 fecal odorants that elicited GC-EAD responses from fly antennae, a blend of indole and one or more of the alcohols phenol, m-/p-cresol and 1-octen-3-ol proved as attractive to flies as canine feces. Unlike young females, gravid females need to locate carrion for oviposition and distinguish between fresh and aging carrion, the latter possibly detrimental to offspring. Gravid female L. sericata accomplish this task, in part, by responding to trace amounts of DMTS emanating from fresh carrion and by discriminating against carrion as soon it begins to produce appreciable amounts of indole, which is also the second-most abundant semiochemical in fresh canine feces, and apparently serves as an indicator of food rather than oviposition resources. Our results emphasize the importance of studying foraging choices by flies in accordance with their physiological stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects undergo significant morphological and physiological changes as they develop. Depending on the nutritional need of a stage, insects may seek and exploit different resources (Hochuli 2001; Stockhoff 1993). For example, mosquito females seek floral nectar and obtain energy (Gary and Foster 2006; Smith and Gadawski 1994). blood hosts that mature their ovaries (Qiu et al. 2011). and standing water for oviposition (Navarro-Silva et al. 2009; Osgood 1971).
Adult blowflies (Diptera: Calliphoridae) rely on floral nectar, animal feces, and carrion for survival and reproduction (Erzinclioglu 1996). Each resource presents a specific nutritional or reproductive benefit to blowflies: floral nectar provides carbohydrates (Grinfel’d 1955; Norris 1965), feces provide protein (Hanski 1987). and carrion commonly serves as an oviposition site (Byrd and Castner 2010; Norris 1965). Whether young or gravid flies respond similarly to each type of resource and readily distinguish between them based on odor profiles has rarely been investigated (but see Brodie et al. 2014).
Animal feces and manure are particularly odiferous protein sources. For example, >140 volatiles emanate from pig manure (O’Neill and Phillips 1992; Yasuhara and Fuwa 1979), three of which [3-methylbutanoic acid, indole, dimethyl trisulfide (DMTS)], in combination, are attractive to houseflies, Musca domestica (Cossé and Baker 1996). Similarly, of the many volatiles that emanate from bird droppings, five (ammonia, methylamine, dimethylamine, trimethylamine, and 1-pyrroline) attract tephritid fruit flies (Epsky et al. 1997; Robacker et al. 2000).
Blowflies frequently visit animal feces and obtain nutrients, but oviposit only on fresh carrion (Hanski 1987; Norris 1965). Gravid, but not recently eclosed, females of the common green bottle fly, Lucilia serricata, orient toward fresh carrion in response to DMTS (Brodie et al. 2014). but DMTS also emanates from feces, indicating that there must be additional semiochemicals that help blow flies distinguish at long range between these different resources. The composition of semiochemicals in a resource may change over time, indicated by older carrion being rejected by gravid blowflies as a suitable oviposition site (Archer and Elgar 2003; George et al. 2012; Hall and Doisy 1993). The semiochemical(s) that mediate the foraging shift by maturing flies from feces to fresh carrion, or those that render older carrion unsuitable for oviposition, are not known. Identifying them and demonstrating their effect on the behavior of flies were the major objectives of this study.
We worked with L. sericata as a representative blowfly species of the northern hemisphere (Hall 1948; Hall and Townsend, 1977) and as an early responder to animal carrion (Byrd and Castner, 2010; Hall and Doisy 1993). We focused on the response of female flies, because females undergo significant physiological changes as they mature and develop their oocytes, and, unlike males, must find opposition sites. We used recently deceased Norway rats, Rattus norvegicus, and mice, Mus musculus, as oviposition sites because they are commonly exploited by L. sericata and are sufficiently small to be deployed as baits in laboratory and field bioassays. We selected canine feces as a proven attractive protein source to flies (Lawson and Gemmell, 1990) because it could readily be collected immediately after defecation.
To unravel the mechanism(s) that underlie the foraging shift by maturing flies from fresh feces to fresh carrion, and the rejection of aged carrion by females, our specific objectives were to: (1) compare the attractiveness of fresh canine feces and fresh rat carrion to young and gravid flies; (2) obtain head space volatile (HSV) extract from fresh feces and bioassay its attractiveness to flies; (3) identify candidate semiochemicals in bioactive HSV extracts; (4) determine the key semiochemical(s) in HSV extracts; (5) monitor time-related changes in the odor profile of aging mouse carrion; and (6) test the effect of key semiochemical(s) on the acceptance or rejection of fresh mouse carrion.
Methods and Materials
Source of Rodents, Canine Feces, and Flies
Norway rats and mice were purchased by, and housed in, the Animal Care Facility of Simon Fraser University (ACF-SFU) or were purchased from a retailer (CTC Predator Feed, Duncan, BC, Canada). Rodents were CO2-euthanized in the ACF-SFU (Permit # 1042-12) and were incised post-mortem (Brodie et al. 2014) to simulate the death of an injured animal. Both rats and mice were used in our study because blowflies seek both for oviposition (Byrd and Castner, 2010) and because both became available from a rodent pheromone research project, when the animals were nearing the natural end of their life. Moreover, only mice were sufficiently small to fit as bait in Oak Stump traps (see below).
Dog feces served as an analytical resource and test stimulus, and originated from two healthy, mixed-breed, 5- and 9- yr-old dogs. The dogs’ diet consisted primarily of kibble (Science Diet® Lamb Meal and Rice Formula; Nutro®), and occasionally of table scrapes and grass. Fresh feces from both dogs was collected daily, combined, and homogenized to standardize odor cues, and tested within 6 h of defecation.
