Abstract
Scents emitted from excretions provide important information about the owner. Volatile compounds with higher levels in a species and/or sex, or that vary among individuals could be odor cues for species and/or sex, or individual recognition. However, such compounds have been identified in only a few vertebrate species. In domestic cats (Felis silvestris catus), it is known that unburied cat feces are territorial markers asserting the border of their home range, but little was known which fecal compounds are scent cues for species, sex, and individual recognition in cats. In the present study, we demonstrated the chemical basis for species, sex, and individual recognition using feces of cats. For males, major contents were fatty acids and 3-mercapto-3-methyl-1-butanol (MMB), a derivative of the unusual amino acid, felinine. MMB emission levels from feces had sex-based differences (male > female) and dynamic temporal changes during aging. Cats distinguished fecal odors with and without MMB, and different fatty acid compositions among individuals. No cat-specific compound, such as MMB, was detectable from their anal odor emitting fatty acids. We concluded that fecal MMB is a male sex recognition pheromone in cats and also provides a temporal trace of the owner. After sensing MMB, they may distinguish individual differences of conspecific feces with variable subsets of fatty acids. In contrast to scent marks, since cats can obtain species information from visual cues before sniffing conspecific anal odors, they may use their efforts to distinguish individual differences of anal odors during sniffing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ability for species, sex, and individual recognition is essential for complex interactions among a number of mammals that share a habitat (Wyatt 2014). Most mammals use metabolic waste products from the body, such as urine and feces, for scent communication. These metabolic wastes contain a variety of volatile and non-volatile organic compounds, some of which function as odor cues providing information regarding their owners (Apfelbach et al. 2005; Hurst and Beynon 2004). Volatile compounds whose concentrations are markedly higher in only certain species and sexes may act as odor cues for species and sex recognition, respectively. Previous studies identified such volatile compounds in experimental mice and some wild mammals, such as (methylthio)methanethiol (MTMT) in male mouse urine (Lin et al. 2005) and 2,3,5-trimethyl-3-thiazoline (TMT) in fox feces (Vernet-Maury et al. 1992). Alternatively, variable subsets of compounds in an animal’s chemical profile function as signature mixtures that are learnt for individual recognition by other animals (Wyatt 2010). For example, the small Indian mongoose (Herpestes auropunctatus) uses individual differences in fatty acid composition emitted from anal sac secretions for individual identification (Gorman 1976, Miyazaki et al. 2018a).
The domestic cat (Felis silvestris catus) performs scent marking by spraying urine onto a vertical surface (Feldman 1994; Natoli 1985). Cat urine contains a large amount of an unusual amino acid known as felinine that is detectable only in small felids, such as the domestic cat, bobcat (Lynx rufus), and lynx (Eurasian lynx), but uncommon in other species, including large felids (Hendriks et al. 1995a; Westall 1953). Odorless felinine decomposes into a volatile mercaptan, 3-mercapto-3-methyl-1-butanol (MMB), with a characteristic sulfur odor (Miyazaki et al. 2006a). Cat urine also emits MMB analogs, 3-mercapto-3-methylbutyl formate and 3-methyl-3-methylthio-1-butanol that have never been detected in mammals other than small felids. These volatile compounds are strong candidate scent signals for conspecific recognition in cats (Hendriks et al. 1995a; MacDonald et al. 1984; Miyazaki et al. 2006b).
Cats also use feces for scent marking (Feldman 1994). Although most cats bury their feces after defecation when within their home range or near nesting sites, their feces are left on the ground without burying in peripheral areas, and are likely used as a territorial marker around the border of their home range (Crowell-Davis et al. 2004). Previous study reported that cats distinguish the fecal odors of familiar individuals and unfamiliar conspecifics (Nakabayashi et al. 2012). However, it is not clear which fecal volatile compounds contribute to species, sex, and individual recognition in cats. We hypothesized that cat feces used for territorial marking emit species-specific volatile compounds, such as MMB, as well as their urine. In addition, such compounds are necessary to alert other mammals in the same area that the scent owners are cats.
Volatile compounds emitted from urine and anal sac secretions have been well-examined in several felids (Apps et al. 2017; Apps et al. 2014; Dehasse 1997; Mattina et al. 1991; Miyazaki et al. 2017; Miyazaki et al. 2018b; Vogt et al. 2016); however, our knowledge of scent communication using feces is limited in felids. The main purpose of this study was to understand the chemical basis of species, sex, and individual recognition using feces as scent marks in domestic cats. We analyzed fecal volatile compounds to identify odor cues for species, sex, and individual recognition in cats. Our recent study found that felinine is excreted into the feces via bile from the liver in cats (Futsuta et al. 2018), suggesting that cat feces also emit MMB. Therefore, we analyzed volatile chemical profiles of male and female cat feces, focusing on the determination of whether MMB is emitted from cat feces. Then, we determined whether cats could distinguish candidate odor cues for species, sex, and individual recognition. We also discussed differences in chemical profiles between fecal odor and body odors around the anus through which cats obtain species and sex information for scent owners from visual cues before sniffing. These studies will improve our understanding of scent communication using scent marks and body odor in mammals other than mice, especially small felids. Furthermore, our findings will help develop deodorants for the neutralization of the distinctive smell of cat feces that the general public finds unpleasant.
