Abstract
In this study, the relationship between physiological conditions, including oogenesis and gravidity, and attraction to carcass odors was examined in order to discover how the attractivity of volatile organic compounds (VOCs) varied in females of the necrophagous fly Lucilia caesar (Linnaeus, 1758) (Diptera: Calliphoridae). Thus we want to shed some light on settlement behavior of scavenger flies. In bioassays with a decomposed mouse (Mus musculus), mixtures of synthetic compounds and flies of several physiological states, we observed rates of attraction and found compound classes that attract the different physiological stages. We found that a protein-poor diet increased the attraction to a carcass, especially between the age of 3 and 7 days. 7-day old females were attracted to cyclic compounds when mated and after exposure to a protein-poor nutrition, whereas they responded to fatty acids when mated after exposure to a protein-enriched diet. Furthermore we found that only with a protein-rich diet did the gonadal development complete with mature eggs in the Xth stadium between seven and 10 days and an overlapping second cycle with eggs in the Vth stadium at day 10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to understand insect behavior, it is essential to know the sex and the stage of development of the individuals under study. This understanding can lead us to the resources required by individuals in different physiological conditions.
Insects must feed, mate and oviposit to complete their life cycle and these requirements are influenced by intrinsic factors (neuronal, hormonal and muscular).
One example is that food resources to sustain vital functions might be different from resources related to reproduction. Special diets may be needed for the development of reproductive organs (Stoffolano et al. 1995). Another factor than temperature, humidity, light, host stimuli, and copulation that influence the neural and hormonal control of ovarian development (Papaj 2000, 2005; Lachmann and Papaj 2001) described Barton-Browne (2001) for Lucilia cuprina (Wiedemann, 1830) (Diptera: Calliphoridae). Females require protein-rich food in order to complete egg maturation that depends on the release of the ovarian ecdysteroidogenic hormone through the corpora cardiaca (Yin et al. 1993; Zou et al. 1989). Without protein the cycle stops at the end of previtellogenesis or at the beginning of vitellogenesis and development of all primary ovarian follicles is arrested (anautogene oogenesis) (Harlow 1956; Aluja et al. 2001). Insects’ attraction to specific resources depends on intrinsic physiological circumstances; therefore resource-orientated behavior is the result of physiological status as well as external stimuli (Barton-Browne 1993). For example show gravid females of blowflies in advanced vitellogenic stages a high response activity to carrion odours (Woodburn and Vogt 1982; Stoffalano et al. 1990; Wall and Warnes 1994; Ashworth and Wall 1995; Archer and Elgar 2003; Aak and Knudsen 2012).
Another fact is that mates are needed in all fly species for fertilization (given there is no parthenogenesis) (Barton-Browne et al. 1987). Full ovarian development, encourages mating (Carsten and Papaj 2005). Simple exposure to food resources or food-associated stimuli can also have a positive effect on egg load e.g., fruit experience for Tephritidae (Diptera) (Papaj 2000, 2005). Stoffolano et al. (1995) showed that sugar, feces or liver feeding in Calliphoridae resulted in different levels of ovarian development and thus in differences pertaining to the readiness for mating in females and also in males. In contrast, Omar (1992) claims that a protein-rich diet did not affect male blowfly mating behavior or their sexual activity (Parker 1968).
Both sexes of Diptera, using ephemeral resources such as dung, ripe fruits or carcasses, can find each other either on food resources (Stoffalano et al. 1990), at oviposition sites (Parker 1968) or via sex pheromones (Shorey et al. 1969). Evidence is accumulating that mating changes the fly’s responsiveness to attractants including semiochemicals and thus their behavior. For instance, given the choice, virgin females of Tephritidae were highly attracted to male pheromones over host fruits, whereas mated females, instead, chose the fruit odour (Jang 1995, 2002). It often happens that males await females on oviposition sites (Borgia 1981; Otronen 1995). For example, Scathophaga (Diptera: Scathophagidae) females visit cattle dung to lay their eggs and mate directly before oviposition on the dung (Parker 1970a, b, 1974).
However, blowflies have no long-range pheromone attractant (Broce 1980) but a mounting stimulant (Parker 1968). They use kairomones to aggregate (Aak and Knudsen 2012) and males most likely use visual cues to finally locate and approach females (Boeddeker et al. 2003).
Now, the fertilized female flies require an oviposition site and it is of interest to know how they find the appropriate resource for their offspring. Sivinski (1988) showed female aggregation in Phoridae (Diptera) through pheromones emitted by other females, this has also been shown for blowfly L. cuprina (Barton-Browne et al. 1969), for Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) (Laurence and Pickett 1985), and for four other different dipteran families (McCall and Cameron 1995). From this it is apparent that there is semiochemical-regulated oviposition behavior in many flies, however, it has been suggested that females are mainly attracted by odors released from characteristic resources (Pfeil and Mumma 1993; Hartlieb and Anderson 1999; Staedler 2002; Stensmyr et al. 2002; Aak et al. 2010; Van der Niet et al. 2011; Paczkowski et al. 2012).