Experimental flies were reared in SFU’s insectary under a L16:D8 photoperiod, at 30–40 % RH and 23–25 °C. Every 12 mo, the colony was re-started using 50 wild-captured gravid female flies, increasing the colony to about 5000 flies at specific times, depending on experimental requirements.
General Bioassay Procedure
Two groups of flies, differing in age and reproductive status, were tested in bioassays: (1) young, 3–5-d-old females, and (2) aged, 10–12-d-old (gravid) females. Considering that recently eclosed young flies primarily seek nectar (carbohydrates; Brodie, unpublished data) and may have had only ephemeral access to animal feces, and that gravid flies will consume considerable protein for maturation of eggs (Barton Browne 2001). the group of young flies was given access only to water and sugar, whereas the group of aged, gravid flies was provided with proteinaceous milk and liver powder (Sigma Aldrich, St. Louis, MO, USA) in addition to sugar and water.
For each experimental replicate, 50 cold-sedated females (Table 1) were placed into an aluminum wire mesh cage (61 × 61 × 61 cm; Fig. 1a) and given 24 h to acclimate before the bioassay. Taking into account that the propensity of flies to respond can vary among days, and thus make experimental treatment effects less apparent, the same numbers of replicates of each treatment in experiments 1–4, 7–12, 13–18, and 19–22 were run concurrently (in parallel) on any given day, with treatments being applied to each cage in random order. Flies were tested only once for their response to a particular experimental treatment.
a Design of laboratory experiments recording alighting of flies on paired Ziploc storage containers (experiments 1–4) and on paired Solo cups (experiments 5–22) containing (i) canine feces (10 g) or nothing (empty control), (ii) rat carrion or nothing, and (iii) filter paper impregnated with (synthetic) semiochemicals or a solvent control; b design of laboratory experiment 23, which recorded captures of flies in paired Oak Stump traps covered in brown construction paper and baited with a mouse carcass (see c) or canine feces (20 g); c Oak Stump trap baited for field experiments 24 and 25 with a mouse carcass in a mesh bag; the white cotton ball was impregnated with either indole (100 μg) dissolved in ether or ether alone (control)
The test stimuli for laboratory experiments 1–4 are described in objective 1 (see below). For laboratory experiments 5–22, each test stimulus was placed on the bottom of a white paper Solo cup (0.5 l; 9 × 8.5 cm; Solo®, Lake Forest, IL, USA), which was then covered with 2-ply white cheesecloth (VWR, Radnor, PA, USA) to standardize visual cues (Fig. 1a). Each semiochemical stimulus (including control) was prepared in pentane and pipetted in 30-μl aliquots onto white filter paper (55 mm diam; Springfield Mill, UK). For all tests, one randomly assigned treatment and control cup were placed 10 cm apart in the center of the cage. Immediately thereafter, the number of flies that alighted on the cheesecloth of each cup was recorded for 5 min. and averaged across all replicates.
Objective 1: Compare the Attractiveness of Fresh Canine Feces and Fresh Rat Carrion to Young and Gravid Females
Experiments 1–4 (Table 1) offered young females (Exps. 1, 2) and gravid females (Exps. 3, 4) a choice between two Ziploc® plastic containers (828 ml; SC Johnson, Racine, WI, USA), covered in 2-ply cheese cloth to standardize visual cues, containing, in one set, canine feces (10 g; see above) or nothing (control) and, in another set, rat carrion or nothing. Ziploc® plastic containers were used instead of Solo cups in experiments 1–4 to house the larger rats (♂ mean = 463 g; ♀ mean = 224 g). Each rat was CO2-euthanized 5 h prior to testing and incised (Brodie et al. 2014) to simulate the death of an injured rat.
Objective 2: Obtain Head Space Volatile (HSV) Extracts from Fresh Canine Feces and Bioassay its Attractiveness to Flies
After placing an homogenized 10-g sample of fresh canine feces in a Pyrex® glass chamber (34 cm high × 12.5 cm wide), a pump drew charcoal-filtered air, at 0.5 l.min−1, for 5 h through the chamber and a glass column (6 mm o.d. × 150 mm) containing 200 mg of Porapak-Q™ (50–80 mesh) adsorbent (Byrne et al. 1975). Feces-derived headspace volatiles captured on Porapak-Q were desorbed in sequence using pentane (1 ml) and ether (1 ml). Pentane and ether extracts were concentrated and combined, such that 30-μl aliquots of HSV extract contained 10 g-hr equivalents of feces volatiles (i.e., the amount of volatiles given off by 10 g of feces over 1 h).
In experiment 5 (Table 1), aliquots of the HSV extract at 10 g-hr equivalents were then tested for attractiveness to flies, following the general experimental design described above. Because only young flies responded well to fresh canine feces (see Results), only they were tested for response to HSV extract of canine feces.