Methods and Materials
Animals
This study used 15 laboratory mixed breed cats (2–4 years old), including nine intact males (ID. M1-M9) and six intact females (ID. F1-F6) that were non-estrus. These cats were individually housed in metabolic cages in an experimental animal room controlled at 22 °C under 12 hr lighting. Cats were maintained on a commercial dry diet (Dr.’s Diet, pH Ade. Novartis Co., Tokyo, Japan) and water. Fresh fecal samples were obtained from the cats by checking each cage hourly between 8:30 am and 3:30 pm. Fresh fecal samples were used for the following studies within 30 min of collection. This study followed local animal ethics guidelines and was approved by the Animal Research Committee of the Faculty of Agriculture of Iwate University.
Thermal Desorption-Gas Chromatography Mass Spectrometry
After introducing fresh cat feces (1 g) into a 20 ml glass vial (GESTEL, Mülheim an der Ruhr, Germany), the headspace of the sample was concentrated into a Tenax TA tube (Shimadzu, Kyoto, Japan) at 40 °C by purging with pure nitrogen gas at 50 ml/min for 90 min. Body odors were sampled in 10 cats (M1-M5 and F1-F5) who were anesthetized with isoflurane. Volatile compounds emitted from the neck and the perianal area were concentrated into the Tenax TA tube using a pump device (GSP-30FT-2, Gastec Co., Kanagawa, Japan) at 50 ml/min for 90 min. Room air samples were also prepared using the same method, and served as blank references. Volatile compounds trapped in the Tenax TA tube were analyzed using the thermal desorption (TD-20, Shimadzu Co.) gas chromatography-mass spectrometry (GC-MS) (QP-2010 Ultra, Shimadzu Co.) system using the following conditions; Stabilwax column (60 m × 0.32 mm i.d. × 0.5 μm film thickness, Restek, Bellefonte, PA, USA); column temperatures, 40 °C (2 min), then increased by 8 °C/min to 250 °C (20 min); carrier gas, and helium (3 ml/min).
GCMS solution software (ver. 4.2, Shimadzu Co.) was used for peak identification from the total ion chromatogram (TIC), tentative identification of compounds using the NIST08 MS library, and quantification of MMB and fatty acids using the characteristic m/z fragments. To identify chemical structures of compounds, authentic compounds were run under the same conditions as samples, and also spiked into samples. Acetic acid (purity >99.5%), propanoic acid (purity >98.0%), 2-methylpropanoic acid (purity >99.0%), butanoic acid (purity >99.0%), 3-methylbutanoic acid (purity >99.0%), pentanoic acid (purity >98.0%), 3-methylpentanoic acid (purity >98.0%), 4-methylpentanoic acid (purity >97.0%), hexanoic acid (purity >98.0%), p-cresol (purity >99.0%) and indole (purity >99.0%) were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). MMB (purity >98%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All peak lists were analyzed using JMP12 software (SAS Institute, Cary, NC, USA) for principal component analysis (PCA) and hierarchical clustering analysis (HCA).
For analyses of temporal changes in emission rates of MMB and total fatty acids from cat feces, fresh feces (aliquot 1 g) obtained from a male cat (M1) were immediately placed in four 20 ml screw-cap vials, and volatile compounds emitted from the feces were sampled into Tenax TA tubes by purging with pure nitrogen gas at 50 ml/min every 90 min up to 30 hr at four different temperatures (4 °C, 10 °C, 20 °C, and 30 °C). Tubes were analyzed on the TD-GC-MS system. The MMB emission rate was calculated by dividing the MMB amount emitted from the feces every 1.5 hr by the total fecal MMB content. The total fatty acid emission rate was expressed as the relative value of the first 1.5 hr emission amounts (100%).
Behavior Bioassays in Laboratory Cats
Bioassays were conducted in a test chamber (54 cm × 74 cm × 60 cm) set in the experimental room. Each cat was introduced into the chamber approximately 5 min prior to beginning the assay. In the first and second experiments, habituation-dishabituation tests were conducted using fecal samples with and without MMB and two artificial fecal odors, between which fatty acid compositions were different, respectively, in male and female experimental cats. This test helped determine, via sniffing duration, whether the animals could habituate to a repeatedly presented odor and whether the animals demonstrated dishabituation when presented a novel odor (Arbuckle et al. 2015).
In the first experiment, we omitted MMB from the headspace of a male feces (M9) by dipping 0.5 g of the fresh feces into 50 ml of 4% copper (II) sulfate solution for 30 min at 25 °C. Fecal samples emitting MMB was prepared by dipping 0.5 g of the same feces into 50 ml of water for 30 min at 25 °C. These fecal samples were placed into a 20 ml capless glass vial and presented to the cats from outside the chamber at approximately 20 cm above the floor level by a researcher wearing plastic gloves. The copper solution-treated fecal sample was presented to eight male (M1-M8) and five female (F1-F5) cats twice in the first and second round for 60 sec at 30 sec intervals sequentially, and the water-treated fecal sample was presented once in the third round for 60 sec.