In summary, it seems that olfactory-mediated behavior depends on a combination of intrinsic factors, which mediate the response to extrinsic stimuli. In the present study we examined the change of attractiveness of carrion across different stages of development of a necrophagous female blowfly, Lucilia caesar (L.) for a better understanding of insect succession on carcasses and the mechanisms responsible.
Decay of carrion is typically accompanied by the emission of volatile organic compounds (VOCs) responsible for the unpleasant odor of a carcass and thus for the attraction of necrophagous insects. The odor is built up by a plethora of compounds including, for example, alcohols, aldehydes, ketones, fatty acids, esters, sulfides and cyclic compounds (Vass et al. 2004) (Fig. 1). Although necrophagous insects are known to exhibit a predictable succession of settlement on a carcass during the decomposition process (Anderson and VanLaerhoven 1996), and multiple studies have investigated how the physiological stage of an insect influences its searching behavior (Crnjar et al. 1990; Ashworth and Wall 1995; Hayes et al. 1999; Wall and Fisher 2001; Aak and Knudsen 2011, 2012; Paczkowski et al. 2012; Zhu et al. 2013), the relationship between the physiological stage of necrophagous flies, and its response to the volatile compounds emitted by different stages of carcass decay is not yet fully understood.
Relative distribution of the volatile compound classes of a mouse decomposed for 10 days under cold and dry conditions (12 °C/40–60 % RH) in ng/ μl, (after Kasper et al. 2012)
However, as large carcasses are more frequently found in rural regions, and garbage, slaughterhouses and feces are important breeding sites for blowflies in urban areas (Norris 1965), there are some other important medical aspects to be considered. Blowflies may lay their eggs in fresh or cooked meat and fish or dairy products and are therefore of the greatest hygienic importance, being potential vectors of bacteria, viruses, protozoan and helminthes (Schuhmann 1965 and 1971).
L. caesar can also be an option for secondary parasites and can cause myiasis (Stevens and Wall 1997). The eggs are laid in open wounds of vertebrates, mostly in sheep, and the larvae feed on necrotic cells, but may also attack the healthy tissue. As attractive VOCs can also originate from breath, feces, or wool (Cragg 1956; Cork and Hall 2007), the analysis of the behavior of L. caesar can have implications for blow fly control in terms of reduced destruction of food (Wall and Warnes 1994; Aak et al. 2011) and prevention of myiasis (Broughan and Wall 2006; Urech et al. 2009; Harvey et al. 2010).
In any case, when studying resource-oriented behavior, the gravid females are the primary interest. Gravid females actively search for an oviposition site, and their larvae cause the main damage on food resources or that they have further implications for forensic entomology in terms of PMI calculations.
Materials and Methods
Breeding
For fly breeding L. caesar L3-stadium larvae, which were close to pupation, were used. To obtain unfertilized females, pupae were reared individually and after eclosion and gender determination, 20–60 females were kept together in breeding boxes. To obtain inseminated females, pupae (50–150 individuals) were reared together in a single breeding box with the chance of both sexes to mate quickly (Pollock 1971). In the following we refer to those two female groups as unmated and mated females.
The breeding boxes were transparent acrylic glass boxes (35 × 20 × 15 cm). Room temperature was held constant 23 ± 2 °C at 55 % humidity. The light–dark cycle was −11:13 h, which was maintained through daylight-lamps during the winter. The flies were kept under two separate feeding conditions. One group received a low-protein diet consisting of sugar-water (1:4), raisins and cornflake-mush and the other received a protein-enriched diet in which peptone powder from meat, dissolved in water was present, in addition to the aforementioned diet. Fresh food was provided every day, and old food removed, to prevent odor formation, thus maintaining female flies naïve to cadaver-odors.
For the different experimental designs flies were tested after 1, 3, 7 and 10 days for the oogenesis and decomposition attraction experiments and after 1, 3 and 7 days for the volatile attraction experiment (Table 1).
Preparation of Ovaries for Ovary Development Status
To prepare ovaries and spermathecae the flies were shock-frozen at −20° for 20 min and the organs were removed. Extracted ovarioles and entire fragments of the ovary were transferred to 96 % ethanol, mounted on a slide, and the stage of the primary ovarian follicles was determined with a phase contrast microscope. The development of the ovular chamber has been divided in ten stages (Adams and Mulla 1967). The Ist germ cell stadium forms a cell differentiation in the basal part of the germarium. The germ cell, separated from the germarium through a thin epithelial layer of cells, changes its conical to a spherical structure. The chromatin of the nurse cell cores experience strong changes up until the IIIrd stadium. Oocytes begin to form within the IVth stadium, where yolk forms and the oocyte starts to grow to the size of a mature egg. The ovular cell grows from initially 30 to 1000 μm. The nurse cells degenerate in the IXth stadium (Adams and Reinecke 1979; Theunissen 1973; Vogt et al. 1974). Oocytes develop synchronously in distinct cycles, but the cycles overlap temporally.