Objective 3: Identify Candidate Semiochemicals in Bioactive HSV Extracts
Aliquots of Porapak-Q HSV extract of canine feces were analyzed by gas chromatographic-electroantennographic detection (GC-EAD) and GC/mass spectrometry (MS), with procedures and equipment previously described in detail (Arn et al. 1975; Gries et al. 2002). For GC-EAD recordings (N = 10), an antenna was carefully pulled from the head of a 3–5-d-old female fly and suspended between two glass capillary electrodes (1.0 × 0.58 × 100 mm; A-M Systems, Carlsborg, WA, USA) filled with saline solution (Staddon and Everton 1980). Volatile components that elicited responses from at least seven out of 10 antennae were considered candidate semiochemicals. The GC-EAD apparatus utilized a Hewlett-Packard 5890 gas chromatograph (GC) fitted with a DB-5 GC column (30 m × 0.32 mm i.d.; J&W Scientific, Folsom, CA, USA). Helium was used as the carrier gas (35 cm.s−1) with the following column oven temperature program used: 50 °C for 5 min., 20 °C.min−1 to 280 °C. The injector port and flame ionization detector (FID) were set at 250 °C. Candidate semiochemicals were analyzed on a Saturn 2000 Ion Trap GC/MS operated in full-scan electron impact mode and fitted with a DB-5 column (50 m × 0.25 mm i.d.). Helium was the carrier gas (35 cm.s−1). The column oven temperature program was 50 °C for 1 min, 10 °C.min−1 to 280 °C (held for 10 min). The injector port and ion trap were set at 250 °C and 260 °C, respectively. Components that elicited responses from fly antennae were identified by comparing retention indices (Van den Dool and Kratz 1963) and mass spectra with those reported in the literature (Adams 1989; Jennings and Shibamoto 1980) and with those of authentic standards.
To confirm the structural assignment of candidate semiochemicals and to prepare synthetic blends (see objective 4), the following compounds were purchased: propanoic acid (>99.5 % chemically pure), 2-methylpropanoic acid (99 %), butanoic acid (>99 %), 2-methylbutanoic acid (>98 %), 3-methylbutanoic acid (99 %), phenylacetaldehyde (90 %), nonanal (95 %), decanal (>95 %), phenol (>95 %), 1-octen-3-ol (>98 %), m- and p-cresol (99 %), sulcatone (>98 %), geranylacetone (>97 %), indole (>99 %), dimethyl trisulfide (>95 %) (all Sigma Aldrich, St. Louis, MO, USA), and (E)-2-octenal (Bedoukian, Danbury, CT, USA).
Objective 4: Determine the Key Semiochemical(s) in Bioactive HSV Extracts
Experiment 6 (Table 1) tested a synthetic blend (SB) of all candidate semiochemicals in HSV extracts of feces that had elicited responses from fly antennae in GC-EAD recordings (see Results). The SB included propanoic acid, 2-methylpropanoic acid, butanoic acid, 2-methylbutanoic acid, 3-methybutanoic acid, phenylacetaldehyde, (E)-2-octenal, nonanal, decanal, phenol, 1-octen-3-ol, m- and p-cresol, sulcatone, geranylacetone, indole, and dimethyl trisulfide (DMTS). As m- and p-cresol could not be separated, they were both included in the SB. All components were prepared in pentane, in a ratio equivalent to that found in GC/MS analyses of HSV extracts, and were tested at 10 g-hr equivalents, following the general bioassay procedure described above.
With evidence that HSV extract of fresh canine feces and the SB were both effective in attracting flies (see ‘Results’), the next set of experiments was designed to determine the essential semiochemical(s) that mediate the response of flies to the SB. Accordingly, parallel-run experiments 7–12 (Table 1) tested the SB (experiment 7), and SBs that lacked groups of chemicals, including alcohols (experiments 8), aldehydes (experiment 9), ketones (experiment 10), acids (experiment 11), and N- or S-containing compounds (experiment 12). All SBs were tested following the general bioassay procedure described above.
With evidence that an alcohol and a N- or S-containing compound (DMTS or indole) were key semiochemicals in feces (see Results), experiments 13–18 (Table 1) tested the SB (experiment 13) and SBs lacking a specific alcohol, namely phenol (experiment 14), 1-octen-3-ol (experiment 15) or m/p-cresol (experiment 16), or lacking DMTS (experiment 17) or indole (experiment 18). All SBs were tested following the general bioassay procedure described above.
With evidence that indole, but not DMTS or any one specific alcohol, were key semiochemicals of feces (see Results), experiments 19–22 (Table 1) investigated potential interactive effects between components, by testing the SB (experiment 19), the three alcohols combined (phenol, 1-octen-3-ol, m-/p-cresol) (experiment 20), indole alone (experiment 21), and a mixture of the alcohols and indole (experiment 22). All synthetic stimuli were tested following the general bioassay procedure described above.
Objective 5: Monitor Changes over Time in Odor Profile of Aging Mouse Carrion
To quantify the amount of indole and DMTS in the headspace of mouse carrion, 10 CO2-euthanized mice (3 replicates) were placed in a Pyrex® glass aeration chamber, capturing the headspace on Porapak-Q (see Objective 2 and Table 1 for detailed methods). After the first 16 h of volatile capture, the Porapak-Q volatile traps were replaced every 12 h, up to 76 h. Aliquots of the Porapak-Q extracts were analyzed by GC/MS, using hexyl acetate as an internal standard.