In the second experiment, two artificial fecal odors, which were prepared by mixing chemicals based on the GC-MS data of two male cats (M7 and M8) (Table S1) were used. Approximately 10 min before the experiment, each solution (10 μl) was placed in the bottom of the 20 ml capless glass vial. Six male (M1-M6) and six female cats (F1-F6) were presented the M7’s sample twice in the first and second round and then the M8’s sample once in the third round. Each sample was presented for 60 sec at 30 sec intervals sequentially. A digital video camera (Handycam HDR-CX560V; Sony, Tokyo, Japan) recorded all experiments, and the duration of sniffing, which is a characteristic odor sampling behavior, was counted while the nose of the cats twitched to sense the odor of samples and they reached their nose to the tube opening. Statistical significance was tested using a repeated measure analysis of variance (ANOVA) followed by a Tukey’s Honestly Significant Difference test in JMP software (ver. 12.0; SAS Institute, Cary, NC, USA).
Field Trials
To examine the olfactory discrimination ability for cat feces with and without MMB in free-roaming cats, we presented two 10 cm plastic dishes containing male cat feces (0.5 g) treated with copper solution or water on the ground at 2 m intervals. For control studies, two 10 cm plastic dishes containing water-treated male cat feces (0.5 g) were placed on the ground at 2 m intervals. Each assay was alternately repeated five times every 3 days at a kindergarten playground in Morioka, Japan, from 8:00 pm and 8:00 am from September to November 2012. Samples for these experiments were prepared using fresh feces obtained from a male cat (M9) on each experimental day. During each test, the video camera attached to an infrared light (HVL-HIRL, Sony) was set to record continuously for 12 hr. The total sniffing duration toward each dish by free-roaming cats was counted. Statistical significance was tested using the Wilcoxson test in JMP software.
Results
Volatile Chemical Profiles of Cat Feces
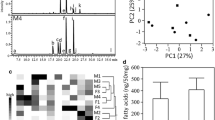
Figure 1a shows a representative TD-GC-MS TIC of volatile compounds emitted from cat feces. Major compounds of fecal samples were fatty acids, such as butanoic acid and pentanoic acid, for both sexes. In addition, there was a major bimodal peak at 17.5 min in all male samples (Fig. 1a, asterisk). Two compounds were completely separated by using mass chromatograms at m/z 69 and m/z 60 extracted from the full-scan data (Fig. 1b). By comparison of mass spectra and retention time between samples and standards (Fig. 1c), we found that the first and second compounds were MMB and 3-methyl butanoic acid, respectively. Approximately 50 ng of MMB were emitted from male feces (wet weight 1 g), whereas it was under the detection threshold in female samples (Fig. 1d). In volatile chemical profiles of gastrointestinal contents of a male cat, MMB was detectable in the colon and rectum samples, but not in the duodenum or jejunoileal samples (Fig. S1). This indicated that felinine degradation to MMB begins in the large intestine.
Volatile chemical profiles of cat feces. a A representative TD-GC-MS TIC of volatile compounds emitted from male cat feces. b A TIC (dotted line) and mass chromatograms at m/z 69 (line) and m/z 60 (gray dotted line) around a bimodal peak detected at 17.5 min. c Mass spectra of the compound detected at 17.4 min (upper) and an authentic sample of MMB (lower). d Box plots of MMB emission amounts from five male (M1-M5) and five female cat (F1-F5) feces. e Principal component analysis (PCA) score plot of the 10 GC-MS results obtained from the 10 cats. f Heat map and dendrograms of the 10 GC-MS results obtained from the 10 cats. Ward’s minimum variance was used for hierarchical clustering. [a: acetic acid, b: propionic acid, c: 2-methyl propanoic acid, d: butanoic acid, e: 3-methyl butanoic acid, f: pentanoic acid, g: 4-methyl pentanoic acid, h: hexanoic acid, i: p-cresol, j: phenol, k: indole, and M: MMB]
We next subjected the GC-MS data from five males (M1-M5) and five females (F1-F5), including eight fatty acids, indole, phenol, p-cresol, and MMB to PCA for the characterization of fecal volatile chemical profiles. Both PC1 and PC2 grouped the 10 GC-MS data into two major groups corresponding to males and females (Fig. 1e). HCA using the same GC-MS data revealed that MMB, propanoic acid, and 4-methyl pentanoic acid were significant contributors; high levels of these compounds were characteristic of male samples (Fig. 1f).