In this study the primary ovarian follicles without opaque (visible) yolk material in the egg chamber are described as previtellogene and were classified as stage I-III (Adams and Mulla 1967). Stages IV-IX were vitellogene follicles and the Xth stage equaled a mature egg (Fig. 2). Females were dissected at different ages (1, 3, 7, and 10 days) and the follicular stages of each age, from each diet, were determined (Table 1).
Age- and nutrition depended oogenesis: Development stages of the egg chamber of 1, 3, 7 and 10 days old Lucilia caesar females with and without protein n = 20 Oocyte development stadiums (roman letters) of the egg chambers with age (in days) and nutrition. A: Females fed with protein-poor nutrition. a) 1 day (II stadium), b) 3 days (III stadium), c) 7 days (III stadium), d) 10 days (III stadium); B: Females fed with protein-rich nutrition. e) 1 day (II stadium), f) 3 days (III stadium), g) 7 days (IX stadium), h) 10 days (V stadium) 2. cycle; Abbriviations: cp, connection pipe; fe, follicle epithelium; fo, follicle; ge, germarium; Oo, oocyte; pf, primary follicle; sf, secondary follicle; tn, trophocytes nuclear; yo, yolk
Mating Status and Oviposition
To verify how many, and at which age, females were inseminated, squeeze preparations of the spermathecae of a fly were made. Twenty females from each age group with the protein-enriched diet were selected and their spermathecae examined.
Protein-fed females, brought together with males in the same breeding box were expected to oviposit. Therefore 20 flies from each age stage (1, 3, 7 and 10 days respectively) (Table 1 ) were separated and placed on a beef mince ball (5 cm in diameter) in a jar. After removal of the flies, 6 h later, the number of laid eggs were counted. After 12 h the number of hatched larvae was determined through subtraction of the remaining unhatched eggs.
Bioassays
Olfactory attraction was recorded using a static-4-chamber olfactometer as described by Steidle and Schöller (1997). The olfactometer was made of acrylic glass and consisted of a cylinder (4 cm high, 19 cm across), was placed on top of the four-chambered cylinder. The walking arena had a plastic gauze (mesh 0.5 mm) base and a glass plate lid. Each chamber was open to the walking arena above so volatiles could diffuse through the gauze and form an odor field.
For each test-run a single fly was released into the walking arena using an aspirator. The fly was left for 10 s in the dark to acclimate. Thereafter, the time the fly spent walking within each of the four odor fields was recorded over a period of 180 s by visual observations and using a digital counter, that calculation of total times with minimal reaction delay. Twenty individuals were tested per treatment combination. The order of the tested parameters (sex, age, nutrition, mating status) was randomized and the olfactometer was cleaned with detergent, rinsed thoroughly and wiped with acetone to avoid odor contamination, always before a different physiological stage was used. The olfactometer was kept in a fully shaded small room and was illuminated vertically from above to exclude unwanted visual disturbances. However, the olfactometer was turned slightly after every test-run to avoid unnoticed effects, such as cardinal directions or ventilation.
Dead mice of both sexes were used in this study, which originated from the same rearing at the University Hospital Charité Berlin, and were of the same size and age and had been fed the same diet (hay food pellets). For ethical reasons we used mice scheduled to be destroyed due to stock size and dispatched them by breaking the spinal cord. Immediately after death, mice were put in an open glass Petri dish (12 cm diameter) and allowed to decompose in a climatic exposure cabinet in a cold and dry environment (12 °C/40–60 % RH) for 10-days (cd10) (Kasper et al. 2012).
-
1
Attraction to mouse carcass
The first bioassay was conducted with mated and unmated females of different ages (1, 3, 7 and 10 days after emerging) and feeding history (reared with and without proteins) with n = 20 for every treatment combination (Table 1). Three chambers were left empty and in the fourth chamber a dead mouse was placed. The mouse was of the highly attractive decomposition stage (cd10) (Kasper et al., accepted) in order to provide a reference point for changes in behavior.
-
2
Attraction to volatile compound mixtures
Bioassay two was designed to measure the response of different stage and state flies to the volatiles typical of a certain decomposition stage.
Six mixtures, each consisting of a different class of compounds (alcohols, fatty acids, esters, sulfides, aldehydes and cyclic compounds; Fig. 1) were produced, each representing a different decomposition stage. The composition of compounds and their quantity used for the mixtures (Table 2) was based on the organic volatile composition of the cd10 mouse (Kasper et al. 2012).
Table 2 Composition of the used mixtures in bioassay 2 in respect of the result after a mouse decomposed for 10 days under cold and dry conditions (12 °C/40–60 % RH) was analyzed in the GC-MS (Kasper et al. 2012) The mixtures were tested for their attraction to males, unmated females and mated females (1, 3 and 7 days after emerging; n = 20) reared on a protein poor diet. Because of the results of the ovarian development and bioassays 1, the 7-days old flies were separated into two groups: with protein-enriched diet and without (Table 1). Three chambers were provided with blank filter paper as reference. The volatiles were dissolved in dichloromethane and 1 μl was dropped onto a fourth piece of paper placed in test chamber four.