Objective 6: Test the Effect of Key Semiochemical(s) on the Acceptance or Rejection of Mouse Carrion
With evidence that young and gravid flies differed in their resource preferences (see Results), and that indole was absent from fresh carrion, but essential for attraction of young flies to canine feces (see Results), experiments 23–25 tested the effect of fresh canine feces, or of indole specifically, on the response of flies to fresh mouse carrion. Laboratory experiment 23 and field experiment 24 (Table 1) tested the response of aged (13-d-old) flies and wild-type flies, respectively, to one mouse carcass at the bloat stage (24 h post CO2-euthanizition at room temperature) and the same type of mouse carrion in combination with fresh canine feces (20 g) applied to the outside of a mesh bag (50 × 63 mm; Fig. 1c) enclosing the carrion. The mesh bag was suspended from a wire above a soap water moat inside an Oak Stump trap covered with brown paper to occlude test stimuli. For each experimental replicate, 50 cold-sedated female flies were allowed to acclimate for 1 h before the two traps were placed 0.45 cm apart in opposite corners of the bioassay cage. After 1 h, the traps were removed, captured flies counted, and their reproductive status (non-gravid, gravid) determined by dissection of the ovaries (Adams and Reinecke 1979).
Field experiment 24 (13–15 September 2014; Table 1) was conducted on a berry farm (49°01′31.55″N, 122°18′43.61″W) next to a poultry farm in Abbotsford, BC, Canada. Testing the same two stimuli as in experiment 23 (mouse carrion with or without canine feces), Oak Stump traps were suspended 0.5 m above ground from a fence in randomized complete blocks with 10 m between traps within and among blocks. Near the end of the photophase of each of the 2 days, the number, sex, and physiological status (see above, experiment 23) of captured specimens of each fly species were determined. Combined data for days 1–2 are reported.
Field experiment 25 (21–23 September 2014; Table 1) had the same design as experiment 24, but the freshly deceased mouse carrion in each trap was combined with a cotton ball (Fig. 1c), impregnated with ether (control) or indole (100 μg) dissolved in ether, and attached to the mesh bag enclosing the carrion.
Statistical Analyses
All data were analyzed with JMP 11® (SAS Institute Inc., Cary, NC, USA). In experiments 1–4, 7–12, 13–18, and 19–22, the mean proportions of fly landings on paired Solo cups were analyzed by one-tailed t-tests, expecting a preferred stimulus to induce >65 % of the fly responses. Data (mean cumulative number of fly landings on treatment stimuli) in experiments 7–12 and 19–22 were log-transformed and evaluated for normality using a Q-Q plot. Within experiments 7–12, 13–18, and 19–22, respectively, the alighting responses of flies to treatments were analyzed by ANOVA followed by Tukey’s HSD test. The alighting responses of flies to treatments in experiments 5 and 6 were compared by a Wilcoxon test. Data in each of experiments 23–25 were analyzed using a non-parametric Wilcoxon signed rank test.
Results
Objective 1: Compare the Attractiveness of Fresh Canine Feces and Fresh Rat Carrion to Young and Gravid Females
Fresh canine feces received more (>65 %) alighting responses from young females than did the control (Fig. 2, Exp. 1: df = 9, t = 30.84, P < 0.001), whereas there was no difference (Fig. 2; Exp. 3: df = 9, t = −1.394, P = 0.896) in alighting responses of gravid females on fresh canine feces over the control. This indicates that fresh canine feces was attractive only to young flies. In contrast, fresh rat carrion received >65 % of the alighting responses from both young (Fig. 2; Exp. 2: df = 9, t = 33.99, P < 0.001) and gravid females (Fig. 2; Exp. 4: df = 9, t = 47.12, P < 0.001), indicating that fresh rat carrion was attractive to both young and gravid flies.
Mean (+SE) cumulative alighting responses by young and gravid female Lucilia sericata in experiments 1–4 (N = 10 each) on paired, cheesecloth-covered Ziploc plastic containers that were left empty (control) or baited with fresh canine feces or fresh rat carrion. In experiments 1, 2, and 4, an asterisk denotes that the treatment received more than 65 % of the alighting responses (one-tailed t-test; Exp. 1: t = 30.84, df = 9, P < 0.001; Exp. 2: t = 33.99, df = 9, P < 0.001; Exp. 4: t = 47.12, P < 0.001)
Objective 2: Obtain Head Space Volatile (HSV) Extract from Fresh Feces and Bioassay its Attractiveness to Flies
Aliquots of Porapak Q HSV extract of fresh canine feces received >65 % of the landing responses of flies, different from that of the control (Fig. 3; Exp. 5: t = 7.83, df = 13, P < 0.001), indicating that the essential semiochemical(s) associated with fresh canine feces were present in HSV extract.
Mean (+SE) cumulative alighting responses by young female Lucilia sericata in experiment 5 (N = 14) and 6 (N = 10) on paired cheesecloth-covered Solo cups containing filter paper impregnated with (i) headspace volatile (HSV) extract of fresh canine feces in pentane/ether at 10-g-hr equivalents (amount of volatiles given off by 10 g of feces over 1 h) (Exp. 5), and (ii) a synthetic blend (SB) of candidate semiochemicals (see Table 1) in pentane/ether vs. pentane/ether (control; Exp. 6). In experiments 5 and 6, an asterisk denotes that the treatment received more than 65 % of the alighting responses (one-tailed t-test; Exp. 5: t = 7.83, df = 13, P < 0.001; Exp. 6: t = 6.69, df = 9, P < 0.001)
Objective 3: Identify Candidate Semiochemicals in Bioactive HSV Extracts
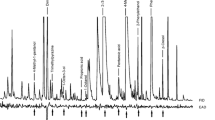
In GC-EAD analyses of HSV extract of fresh canine feces, 12 compounds consistently elicited responses from fly antennae (Fig. 4): phenylacetaldehyde, (E)-2-octenal, nonanal, decanal, phenol, 1-octen-3-ol, m- and p-cresol, sulcatone, geranylacetone, indole, and DMTS. Antennal responses to propanoic acid, 2-methylpropanoic acid, butanoic acid, 2-methylbutanoic acid, and 3-methybutanoic acid varied greatly between runs, likely due to poor chromatography of these acids on the GC column.