Temporal Changes of MMB Emission from Aging Feces
We examined how long male cat feces emitted MMB into the atmosphere. In this experiment, only the incubation temperature was controlled, although other environmental factors, such as humidity, wind, and sunshine duration, could influence MMB emission. However, we believed that temperature would be strongly involved in the emission rate of volatile compounds. There were dynamic temporal changes in MMB emission rates for aging feces, except for samples incubated at 4 °C (Fig. 2a). In feces at over 10 °C, MMB emission rates were significantly higher in samples between 1.5 and 3 hr old than in the first 1.5 hr in each sample. MMB emission rates were from high to low in the order of 30 °C > 20 °C > 10 °C until 10.5 hr, but reached 0% earlier at 30 °C than at other temperatures. At 30 °C, over 90% of MMB were emitted from the feces by 10.5 hr, and MMB was exhausted in the feces at 15 hr. At 20 °C, MMB was barely detectable over 24 hr. In fecal samples incubated under 10 °C, MMB was not exhausted until 28.5 hr, and the remaining MMB was able to be completely ejected from the feces after incubation at 40 °C. At 10 °C, MMB emission rates at 24 hr were approximately 90%. At 4 °C, only approximately 40% of MMB was emitted from the feces into the atmosphere, and over half of the first fecal contents of MMB were retained in the feces for over 24 hr. Figure 2b shows the ratio of MMB to total amounts of fatty acid in the headspace of cat feces at 20 °C. In contrast to MMB, over 40% of the first 1.5 hr emission amounts of total fatty acids were emitted from the feces every 90 min until 28.5 hr in similar compositions of fatty acids (Fig. S2), indicating that MMB makes a great contribution for the production of fecal odor until 24 hr at 20 °C.
Temporal changes of emission rates of MMB and fatty acids from male cat feces. a MMB emission rates from a male feces were determined by TD-GC-MS every 90 min for up to 30 hr at 4 °C, 10 °C, 20 °C, or 30 °C. The rates were calculated by dividing emission amounts of MMB and fatty acids from 1 g feces every 90 min by the total fecal contents of MMB and fatty acids, respectively. In fecal samples incubated at 10 °C and 4 °C, the MMB that was not exhausted from feces until 28.5 hr was completely ejected from the feces by incubation at 40 °C (asterisk). b Fecal MMB to fecal total volatile ratio was calculated by dividing the emission amount of MMB by the sum of MMB and fatty acids every 90 min. Fig. S2 shows temporal changes in chemical profiles of fatty acids emitted from the feces
Olfactory Discrimination Ability for Fecal MMB in Cats
In olfactory habituation-dishabituation tests, we examined whether cats could discriminate between fecal odors with and without MMB. By comparison of volatile chemical profiles between the copper solution-treated and the water-treated feces, we found that although fatty acids, p-cresol, and indole were detected at similar levels in both samples, MMB was detectable in the water-treated feces, but not in the copper solution-treated feces (Fig. 3a and b). These results confirmed that both fecal samples exhibited the same chemical profiles of volatile compounds, except for MMB. In the habituation trial, the sniffing duration toward the copper solution-treated feces decreased significantly in both sexes when the sample was sniffed in the second round (Fig. 3c). By exposing the cats to the water-treated feces, a significant (p < 0.05) increase in sniffing duration toward the sample (dishabituation) was observed in the eight male cats. This indicated that male cats could discriminate between fecal odors with and without MMB. In female cats overall, a statistically significant increase in sniffing duration toward the water-treated feces was not observed (Fig. 3d), although it was observed in three of the five female cats.
Olfactory discrimination ability for fecal MMB in cats. a Data acquired from TD-GC-MS TICs of volatile compounds emitted from water- and copper solution-treated fecal samples used for behavior assays. Insets show mass chromatograms at m/z 120, m/z 86, and m/z 69 for MMB. [a: acetic acid, b: propionic acid, c: 2-methyl propanoic acid, d: butanoic acid, e: 3-methyl butanoic acid, f: pentanoic acid, g: 4-methyl pentanoic acid, h: p-cresol, i:, indole, and M: MMB] b. Mass spectra of the compound detected at 20.1 min in water- and copper solution-treated feces. Fragmentation patterns obtained from water-treated feces (Water), but not from copper solution-treated feces (Feces-Cu), matched that of authentic MMB. c and d Olfactory habituation/dishabituation tests with 8 males (c) and 5 females (d) using copper solution-treated feces (Feces-Cu) for habituation trials and water-treated feces (Feces) for dishabituation tests. e A picture shows outdoor experiments, in which a free-roaming cat came into the video recording location (kindergarten sandbox) presenting water- (a) and copper solution-treated feces (b). f The sniffing duration of free-roaming cats in outdoor experiments using water- and copper solution-treated feces. g Sniffing duration of free-roaming cats in outdoor experiments only using water-treated feces. Different letters indicate significant differences among the groups (p < 0.05, repeated ANOVA followed by a Tukey Honestly Significant Difference test)
In addition to laboratory cats, olfactory discrimination ability for MMB was also examined in free-roaming cats. Because it would be difficult to test habituation-dishabituation tests in captured free-roaming cats because of capture-induced stress, we compared their sniffing duration of cat feces with and without MMB for free-roaming cats in their territories. In the test presenting both copper solution-treated and water-treated feces at the kindergarten playground (Fig. 3e), the video camera captured five mature cats of unknown sex 15 times. For data analyses, we used nine captured movies, in which they sniffed both samples sequentially within 5 min. Three of the five cats were observed more than once on different trial days (Table S2). A mean, minimum, and maximum numbers of observations/cat were 1.8, 1, and 3 times, respectively. There was no significant difference in the total sniffing duration of cats between copper solution-treated and water-treated feces (Fig. 3f). In a second test, presenting only water-treated feces at two locations, four of the five cats were captured 16 times. In analysis of 9 of the 16 movies in which they sniffed both samples, there was a significant difference of sniffing duration between the two samples (Fig. 3g). Three of the four cats observed more than once on different trial days (Table S3). A mean, minimum, and maximum numbers of observations/cat were 2.5, 1, and 3 times, respectively. These results suggested that free-roaming cats recognized the two samples as the same odor in the control assays, but fecal odors with and without MMB as different odors in the test assay.