Data Analysis
Differences in abidance time across the four fields were tested using the Friedman test based upon the mean rank differences of the fields (Siegel and Castellan 1988).
If the Friedman test was significant (P < 0.05) a post hoc Wilcoxon signed-rank test was used to test for a specific difference in the time spent above chamber four, with the dead mouse, versus chamber two, which has no direct border to the test field. Control tests with four blank fields were conducted to confirm the influence of the treatments. As none of the control tests showed any significant differences between chambers they are not discussed further.
A non-parametric one-way analysis of variance by ranks, the Kruskal-Wallis-test was used to examine the effect of the group variables (age, diet and gravidity) upon the time spent on test field four.
The parametric equivalent, the one-way analysis of variance (ANOVA), was used to test the differences between combination of diet, age and mating status. In order to indicate which parameter influenced the behavior of the flies, the ANOVA was followed by post-hoc pairwise comparison, using the least significant difference (LSD) test.
Results
Developmental Status of Ovaries
Microscopic preparation showed that all follicles of paired ovaries developed synchronously within an individual. Flies with similar nutrition reached a consistent egg development stage at each age step, with no more than one stadium of difference between individuals within a group. The previtellogenous phase lasted 3 days for all examined flies, independent of their nutrition (with or without proteins) and independent of the presence of male flies.
By day 7 and day 10, diet clearly influenced the progress of oogenesis (Fig. 2). Eggs from females with protein-poor diets reached the IIIrd stadium (at most), i.e., the stage before the yolk proteins are ingested in the oocytes. After 10 days the ovaries still remained in the previtellogenesis stage. Only with a protein-rich diet was the gonadal development completed. The dissected female flies produced mature eggs in the Xth stadium between 7 and 10 days and a second cycle had begun. At day 10, when the first cycle was completed and the eggs in the Xth stadium were ready to be laid a second gonotrophic cycle emerged with eggs in the Vth stadium.
Mating Status and Oviposition
In 10 % of the tested protein-deprived females sperm was found in the spermathecae after 24 h (Fig. 3a). With increasing age, the number of inseminated females also increased gradually, up to 80 % of the tested females after 10 days. None of the protein-deprived female flies deposited their eggs. However, 10 % of the protein-fed females deposited their mature eggs after 7 days, all of which hatched (Fig. 3b). The eggs were laid predominately in clusters, as is characteristic for this species. After 10 days 40 % of the tested flies laid their eggs, most of which hatched within approximately 12 h and the last after 1 day. Less than 1.7 % of eggs remained unhatched.
Mouse Carcass Bioassay
Comparing the mean time spent on each field none of the 1-day-old flies showed any preference for the test field with the dead mouse (Friedman test and Wilcoxon test P > 0.05). From day three for mated and day seven for unmated females, all flies were attracted to the dead mouse regardless of their diet (Friedman test and Wilcoxon test P < 0.05) (Fig. 4).
Mean time spent on test field 4 above the dead mouse (± standard error SE) of mated and unmated female flies reared on protein-poor and protein- enriched diets at four different ages; maximum possible time = 180 s, random expectation of one-quarter the time = 45 s. There was significantly greater attraction to the carcass with 3 to 10 days old flies, and a Kruskal Wallis-Test showed a significant difference within the 3-day-old protein-fed flies between the mated and the unmated females (ab). A LSD test showed the significant differences for the parameter combinations nutrition and mating status between the four age groups demonstrated by asterisks
The two different mating conditions of the protein-deprived females showed no significant difference of the behavior within one age group, whereas the protein-fed females showed a significant difference (Kruskal Wallis test P = 0.007, n = 20) 3 days after eclosion (Fig. 4).
Tests within the unmated protein-fed and protein deprived as well as mated protein-fed and protein deprived tested for the four age groups showed significant different responses to the test field (Kruskal-Wallis test P ≤ 0.001 n = 20). The analysis of variance confirmed this (ANOVA P < 0.001, n = 20) with ad-hoc LSD test for the single age groups with P < 0.05 displayed in Fig. 4. The nutrition and the mating stage had no significant influence on their own.
Combinations of the three parameters (nutrition, age and mating) result in ANOVA P < 0.05 for “nutrition and mating,” as well as for “nutrition and age”. That means the nutrition in combination with mating OR age has an influence of the behavior with LSD P < 0.01 for the ages compared 1:3, 7, 10 days and 3:7 days. In summary a protein-poor diet increased the attraction to the dead mouse, especially between 3 and 7 days.
VOCs Bioassays
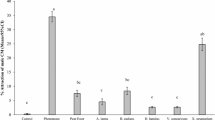
Male responses to the compound mixtures were low and although they were mainly attracted to sulfides and cyclic compounds this was not always significant (Friedman test and Wilcoxon test P < 0.05) (Fig. 5). The responses to fatty acids varied and interestingly the attraction to alcohol increased with age.