Representative recordings from a gas chromatographic flame ionization detector (FID) and an electroantennographic detector (EAD; of female Lucilia sericata antenna) to an aliquot of Porapak Q headspace extract of fresh canine feces. Antennal responses to propanoic acid (1), 2-methylpropanoic acid (2), butanoic acid (3), 3-methybutanoic acid (4), and 2-methylbutanoic acid (5) varied greatly between runs due to poor chromatography of these acids and are not depicted here. Other EAD-active odorants: dimethyl trisulfide (6), phenol (7), 1-octen-3-ol (8), sulcatone (9), phenylacetaldehyde (10), (E)-2-octenal (11), meta- and/or para-cresol (12, 13), nonanal (14), decanal (15), indole (16), and geranylacetone (17)
Objective 4: Determine the Key Semiochemical(s) in Bioactive HSV Extracts
The SB of candidate semiochemicals from fresh canine feces had more (Fig. 3; Exp. 6: t = 6.69, df = 9, P < 0.001) landing than the solvent control, indicating that the SB contained key semiochemicals associated with canine feces. Moreover, the mean response of flies to the SB (Exp. 6: 70.0 ± 7.3 landings) and to HSV extract of feces (Exp. 5: 62.6 ± 5.1 landings) did not differ (Z = 140.5, df = 1, P = 0.380), indicating further that the SB contained all the key volatile semiochemical(s) of canine feces.
The response of young females to the complete SB or to SBs lacking specific components differed (Exps. 7-12; F (5,54) = 4.45, P = 0.002; Fig. 5). In comparison to the control, the complete SB and SBs lacking acids, aldehydes, or ketones all received >65 % of landing responses from flies (df = 9 each; Exp. 7: t = 7.45, P < 0.001; Exp. 8: t = 6.46, P < 0.001; Exp. 9: t = 7.09, P < 0.001; Exp. 11: t = 5.79, P < 0.001; Fig. 5). A preferred response was not evident for SBs lacking alcohols (Exp. 10: t = 1.29; P < 0.115) or indole and DMTS (Exp. 12: t = −1.46; P < 0.911), indicating that at least one alcohol, as well as indole and/or DMTS, mediate attraction of young females to fresh canine feces.
Mean (+SE) cumulative number of alighting responses by young females of Lucilia sericata in experiments 7–22 (N = 10 each) on paired cheesecloth-covered Solo cups containing filter paper impregnated with (i) a synthetic blend (SB; see Table 1) of the 17 components that elicited antennal responses in headspace volatile extracts of canine feces (see Fig. 4), (ii) partial blends of these components, or (iii) 1–3 select key components. Filter paper impregnated with the corresponding amount of pentane was the control. In each experiment, an asterisk denotes a treatment that received more than 65 % of alighting responses (one-tailed t-test: P < 0.001). Within experiments 7–12, 13–18, and 19–22, bars with different letters indicate differences in the number of alighting responses by young female flies (Tukey’s HSD: P < 0.05)
The response of young females to the complete SB or to SBs lacking a specific component differed (Exps. 13–18; F (5,54) = 10.5, P < 0.001; Fig. 5). In comparison to the control, the complete SB and SBs lacking phenol, 1-octen-3-ol, p/m-cresol, or DMTS all received >65 % of the landing responses of flies (df = 9 each; Exp. 13: t = 4.73, P = 0.001; Exp. 14: t = 5.96, P < 0.001; Exp. 15: t = 2.73, P = 0.023; Exp. 16: t = 3.82, P = 0.004; Exp. 17: t = 7.30, P < 0.001; Fig. 5), indicating that neither of these odorants alone mediates attraction of young females to fresh canine feces. In contrast, the SB lacking indole failed to receive >65 % of the landing responses of flies (Exp. 18: t = 0.70, P = 0.22; Fig. 5), indicating that the presence of indole is important for attracting young females to fresh canine feces.
The response of young females to test stimuli consisting of the complete SB, three alcohols (phenol, 1-octen-3-ol, m- and p-cresol), indole, or a mixture of the alcohols and indole differed (Exps. 19–22; F (3,36) = 18.03, P < 0.001; Fig. 5). In comparison to the solvent control, the complete SB and the mixture of alcohols and indole both received >65 % of the landing responses of flies (df = 9 each; Exp. 19: t = 11.81, P < 0.001; Exp. 22: t = 9.62, P < 0.001; Fig. 5). The same type of preferred response was not evident when we tested the alcohols (Exp. 20: df = 9, t = 0.44, P = 0.439; Fig. 5) or indole (Exp. 21: df = 9, t = 0.04, P = 0.489; Fig. 5), which received 12.9 ± 3.3 fly landings and 12.6 ± 4.6 fly landings, respectively, five times fewer than that received by the mixture of alcohols and indole, indicating synergistic attractiveness between indole and one or more of the alcohols.
Objective 5: Monitor Changes over Time in Odor Profiles of Aging Mouse Carrion
During 16 hr post mortem, indole was detectable in only one of three replicates (10 mice carcasses per replicate) and in only a small amount (mean of <0.01 μg; Fig. 6), whereas DMTS was detectable in all three replicates, averaging 1.33 μg (Fig. 6). During the subsequent 12-h periods, the amounts of DMTS and indole released from mice carcasses increased substantially. At each sampling interval, the amount of DMTS exceeded the amount of indole by 13–3 times (Fig. 6).