Olfactory Discrimination Ability for Different Fatty Acid Compositions in Cats
In habituation-dishabituation tests, olfactory discrimination ability for two artificial fecal odors that had different fatty acid compositions was examined in six male (M1-M6) and six female cats (F1-F6). Chemical profiles of fatty acids used in this test were markedly different between the two samples (Fig. 4a). In the habituation trial, the sniffing duration toward the M7’s sample was significantly decreased by repeated exposures in both sexes (Fig. 4b and c). Then, during dishabituation, the recovery of sniffing duration toward the M8’s sample, was observed in all cats. These results indicated that male and female cats could distinguish the two artificial fecal odors by sensing different fatty acid compositions.
Olfactory discrimination ability for two artificial fecal odors in cats. a GC-MS TICs of two artificial fecal odor that were prepared by mixing authentic free fatty acids based on the GC-MS data of two male cat feces (M7 and M8). [a: acetic acid, b: propionic acid, c: 2-methyl propanoic acid, d: butanoic acid, e: 3-methyl butanoic acid, f: pentanoic acid, g: 4-methyl pentanoic acid, h: hexanoic acid] b Olfactory habituation/dishabituation tests in six laboratory male (M1-M6) and six female cats (F1-F6). The M7’s sample was presented twice for 60 sec with a 30 sec interval (habituation trial); and then M8’s sample was presented once for 60 sec. Sniffing durations were measured. Different letters indicate significant differences among the groups (p < 0.05, repeated ANOVA followed by a Tukey Honestly Significant Difference test)
Volatile Chemical Profiles of Body Odors in Cats
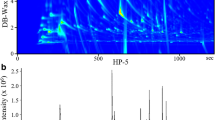
We questioned whether volatile compounds emitted from bodies also contain species-specific volatile compounds such as MMB, as well as excretions such as urine and feces. To address this question, we analyzed volatile compounds emitted from around the neck and the perianal area where cats sniff each other preferentially. In TD-GC-MS TICs of volatile compounds emitted from around the neck and the anus, there was no peak with levels significantly higher in the neck region compared to room air, except from isoflurane used for anaesthesia during sampling (Fig. 5a). In the perianal area, several fatty acids were detected, as well as cat feces, but MMB was not detected. In the PCA and HCA of GC-MS data from five male (M1-M5) and five female cats (F1-F5) for perianal odors, their chemical profiles markedly showed individual differences, but there was no sex polarization as described for fecal odors (Fig. 5b and c).
Chemical profiles of volatile compounds emitted from cat bodies. a Representative TD-GC-MS TICs obtained from Tenax-TA after concentrating the volatile compounds in a room without cats and from the neck and perianal regions of a male cat. The asterisk denotes the background peak of isoflurane. Inset pictures show the sampling of volatile compounds desorbed using Tenax TA tubes. b PCA score plot of the 10 GC-MS results obtained from the perianal regions of five male and five female cats. c Heat map and dendrograms of the 10 GC-MS results obtained from five male (M1-M5) and five female (F1-F5) cats. Ward’s minimum variance was used for hierarchical clustering. [a: acetic acid, b: propionic acid, c: 2-methyl propanoic acid, d: butanoic acid, e: 3-methyl butanoic acid, f: pentanoic acid, g: 3-methyl pentanoic acid, h: hexanoic acid]
Discussion
Previous studies had shown that unburied cat feces are territorial markers asserting the border of their home range, but little was known which fecal compounds may function as scent cues for species, sex, and individual recognition in cats. The present study demonstrated scent communication using feces from chemicals to behavioral responses in domestic cats. A major finding is that the unusual amino acid, felinine, which is found in certain Felidae species, is decomposed to the volatile mercaptoethanol, MMB, in the large intestine. MMB is then emitted from feces, whereas it was previously assumed that MMB is found only in the urine (Miyazaki et al. 2006a). Further studies suggest that variable subsets of short chain-fatty acids provide individual identification to the fecal samples. In contrast to fatty acids that are major fecal components of mammals, including humans (Kotani et al. 2009), MMB has never been identified in other species including dogs, rabbits, and mice (Arnould et al. 1998; Goodrich et al. 1990; Goodrich et al. 1981). These studies indicate that MMB contributes to a cat-specific odor while the general odor of mammalian feces is produced by fatty acids and indole. Previous studies suggested that MMB is a pheromone in cats (MacDonald et al. 1984; Hendriks et al. 1995a; Miyazaki et al. 2006b), but direct evidence had been lacking. Considering discrimination ability for fecal MMB in cats, we propose that MMB is a male sex recognition pheromone in cats, which provides useful information for other males, perhaps to avoid conflictions with scent owners.