Response of the different physiological fly stages (m: males and f: females, 1, 3, 7: age in days, p: fed with protein) to the compound mixtures: mean time spent on test field 4 above the compound (± standard error). Maximum possible time = 180 s, random expectation of one-quarter the time = 45 s. Significant attractiveness tested with Friedmann test and confirmed by Wilcoxon test are demonstrated by asterisks
The attractiveness to the VOC mixtures changed from fatty acids over ester and sulfides to aromatic cyclic compounds with increasing age (Friedman test and Wilcoxon test P < 0.05).
Although 7 day old mated females reacted almost identically to the carcass, regardless of their diet and their egg load, the volatile composition to which they responded was very different (Fig. 5). The protein-deprived females were attracted to cyclic compounds only (Friedman test P = 0.003), whereas the gravid, protein-fed females were interested in fatty acids Friedman test P = 0.001) and sulfides (Friedman test P = 0.022) (Wilcoxon test for all < 0.05).
Discussion
Having confirmed consistent and synchronous egg development in our rearing depending on protein nutrition (as described in Mackerras 1933; Vogt et al. 1974; Stoffolano et al. 1995; Barton-Browne 2001) we can interpret the searching behavior for carcasses as a search for food resource and mating or oviposition sites with respect to the physiological status of the female flies.
We refer to the females reared together with males as mated females as they had the chance to mate quickly (Pollock 1971). However, only 25 % of our 3 day old females were inseminated and 52 % after 7 days (Fig. 3). Those females were protein-deprived and it is likely that protein-fed females had a higher percentage of insemination as the egg load has a positive effect on the female to mate (Papaj 2000 and 2005; Aak and Knudsen 2012).
The mouse, despite the fine gauze, is a visual cue and could have influenced the behavior as olfactory and visually mediated behaviors are often associated (Morehead and Feener 2000; Wall and Fisher 2001; Gomes et al. 2007; Aak and Knudsen 2011).
We are also aware that attraction is generally considered a result of blends including the ratio between central compounds of chemicals (Ashworth and Wall 1994; Morris et al. 1998; Hansson 1999; Urech et al. 2004; Bruce et al. 2005; Cork and Hall 2007; Aak et al. 2010; Aak and Knudsen 2012) and that the mixtures of compound classes give only limited answers to what is important for different physiological stages, and that further experiments with single compounds and compound combinations are necessary.
Nevertheless are the results of this study, including the detailed analysis of attractive compounds, important to indicate the attraction of VOCs to gravid necrophagous flies of public health concern. The quantity and ratio of the tested compounds reflect those of an attractive decomposition stage, even if they are split in compound classes, characterized by a high amount of polysulfides, intermediate amounts of alcohol, fatty acids and cyclic compounds, and low amounts of ester and aldehydes.
The response to the mouse carcass was low and the differences between all females were minimal 1 day after the adults emerged, as expected, because there was little time to mate and the diet had not much influence on the egg development thus far.
However, the unmated protein-deprived females responded most strongly to fatty (carboxylic) acids 1 day after emergence, confirming results shown for houseflies by Cossé and Baker (1996) and Kelling (2001) and for blowflies Kasper et al. accepted (butanoic acid and propanoic acid). It is possible that their physiological condition requires the fatty acids and they incorporate them into their tissue; generally without modification of the fatty acids (sugars and certain amino acids are converted into fatty acids before incorporated into tissues) (Stanley-Samuelson et al. 1988; Simopoulos and Salem 1992). In addition, there is continuous fatty acid turnover in tissue lipids by hydrolytic and transferring enzymes (Spike et al. 1991).
As the response of protein-fed unmated females to the offered carcass was low compared to the other females, we assume their behavior was indifferent because they do not require more proteins than they obtained from their food. Their egg maturation developed well (see Fig. 2) but as they had not yet mated, no oviposition site was required either (Ashworth and Wall 1994).
In contrast, the unmated females reacted strongly to the carcass if no protein was given during their rearing. Without protein, and therefore with undeveloped eggs, the attraction of protein-rich resources occurred always 3 days after the female flies emerged regardless of the state of gravidity. Especially between 3 and 7 days the response was high because during these days, before the yolk proteins are ingested in the oocytes (stage IV), proteins are required (Stoffolano et al. 1995; Barton-Browne 2001) and a protein-deficiency triggers a searching behavior for the same (Dethier 1961; Aak and Knudsen 2012).
Three days after emergence the mated flies were significantly attracted to sulfides and esters when no protein-enriched diet was taken. It is mainly the emission of volatile sulfides, which indicates protein, because it occurs subsequently to protein degradation caused by enterobacterial degradation of cysteine and methionine (Wahl et al. 1999; Dent et al. 2004).
The role of sulfides as key compounds for necrophagous insects in detecting carrion and as a resource for reproduction is already well examined (Emmens and Murray 1982; Nilssen et al. 1996; Stensmyr et al. (2002), Woodard 2006; Kalinová et al. 2009; Podskalská et al. 2009; Paczkowski et al. 2012; Kasper et al., accepted).
Emmens and Murray (1982), for example, demonstrated that the oviposition behavior of L. cuprina was strongly affected by bacterial products, especially by sulfur compounds (also see Ashworth and Wall 1994).