Objective 6: Test the Effect of Key Semiochemical(s) on the Acceptance or Rejection of Mouse Carrion
In laboratory experiment 23 with (>90 %) gravid females, traps baited with both mouse carrion and canine feces captured fewer females (Z = −3.745, df = 1, P < 0.001; Fig. 7) and fewer gravid females (Z = −3.75, df = 1, P < 0.001; Fig. 7) than traps baited with mouse carrion alone, revealing a repellent effect of canine feces on the responses of oviposition-site-seeking flies. In contrast, non-gravid females responded equally to both baits (Z = −1.793, df = 1, P = 0.073; Fig. 7), consistent with prior results (Fig. 2, exps. 1, 2) that canine feces and recently deceased rat carrion are equally attractive to protein-hungry flies with immature oocytes.
Mean (±SE) number of Lucilia sericata females captured in paired Oak Stump traps baited with (i) one mouse carcass alone or in combination with fresh canine feces (20 g) (laboratory experiment 23, 24-h-old mouse carcass; field experiment 24, fresh mouse carcass), or (ii) one fresh mouse carcass alone or with indole (100 μg) dissolved in ether and applied to a cotton ball (field experiment 25). In each experiment, an asterisk denotes the stimulus that attracted more flies of a particular reproductive stage (Wilcoxon signed rank test; P < 0.05).
The type of trap bait had an effect on captures of wild female flies (Z = −2.293, df = 1; P = 0.022, Fig. 7, exp. 24). Traps baited with both mouse carrion and canine feces attracted more non-gravid flies (Z = −2.973, df = 1, P = 0.003) than traps baited with mouse carrion alone, indicating a preference of these flies to the proteinaceous feces. However, there was no preference for either bait by gravid flies (Z = −0.873, df = 1, P = 0.383).
In field experiment 25, the type of trap bait had no effect on the overall number of wild flies captured (Z = 0.325, df = 1, P = 0.985, Fig. 7). However, traps baited with both mouse carrion and indole (an indicator semiochemical of feces) captured fewer gravid female flies than traps baited with mouse carrion alone (Z = −2.89, df = 1, P = 0.004). In contrast, captures of non-gravid females in the same paired traps were not affected by the presence of indole (Z = −0.38, df = 1, P = 0.704).
Discussion
Our data demonstrate that: (1) the physiological state of L. sericata females affects their resource preference, with fresh canine feces attracting young (protein-hungry) flies but not gravid flies, and fresh rat carrion attracting both young and gravid flies; (2) attraction of young flies to canine feces is mediated by semiochemicals, of which indole and one or more of phenol, m-/p-cresol, and 1-octen-3-ol play key roles; (3) at an advanced, but not early, stage of decay, mouse carrion produces indole; and (4) fresh canine feces, or indole, repels gravid females seeking oviposition sites, indicating that indole signifies the presence of animal feces (protein resources) rather than an oviposition site, or that carrion is at an advanced stage of decay and thus unsuitable for oviposition.
Indole was the second most abundant (18 %) odorant in feces that elicited responses from L. sericata antennae. It has a strong fecal odor (Jensen et al. 1995) and is produced during the degradation of tryptophan, a major building block of proteins (Whitley and Thornton 2012). by intestinal bacteria, such as Escherichia coli (Dawes 1948; Schulz and Dickschat 2007). Enterobacter spp., Klebsiella spp. (Schulz and Dickschat 2007). Lactobaccillus spp., and Clostridium spp. (Jensen et al. 1995) that are present in most animal feces. Both its relative abundance and the fly’s inherent sensitivity could make indole a reliable foraging cue for L. sericata females seeking feces for protein.
While indole is essential for attracting L. sericata females, its attractiveness hinges on the presence of one or more of the alcohols phenol, m-/p-cresol, or 1-octen-3-ol. Unlike indole, which has moderate volatility and thus likely serves as a long-range attractant, the more volatile alcohols could function as close-range attractants to help blowflies pinpoint the location of feces. An analogous system was reported for the aggregation pheromone of the bark beetle Ips typographus, in which the less volatile pheromone component (4S)-cis-verbenol attracts beetles over long distances, whereas the more volatile pheromone component 2-methyl-3-buten-2-ol attracts beetles at short range (Bakke et al. 1977; Schlyter et al. 1987).
The three fecal alcohols exhibit some degree of redundancy, because omitting any one from our SB did not reduce blend attractiveness (Fig. 5, Exps. 14–16). Intense fecal odor typically is associated with recently deposited, and thus moist, feces, and less so with dry feces. As the alcohols may dissipate more quickly than other components such as indole, their decreasing contribution to the odor bouquet might reflect the decreasing moisture content of feces. This would be important to foraging blowflies that have difficulty feeding on dry feces (Hanski 1987).
There are contrasting reports on the response of calliphorid flies to indole or feces. Indole previously was not known to attract calliphorid flies to animal feces, but was known to be part of a semiochemical blend that induced attraction to suboptimal oviposition sites, such as larval waste from artificial rearing material (Chaudhury et al. 2014) and fleece from an oviposition host (Eisemann 1995). Indole also had no effect on the attraction of blowflies in studies that investigated the attractiveness of oviposition sites rather than food sources, and that exclusively bioassayed the response of protein-fed flies (Easton and Feir 1991; Frederickx et al. 2011). The results of these previous studies and our own data indicate that age, physiological need, and reproductive status of blowflies affect their propensity to respond to semiochemicals from specific resources.