MMB levels were much higher in male cat feces than female cat feces. We suggest that the measured sex-based differences in MMB emission levels from cat feces is caused by sex-dependent felinine biosynthesis (Hendriks et al. 2008; Hendriks et al. 1995b). Because our recent studies showed that the biliary contents of felinine precursors, 3-methylbutanol-glutathione and 3-methylbutanol-cysteinylglycine, were significantly higher in males than in females; nevertheless, there was no significant difference in felinine content between male and female fecal samples (Futsuta et al. 2018). These results suggest that more felinine is decomposed to MMB in the large intestine of males than females, which results in higher MMB levels in males than females. However, the remaining levels of felinine that do not decompose to MMB in feces are similar in either sex.
Female cats have less olfactory discrimination ability for fecal MMB than male cats have. The biological significance of fecal MMB may be different between males and females. Males have significantly larger home ranges than females, and the overlap ratio of home ranges between cats is higher in males than females (Yamane et al. 1994). Therefore, males may expose feces with large amounts of MMB for territorial defense in overlapping areas of territories, and such feces emitting MMB may lead to avoidance by other males, with whom contact could lead to fighting and injury. Olfactory discrimination ability for fecal MMB may be more crucial for territorial behavior in male cats than female cats.
Scent signals have advantages over visual and auditory cues in that scent marks remain in the environment for a long time, even if the owners are not present. For example, male mice mark their territories using urine with major urinary proteins (MUPs) that are scent signals for individual recognition and remain stable in the marks over time without degradation (Hurst and Beynon 2004). European badgers (Meles meles) have subcaudal gland secretions containing medium- and long chain-fatty acids (Buesching et al. 2002a). Chemical compositions of most of the fatty acids encoding group membership and individuality remain stable in the secretions over days and seasons, but contents of some fatty acids with temporal changes during aging of the secretions encode the age of scent marks (Buesching et al. 2002a, b). In addition to these reports, our studies provide the knowledge on how a volatile compound encoding species and sex also provide information on the age of scent marks. In contrast to non-volatile MUPs and semi-volatile fatty acids, emission rates of volatile MMB are not fixed during aging of fecal samples. Therefore, MMB may be used for time prediction, i.e., the time the other cat left after depositing the feces. Cats use such scent information to decide whether they penetrate the overlapping territorial area with other cats. If cats sense a high level of MMB in feces, they can predict that the scent owners may be present nearby. In contrast, a low level of MMB may let cats know that scent owners may have left the location some time ago. A male identification pheromone would be important for the maintenance of distance in time and space, and thus can help avoid conflicts between cats.
Prey animals of cats may also use MMB for discriminating the resident cat’s presence in the environment. Brandt’s voles (Lasiopodomys brandtii) exhibited higher levels of defensive responses to freshly collected cat feces than old feces (stored for a few days), suggesting the contribution of volatile compounds emitted from fresh cat feces to these responses (Hegab et al. 2014). There are reports that felinine serves as a predator signal and has negative impacts on mouse reproduction; thus, suggesting that mice recognize felinine as an indication of the presence of cats, their primary predator (Voznessenskaya 2014). Considering that felinine is a non-volatile compound, volatile MMB would be a candidate signal for prey species to avoid encounters with predators.
Based on the present study and our previous studies, we suggest that olfactory information, which animals acquire by sniffing scent marks, such as urine and feces, and body areas, differ from each other (Fig. 6). When cats encounter scent marks of unknown owners, it is difficult to determine species of the owners only by the appearance of the feces and dried urine. They may recognize species and sex of the owners by sensing MMB. Individual recognition may be carried out by sensing subsets of fatty acids that differ among individuals. In contrast, when the animals meet each other, they can obtain species and sex information via visual cues before sniffing body odor. They may use their efforts to distinguish individual differences in body odors with individual patterns of fatty acids, rather than MMB. Because major volatile contents of cat anal sac secretions are fatty acids without MMB (Miyazaki et al. 2017b), as well as those of the perianal area, anal sac secretions might contribute to body odors of the perianal area. In addition to visual cues, olfactory cues may be useful for individual identification of other cats, even if their appearances are similar.
Schematic images to explain differences of odor cues emitted from between scent marks and bodies. a For scent marking, cats spray urine onto a vertical surface and left feces on the ground without burying. When cats encounter scent marks, since they cannot determine species of scent owners only by the outside appearance of feces and dried urine, they utilize MMB to recognize species and sexes of scent owners before individual recognition via individual patterns of fatty acids. b Before sniffing body odor of other individuals, they obtain species and sex information on scent owners from visual cues. Cats use their efforts to distinguish individual patterns of fatty acids during sniffing
The identification of MMB in cat feces addresses a question that concerns the public, especially cat owners: why do their feces emit a distinctive, pungent smell to the atmosphere? The present study showed that a copper ion solution removes the MMB odor from cat feces, because a solution containing copper ions binds to volatile compounds containing a thiol (-SH), and prevents their volatilization from the solution. In other studies, the addition of copper sulfate to solvent reduced the headspace concentration of a volatile thiol compound, 3-mercapto-2-butanol (Vaughn et al. 2011). Deodorants containing copper and silver ions will help to neutralize the distinctive smell of cat feces.