The attractiveness of sulfides to necrophagous flies was also suggested by Stensmyr et al. (2002), who showed that blowflies respond to dimethyl-mono-, dimethyl-di- and dimethyl-trisulfide, emitted by both plants and meat in an identical manner.
In contrast, more tests are required in order to better understand the role of esters in the nutrition and searching behavior of flies. In the current study, only 3-day-old females preferred the ester mixture. Esters have a pleasant fruity odor (Kim et al. 1998), and occur after fermentation, which is attractive for several insects (El-Sayed et al. 2005; Ponnusamy, et al. 2008). Overall, the amount of esters emitted by the tested carcass was very low (4 % of the total emitted VOCs). The predominant ester was ethylbutyrate (Kasper et al. 2012), which in ripe fruits, for example, attracts gravid female fruit flies (Diptera: Tephritidae) (Eisemann and Rice 1992; Malo et al. 2005). Insect pheromones also often contain esters (Leal 1998), whether ethylbutyrate invokes attractiveness for mated females of L. caesar to carcasses as an oviposition site, has yet to be confirmed.
After 7 days, the critical stage of egg development (stage IV) was reached, although the eggs had still not maturated without protein given, the mated flies’ response changed in favor of cyclic hydrocarbons.
Interestingly, the protein-deprived males showed the same response at the seventh day. However, the males showed, regardless of age and nutrition, similar attraction to odors of cyclic hydrocarbons (e.g., phenol and indole (see Table 2) and sulfides. In addition to carcasses, these compounds are known from feces and manure, which is a regular food resource of many dipterans, in particular for Calliphoridae and Muscidae, and may also act as aggregation sites (Cossé and Baker 1996; Johnson and Jürgens 2010) and is preferred to carrion (Stoffalano et al. 1990). Other resources known for containing cyclic hydrocarbons are feces and manure, which are attractive for (Cossé and Baker 1996; Johnson and Jürgens 2010). Also Archer and Elgar (2003) found that males visited carcasses only early after death.
A searching behavior of the males, indicative of the need to obtain protein to build up their readiness for mating (Nakagawa et al. 1994; Stoffolano et al. 1995), was not noticeable.
Although the attractiveness to the alcohol/ketones increased with age in both sexes regardless the diet, those substances did never result in a significant attraction. However, 3-hydroxybutanone, also known as acetion, which is a by-product of bacterial activity after decarboxylation of proteins, was thought to be a key compound group for necrophagous insects in the detection of oviposition sites and resources for the offspring and brood care (Robacker and Lauzon 2002; Robacker et al. 2004; Johansen et al. 2014).
In addition the mouse carcass emitted 1-octene-3-ol and hexanol (Table 2), which are characteristic of fungal decomposition (Pfeil and Mumma 1993), and which is predominantly produced during decomposition in the cold and dry environment. It seems important to enable flies to identify their resources as previously shown by Cork (1994), Cork and Hall (2007) and Schoefiel et al. (1995). In order to explain the role of the alcohol/ketones compounds in terms of resource searching behavior, further investigations are necessary.
Also the aldehydes were not significantly frequented by any of the tested flies regardless of their physiological condition. In previous studies (Paczkowski et al. 2012; Johansen et al. 2014) had shown that heptanal and nonanal were key compounds for Calliphora vicina Robineau-Desvoidy (Diptera: Calliphoridae). Experiments with single compounds, possibly including electro-antenno-grammes, are aspired for Lucilia species.
The mated females fed with protein responded in a similar manner to the mated, protein-deprived flies (Wall and Warnes 1994). They reacted almost identically to the carcass after 7 days. Strikingly, the volatile composition, to which they responded, was very different. When reared with protein the mated females preferred fatty acids and sulfides. Here, the difference in the elicitor is particularly obvious, as gravid females might search for an oviposition site, which offers fatty acids for their offspring or they build it into the egg yolk directly. Cork and Hall (2007) and Urech et al. (2004) showed that blowflies were attracted to short-chained fatty acids, which might lead to the same assumption. We assume the ratio of both sulfides and fatty acids is important for recognizing the right stage for the offspring. They continued to show a high response after 10 days (Hammak et al. 1987) as they have still developed eggs, whereas the attraction to the mouse carcass decreased in the mated, protein-deprived females.
In summary these results confirmed that the combination “nutrition and mating” was relevant at day three, whereas after that, mating without developed eggs did not strongly influence the behavior of the females. In contrast, a protein-enriched diet enabled egg maturation, and subsequent mating triggered a significantly different response. In addition the combination “nutrition and age” influenced the behavior too, and a protein-poor diet increased the attraction to the dead mouse, especially between days 3 and 7. With different elicitors due to these different physiological conditions, we demonstrated that the key compounds of the olfactory mediated behavior changed drastically.