Our conclusion that fecal semiochemicals, in general, and indole, in particular, are repellent to gravid, oviposition site-seeking L. sericata females is supported by both laboratory and field data. Gravid L. sericata females were not more attracted to canine feces than they were to unattractive control stimuli, but strongly preferred mouse carrion over canine feces, and discriminated against mouse carrion when presented in combination with indole. In contrast, young flies were strongly attracted to canine feces, headspace extract of canine feces, and to various blends of synthetic fecal semiochemicals. Avoiding aging carrion, through perception of indole, as oviposition sites may help gravid L. serricata females minimize adverse fitness effects for their progeny. If females were to oviposit on animal carrion during later stages of decay, they might subject their offspring to predation by scavenging vertebrates or to resource competition by detritus-consuming insects, fungi, or microbes (DeVault et al. 2003). These might explain why gravid female flies typically do not oviposit on carrion at advanced stages of decomposition (Huntington et al. 2008). That gravid females and other flies still respond to these resources might be explained by their searching for food (which liquefied carrion provides) or for mates (which often gather on or near food resources) (Archer and Elgar 2003).
In summary, the resource preference of L. sericata females depends on their physiological state. Young, protein-seeking L. sericata females are strongly attracted to feces and to carrion. We demonstrated that they respond as readily to fresh canine feces as they do to a semiochemical blend of indole and one or more of the alcohols phenol, m-/p-cresol, and 1-octen-3-ol. In contrast, gravid females, which have fed already on protein, need to locate carrion for oviposition and to distinguish between fresh and aging carrion, with recently deceased carcasses (oviposition resources) likely providing less resource competition for offspring. Female L. sericata appear to accomplish this, in part, by responding to trace amounts of DMTS that emanate from fresh carrion and by discriminating against carrion as soon it begins to produce appreciable amounts of indole, which is the second most abundant semiochemical in fresh canine feces and apparently serves as an indicator of food rather than oviposition resources. Our results emphasize the importance of studying foraging choices by flies, and possibly insects, in accordance with physiological stage. Being cognizant of the physiological state of bioassay insects should facilitate better interpretation of experimental data, more accurate conclusions about foraging choices, and help facilitate development of Earth-friendly management of insect pests in urban, agricultural, or forest settings.
References
Adams RP (1989) Identification of essential oils by ion trap mass spectroscopy. Academic Press, Inc., San Diego, p 302
Adams TS, Reinecke JM (1979) Reproductive physiology of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae). 1. Oogenesis. J Med Entomol 15:472–483
Archer MS, Elgar MA (2003) Effects of decomposition on carcass attendance in a guild of carrion breeding flies. Med Vet Entomol 17:263–271
Arn H, Städler E, Rauscher S (1975) Electroantennagraphic detector—selective and sensitive tool in gas-chromatographic analysis of insect pheromones. Z Naturforsch C 30:722
Bakke A, Froyen P, Skattebol L (1977) Field response to a new pheromonal compound isolated from Ips typographus. Naturwissenschaften 64:98–99
Barton Browne L (2001) Quantitative aspects of the regulation of ovarian development in selected anautogenous Diptera: Integration of endocrinology and nutrition. Entomol Exp Appl 100:137–149
Brodie B, Gries R, Martins A, VanLaerhoven S, Gries G (2014) Bimodal cue complex signifies suitable oviposition sites to gravid females of the common green bottle fly. Entomol Exp Appl 153:114–127
Byrd JL, Castner JH (2010) Insects of forensic importance. In: Byrd JH, Castner JL (eds) Forensic entomology: the utility of arthropods in legal investigations. CRC Press/ Taylor & Francis Group, Boca Raton, pp 41–73
Byrne KJ, Gore WE, Pearce GT, Silverstein RM (1975) Porapak-Q collection of airborne organic compounds serving as models for insect pheromones. J Chem Ecol 1:1–7
Chaudhury MF, Zhu JJ, Sagel A, Chen H, Skoda SR (2014) Volatiles from waste larval rearing media attract gravid screwworm flies (Diptera: Calliphoridae) to oviposit. J Med Entomol 51:591–595
Cossé AA, Baker TC (1996) House flies and pig manure volatiles: wind tunnel behavioral studies and electrophysiological evaluations. J Agric Entomol 13:301–317
Dawes EA (1948) Production of indole by Escherichia coli. Nature 162:229–231
DeVault TC, Rhodes OE Jr, Shivik JA (2003) Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 102:225–234
Easton C, Feir D (1991) Factors affecting the oviposition of Phaenicia sericata (Meigen) (Diptera: Calliphoridae). J Kansas Entomol Soc 64:287–294
Eisemann CH (1995) Orientation by gravid Australian sheep blowflies, Lucilia cuprina (Diptera: Calliphoridae), to fleece and synthetic chemical attractants in laboratory bioassays. Bull Entomol Res 85:473–477