In conclusion, fecal MMB is a male sex recognition pheromone in cats. Temporal changes of MMB emission rates from feces also provide information about how recently the scent owner was in the vicinity. Moreover, cat feces emit mixtures of short chain-fatty acids encoding individuality of scent marks. In contrast to metabolic waste products using scent marks, their body odors around perianal area emit fatty acids but not specific compounds such as MMB. In summary, our findings improve our understanding of scent communication in mammals, particularly in the domestic cat.
References
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144. https://doi.org/10.1016/j.neubiorev.2005.05.005
Apps P, Mmualefe L, Jordan NR, Golabek KA, McNutt JW (2014) The “tomcat compound” 3-mercapto-3-methylbutanol occurs in the urine of free-ranging leopards but not in African lions or cheetahs. Biochem Syst Ecol 53:17–19. https://doi.org/10.1016/j.bse.2013.12.013
Apps P, Claase M, Yexley B, McNutt JW (2017) Interspecific responses of wild African carnivores to odour of 3- mercapto-3-methylbutanol, a component of wildcat and leopard urine. J Ethol 10:153–159. https://doi.org/10.1007/s10164-016-0503-7
Arbuckle EP, Smith GD, Gomez MC, Lugo JN (2015) Testing for odor discrimination and habituation in mice J Vis Exp e52615 doi:https://doi.org/10.3791/52615
Arnould C, Malosse C, Signoret J-P, Descoins C (1998) Which chemical constituents from dog feces are involved in its food repellent effect in sheep? J Chem Ecol 24:559–576. https://doi.org/10.1023/A:1022321104758
Buesching CD, Waterhouse JS, Macdonald DW (2002a) Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles) part I: chemical differences related to individual parameters. J Chem Ecol 28:41–56. https://doi.org/10.1023/A:1013558718057
Buesching CD, Waterhouse JS, Macdonald DW (2002b) Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles) Part II: time-related variation in the individual-specific composition. J Chem Ecol. 28:57–69. https://doi.org/10.1023/A:1013510802127
Crowell-Davis SL, Curtis TM, Knowles RJ (2004) Social organization in the cat: a modern understanding. J Feline Med Surg 6:19–28. https://doi.org/10.1016/j.jfms.2003.09.013
Dehasse J (1997) Feline urine spraying. Appl Anim Behav Sci 52:365–371. https://doi.org/10.1016/S0168-1591(96)01135-5
Feldman H (1994) Methods of scent marking in the domestic cat. Can J Zool 72:1093–1099. https://doi.org/10.1139/z94-147
Futsuta A, Hojo W, Miyazaki T, Yamashita T, Miyazaki M (2018) LC-MS/MS quantification of felinine metabolites in tissues, fluids, and excretions from the domestic cat (Felis cutus). J Chromatogr B 1072:99–94. https://doi.org/10.1016/j.jchromb.2017.11.006
Goodrich B, Hesterman E, Shaw K, Mykytowycz R (1981) Identification of some volatile compounds in the odor of fecal pellets of the rabbit, Oryctolagus cuniculus. J Chem Ecol 7:817–827. https://doi.org/10.1007/BF00992380
Goodrich B, Gambale S, Pennycuik PR, Redhead T (1990) Volatiles from feces of wild male house mice. J Chem Ecol 16:2091–2106. https://doi.org/10.1007/BF01026922
Gorman ML (1976) A mechanism for individual recognition by odour in Herpestes auropunctatus (Carnivora: Viverridae). Anim Behav 24:141–145. https://doi.org/10.1016/S0003-3472(76)80107-8
Hegab IM, Jin Y, Ye M, Wang A, Yin B, Yang S, Wei W (2014) Defensive responses of Brandt's voles (Lasiopodomys brandtii) to stored cat feces. Physiol Behav 123:193–199. https://doi.org/10.1016/j.physbeh.2013.10.030
Hendriks WH, Moughan PJ, Tarttelin MF, Woolhouse AD (1995a) Felinine: a urinary amino acid of Felidae. Comp Biochem Physiol B Biochem Mol Biol 112:581–588. https://doi.org/10.1016/0305-0491(95)00130-1
Hendriks WH, Tarttelin MF, Moughan PJ (1995b) Twenty-four hour felinine excretion patterns in entire and castrated cats. Physiol Behav 58:467–469. https://doi.org/10.1016/0031-9384(95)00084-V
Hendriks WH, Rutherfurd-Markwick KJ, Weidgraaf K, Ugarte C, Rogers QR (2008) Testosterone increases urinary free felinine, N-acetylfelinine and methylbutanolglutathione excretion in cats (Felis catus). J Anim Physiol Anim Nutr 92:53–62. https://doi.org/10.1111/j.1439-0396.2007.00710.x
Hurst JL, Beynon RJ (2004) Scent wars: the chemobiology of competitive signalling in mice BioEssays: news and reviews in molecular, cellular and. Dev Biol 26:1288–1298. https://doi.org/10.1002/bies.20147
Kotani A, Miyaguchi Y, Kohama M, Ohtsuka T, Shiratori T, Kusu F (2009) Determination of short-chain fatty acids in rat and human feces by high-performance liquid chromatography with electrochemical detection. Anal Sci 25:1007–1011. https://doi.org/10.2116/analsci.25.1007