References
Aak A, Knudsen GK (2011) Sex differences in olfaction-mediated visual acuity in blowflies and its consequences for gender-specific trapping. Entomol Exp et Applicata 139:25–34
Aak A, Knudsen GK (2012) Egg developmental status and the complexity of synthetic kairomones combine to influence attraction behaviour in the blowfly Calliphora vicina. Physiol Entomol 37:127–135
Aak A, Knudsen GK, Soleng A (2010) Wind tunnel behavioural response and field trapping of the blowfly Calliphora vicina. Med Vet Entomol 24:250–257
Aak A, Birkemoe T, Knudsen GK (2011) Efficient mass trapping - catching the pest species Calliphora vicina (Diptera, Calliphoridae) in Norwegian stockfish production. J Chem Ecol 37:924–931
Adams TS, Mulla MS (1967) The reproductive biology of Hippelates collusor (Diptera: Chloropidae). II Gametogenesis. Ann Entomol Soc Am 60:1177–1182
Adams TS, Reinecke JP (1979) The reproductive physiology of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae). I Oogenesis. J Med Entomol 15(5–6):472–483
Aluja M, Díaz-Fleischer F, Papaj DR, Lagunes G, Sivinski J (2001) Effects of age, diet, female density, and the host resource on egg load in Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae). J Inst Physiol 47:975–988
Anderson GS, VanLaerhoven L (1996) Initial studies on insect succession on carrion in Southwestern British Columbia. J Forensic Sci 41:617–625
Archer MS, Elgar MA (2003) Effects of decomposition on carcass attendance in a guild of carrion-breeding flies. Med Vet Entomol 17:263–271
Ashworth JR, Wall R (1994) Responses of the sheep blowflies Lucilia sericata and L. cuprina to odor and the development of semiochemical baits. Med Vet Entomol 8:303–309
Ashworth JR, Wall R (1995) Effects of ovarien development and protein deprivation on the activity and locomotor responses of the blowfly Lucilia sericata, to liver odour. Physiol Entomol 20:281–285
Barton-Browne L (1993) Physiologically induced changes in resource-oriented behavior. Ann Rev Entomol 38:1–23
Barton-Browne L (2001) Quantitative aspects of the regulation of ovarian development in selected anautogenous Diptera: integration of endocrinology and nutrition. Entomol Exp Appl 100:137–149
Barton-Browne L, Bartell RJ, Shorey HH (1969) Pheromone-mediated behavior leading to group oviposition in the blowfly Lucilia cuprina. J Ins Physiol 15:1003–1014
Barton-Browne L, Van Gerwen ACM, Smith PH (1987) Relationship between mated status of females and their stage of ovarian development in field populations of the australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Bull Entomol Res 77:609–615
Boeddeker N, Kern R, Egelhaaf M (2003) Chasing a dummy target: smooth pursuit and velocity control in male blowflies. Proc R Soc B 270:393–399
Borgia G (1981) Mate selection in the fly Scatophaga stercoraria: female choice in a male-controlled system. Anim Behav 29:71–80
Broce AB (1980) Sexual behavior of screwworm flies stimulated by swormlure-2. Ann Entomol Soc Am 73:386–389
Broughan JM, Wall R (2006) Control of sheep blowfly strike using fly-traps. Vet Parasitol 135:57–63
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Carsten LD, Papaj DR (2005) Effects of reproductive state and host resource experience on mating decisions in a walnut fly. Behav Ecol 16:528–533
Cork A (1994) Identification of electrophysiologically-active compounds for new-world screwworm, Cochliomyia hominivorax, in larval wound fluid. Med Vet Entomol 8:151–159
Cork A, Hall MJR (2007) Development of an odour-baited target for female New world screwworm, Cochliomyia hominivorax: studies with host baits and synthetic wound fluids. Med Vet Entomol 21:85–92
Cossé AA, Baker TC (1996) House flies and pig manure volatiles: wind tunnel behavioral studies and electrophysiological evaluations. J Agri Entomol 13:301–317
Cragg JB (1956) The olfactory behavior of Lucilia species (Diptera) under natural conditions. Ann Appl Biol 44:467–477
Crnjar R, Yin CM, Stoffolano JG Jr, Tomassini BI, Liscia A, Angioy AM (1990) Influence of age on the electroantennogram response of the female blowfly Phormia-regina Diptera Calliphoridae. J Ins Physiol 36:917–922
Dent BB, Forbes SL, Stuart BH (2004) Review of human decomposition process in soil. Environ Geol 45:576–585
Dethier VG (1961) Behavioral aspects of protein ingestion by the blowfly, Phormia regina Meigen. Biol Bull Mar Biol Lab W H 121:456–470
Eisemann CH, Rice MJ (1992) Attractants for the gravid Queensland fruit fly Dacus tryoni. Entomol Exp Appl 62:125–130
El-Sayed AM, Heppelthwaite VJ, Manning LM, Gibb AR, Suckling DM (2005) Volatile constituents of fermented sugar baits and their attraction to Lepidopteran Species. J Agric Food Chem 53:953–958