Epsky ND, Dueben BD, Heath RR, Lauzon CR, Prokopy RJ (1997) Attraction of Anastrepha suspensa (Diptera: Tephritidae) to volatiles from avian fecal material. Fla Entomol 80:270–277
Erzinclioglu Z (1996) Blowflies. The Richmond Publishing Co. Ltd, Great Britain, pp 1–69
Frederickx C, Dekeirsschieter J, Verheggen FJ, Haubruge E (2011) Responses of Lucilia sericata Meigen (Diptera: Calliphoridae) to cadaveric volatile organic compounds. J Forensic Sci 57:386–390
Gary RE, Foster WA (2006) Diel timing and frequency of sugar feeding in the mosquito Anopheles gambiae, depending on sex, gonotrophic state and resource availability. Med Vet Entomol 20:308–316
George KA, Archer MS, Toop T (2012) Effects of bait age, larval chemical cues and nutrient depletion on colonization by forensically important calliphorid and sarcophagid flies. Med Vet Entomol 26:188–193
Gries R, Khaskin G, Gries G, Bennett RG, King SGG, Morewood P, Slessor KN, Morewood WD (2002) (Z, Z)-4,7-Tridecadien-(S)-2-yl-acetate: sex pheromone of Douglas-fir cone gall midge, Contarinia oregonesis. J Chem Ecol 28:2283–2297
Grinfel’d EK (1955) Diptera feeding on nectar and pollen and their role in the pollination of plants. Vestn Leningr Univ 10:15–25
Hall DG (1948) The blowflies of North America. Thomas Say Foundation, Baltimore, pp 1–477
Hall RD, Doisy KE (1993) Length of time after death—effect on attraction and oviposition or larviposition of midsummer blow flies (Diptera, Calliphoridae) and flesh flies (Diptera, Sarcophagidae) of medicolegal importance in Missouri. Ann Entomol Soc Am 5:589–593
Hall RD, Townsend LH (1977) The blow flies of Virginia (Diptera: Calliphoridae). Va Polytech Inst Bul 123:1–48
Hanski I (1987) Nutritional ecology of dung- and carrion-feeding insects. In: Slansky F, Rodriguez JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. John Wiley & Sons, New York, pp 837–884
Hochuli DF (2001) Insect herbivory and ontogeny: how do growth and development influence feeding behaviour, morphology, and host use? Austral Ecol 26:563–570
Huntington TE, Carter DO, Higley LG (2008) Testing multigenerational colonization of carrion by blow flies in the great plains. Great Plains Res 18:33–38
Jennings W, Shibamoto T (1980) Qualitative analysis of flavor and fragrance volatiles by glass capillary gas chromatography. Academic, New York, p 465
Jensen MT, Cox RP, Jensen BB (1995) 3-Methylindole (skatole) and indole production by mixed populations of pig fecal bacteria. Appl Environ Microbiol 61:3180
Lawson JR, Gemmell MA (1990) Transmission of taeniid tapeworm eggs via blow flies to intermediate hosts. Parasitology 1:143–146
Navarro-Silva MA, Marques FA, Duque JE (2009) Review of semiochemicals that mediate the oviposition of mosquitoes: a possible sustainable tool for the control and monitoring of Culicidae. Rev Bras Entomol 53:1–6
Norris KR (1965) The bionomics of blow flies. Annu Rev Entomol 10:47–68
O’Neill DH, Phillips VR (1992) A review of the contor of odour nuisance from livestock buildings: part 3, properties of the odorous substances which have been identified in livestock wastes or in the air around them. J Agric Eng Res 53:23–50
Osgood CE (1971) An oviposition pheromone associated with the egg rafts of Culex tarsalis. J Econ Entomol 64:1038–1041
Qiu YT, Smallegange RC, Van Loon JJ, Takken W (2011) Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med Vet Entomol 25:247–255
Robacker DC, Garcia JA, Bartelt RJ (2000) Volatiles from duck feces attractive to Mexican fruit fly. J Chem Ecol 26:1849–1867
Schlyter F, Löfqvist J, Byers JA (1987) Behavioural sequence in the attraction of the bark beetle Ips typographus to pheromone sources. Physiol Entomol 12:185–196
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Prod Rep 24:814–842
Smith SM, Gadawski RM (1994) Nectar feeding by the early-spring mosquito Aedes provocans. Med Vet Entomol 8:201–213
Staddon BW, Everton IJ (1980) Hemolymph of the milkweed bug Oncopeltus fasciatus (Heteroptera: Lygaeidae) inorganic constituents and amino-acids. Comp Biochem Physiol A Physiol 65:371–374
Stockhoff BA (1993) Ontogenetic change in dietary selection for protein and lipid by gypsy moth larvae. J Insect Physiol 39:677–686
Van den Dool H, Kratz PD (1963) A generalization of retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11:463–471
Whitley BL, Thornton SH (2012) Tryptophan: dietary sources, functions and health benefits. Nova Science Publishers Inc, New York, p 42
Yasuhara A, Fuwa K (1979) Volatile and odorous components in solid swine manure. Agric Biol Chem 43:313–316
Acknowledgments
We thank two anonymous reviewers for constructive comments; the Dhaliwal family for granting permission to set up field experiments on their farm; and staff members of SFU’s Animal Care Facility for providing rodents and assisting with euthanizing experimental rodents. The research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC)–Industrial Research Chair to G.G., with The Scotts Miracle-Gro Company (Canada) as the industrial sponsor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brodie, B.S., Babcock, T., Gries, R. et al. Acquired Smell? Mature Females of the Common Green Bottle Fly Shift Semiochemical Preferences from Feces Feeding Sites to Carrion Oviposition Sites. J Chem Ecol 42, 40–50 (2016). https://doi.org/10.1007/s10886-015-0658-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0658-7