Lin DY, Zhang SZ, Block E, Katz LC (2005) Encoding social signals in the mouse main olfactory bulb. Nature 434:470–477. https://doi.org/10.1038/nature03414
MacDonald ML, Rogers QR, Morris JG (1984) Nutrition of the domestic cat, a mammalian carnivore. Annu Rev Nutr 4:521–562. https://doi.org/10.1146/annurev.nu.04.070184.002513
Mattina MJ, Pignatello JJ, Swihart RK (1991) Identification of volatile components of bobcat (Lynx rufus) urine. J Chem Ecol 17:451–462. https://doi.org/10.1007/BF00994344
Miyazaki M, Yamashita T, Suzuki Y, Saito Y, Soeta S, Taira H, Suzuki A (2006a) A major urinary protein of the domestic cat regulates the production of felinine, a putative pheromone precursor. Chem Biol 13:1071–1079. https://doi.org/10.1016/j.chembiol.2006.08.013
Miyazaki M, Yamashita T, Taira H, Suzuki A (2006b) The biological function of cauxin, a major urinary protein of the domestic cat (Felis catus). In: Hurst J, Beynon R, Roberts S, Wyatt T (eds) Chemical Signals in Vertebrates 11, vol 11. Springer, New York, pp 51–60. https://doi.org/10.1007/978-0-387-73945-8_4
Miyazaki M, Nishimura T, Hojo W, Miyazaki T, Laine R, Yamashita T (2017) Potential use of domestic cat (Felis catus) urinary extracts for manipulating the behavior of free-roaming cats and wild small felids. Appl Anim Behav Sci 196:52–60. https://doi.org/10.1016/j.applanim.2017.07.003
Miyazaki T, Nakata K, Nishimura T, Abe S, Yamashita T, Miyazaki M (2018a) Identification of 2-phenylethanol with a rose-like odor from anal sac secretions of the small Indian mongoose (Herpestes auropunctatus). Biosci Biotechnol Biochem 82:232–237. https://doi.org/10.1080/09168451.2017.1419854
Miyazaki T, Nishimura T, Yamashita T, Miyazaki M (2018b) Olfactory discrimination of anal sac secretions in the domestic cat and the chemical profiles of the volatile compounds. J Ethol 36:99–105. https://doi.org/10.1007/s10164-017-0532-x
Nakabayashi M, Yamaoka R, Nakashima Y (2012) Do faecal odours enable domestic cats (Felis catus) to distinguish familiarity of the donors? J Ethol 30:325–329. https://doi.org/10.1007/s10164-011-0321-x
Natoli E (1985) Behavioural responses of urban feral cats to different types of urine marks. Behaviour 94:234–243. https://doi.org/10.1163/156853985X00208
Vaughn S, Berhow M, Winkler-Moser J, Lee E (2011) Formulation of a biodegradable, odor-reducing cat litter from solvent-extracted corn dried distillers grains. Ind Crops Prod 34:999–1002. https://doi.org/10.1016/j.indcrop.2011.03.005
Vernet-Maury E, Constant B, Chanel J (1992) Repellent effect of trimethyl thiazoline in the wild rat Rattus norvegicus Berkenhout. Chemical signals in vertebrates VI. Plenum Press, New York. https://doi.org/10.1007/978-1-4757-9655-1_49
Vogt K, Boos S, Breitenmoser U, Kölliker M (2016) Chemical composition of Eurasian lynx urine conveys information on reproductive state, individual identity, and urine age. Chemoecology 26:205–217. https://doi.org/10.1007/s00049-016-0220-2
Voznessenskaya VV (2014) Influence of cat odor on reproductive behavior and physiology in the house mouse (Mus Musculus). In: Mucignat-Caretta C (ed) Neurobiology of chemical communication, vol Chapter 14. Frontiers in Neuroscience. CRC press, Boca Raton, p Chapter 14
Westall RG (1953) The amino acids and other ampholytes of urine. 2. The isolation of a new Sulphur-containing amino acid from cat urine. Biochem J 55:244–248. https://doi.org/10.1042/bj0550244
Wyatt TD (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol A 196:685–700. https://doi.org/10.1007/s00359-010-0564-y
Wyatt TD (2014) Pheromones and animal behavior vol Second Edition, 2nd edn. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781139030748
Yamane A, Ono Y, Doi T (1994) Home range size and spacing pattern of a feral cat population on a small island. J Mammal Soc Japan 19:9–20. https://doi.org/10.11238/jmammsocjapan.19.9
Acknowledgements
We thank Dr. T. Wyatt for invaluable discussion. This research was funded by JSPS KAKENHI Grant Numbers 17H03937 and 17 K19215.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 228 kb)
Rights and permissions
About this article
Cite this article
Miyazaki, M., Miyazaki, T., Nishimura, T. et al. The Chemical Basis of Species, Sex, and Individual Recognition Using Feces in the Domestic Cat. J Chem Ecol 44, 364–373 (2018). https://doi.org/10.1007/s10886-018-0951-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-0951-3