Emmens RL, Murray MD (1982) The role of bacterial odours in oviposition by Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), the Australian sheep blowfly. Bull Entomol Res 72:367–375
Gomes L, Gomes G, Casarin FE, Da Silva IM, Sanches MR, Von Zuben CJ, Fowler HG (2007) Visual and olfactory factors interaction in resource-location by the blowfly, Chrysomya megacephala (Fabricius) (Diptera : Calliphoridae), in natural conditions. Neotrop Entomol 36:633–639
Hammak L, Bromel M, Duh FM, Gassner G (1987) Reproductive factors affecting responses of the screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae), to an attractant of bacterial origin. Ann Entomol Soc Am 80:775–780
Hansson BS (1999) Insect olfaction. Springer, Berlin
Harlow PM (1956) A study of ovarian development and its relation to adult nutrition in the blowfly Protophormia terrae-novae (R.D.). J Exp Biol 33:777–97
Hartlieb E, Anderson P (1999) Chapter 11: Olfactory-released behavuiours. In: Hansson B (ed) Insect olfaction. Springer Verlag, Berlin Heidelberg New York, pp 315–349
Harvey B, Bakewell M, Felton T, Stafford K, Coles GC, Wall R (2010) Comparison of traps for the control of sheep blowfly in the UK. Med Vet Entomol 24:210–213
Hayes EJ, Wall R, Smith KE (1999) Mortality rate, reproductive output, and trap response bias in populations of the blowfly Lucilia sericata. Ecol Entomol 24:300–307
Jang EB (1995) Effects of mating and accessory gland injections on olfactory-mediated behavior in the Female Mediterranean Fruit fly, Ceratitis capitata. J Ins Physiol 41:705–710
Jang EB (2002) Physiology of mating behavior in Mediterranean fruit fly (Diptera: Tephritidae): chemoreception and male accessory gland fluids in female post-mating behavior. Flor Entomol 85:89–93
Johansen H, Solum M, Knudsen GK, Hågvar EB, Norli HR, Aak A (2014) Blow fly responses to semiochemicals produced by decaying carcasses. Med Vet Entomol 28:26–34
Johnson SD, Jürgens A (2010) Convergent evolution of carrion and faecal scent mimicry in fly-pollinated angiosperm flowers and a stinkhorn fungus. S Afr J Bot 76:796–807
Kalinová B, Podskalská H, Růžička J, Hoskoec M (2009) Irresistible bouquet of death – how are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwiss 96:889–899
Kasper J, Caspers B, Wessel A et al The attraction of different decomposition stages to Lucilia caesar (Diptera: Calliphoridae). Physiol. Entomol. accepted
Kasper J, Mumm R, Ruther J (2012) The composition of carcass volatile profiles in relation to storage time and climate conditions. Forensic Sci Int 223:64–71
Kelling FJ (2001) Olfaction in houseflies – morphology and electrophysiology, 7th edn. Universty Library Groningen, Proefschrift Rijksuniversiteit Groningen, pp 105–119
Kim J, Altreuter DH, Clark DS, Dordick JS (1998) Rapid synthesis of fatty acid esters for use as potential food flavors. JAOCS 75:1109–1113
Lachmann AD, Papaj DR (2001) Effect of host stimuli on ovariole development in the walnut fly, Rhagoletis juglandis (Diptera, Tephritidae). Physiol Entomol 26:38–48
Laurence BR, Pickett JA (1985) An oviposition pheromone for Culex quinquefasciatus Say (Diptera: Culicidae). Bull Entomol Res 75:283–290
Leal WS (1998) Chemical ecology of phytophagous scarab beetles. Annu Rev Entomol 43:39–61
Mackerras MJ (1933) Observations on the life-histories. Nutritional requirements and fecundity of blowflies. Bull Entomol Res 24:353–362
Malo EA, Cruz-López L, Toledo J, Del Mazo A, Virgen A, Rojas JC (2005) Behavioral and electrophysiological responses of the mexican fruit fly (Diptera: Tephritidae) to guava volatiles. Flor Entomol 88:364–371
McCall PJ, Cameron MM (1995) Oviposition pheromones in insect vectors. Parasitol Today 11:352–355
Morehead SA, Feener DH (2000) Visual and chemical cues used in host location and acceptance by a dipteran parasitoid. J Ins Behav 13:613–625
Morris MC, Morrison L, Joyce MA, Rabel B (1998) Trapping sheep blowflies with lures based on bacterial cultures. Aust J Exp Agric 38:125–130
Nakagawa A, Iwama A, Mizukami A (1994) Age-dependant changes related to reproductive development in the odor preference of blowflies, Phormia regina and fleshflies, Boetteherisca peregrina. Zool Sci 11:725–730
Nilssen AC, Tømmerås BÅ, Schmid R, Evensen SB (1996) Dimethyl trisulphide is a strong attractant for some calliphorids and a muscid but not for the reindeer oestrids Hypoderma tarandi and Cephenemyia trompe. Entomol Exp Appl 79:211–218
Norris KR (1965) The bionomics of blowflies. Ann Rev Entomol 10:47–68
Omar AH (1992) Protein in the diet of male blowfly, Chrysomyia albiceps (Wiedemann). J Egypt Soc Parasitol 22:137–44
Otronen M (1995) Energy reserves and mating success in males of the yellow dung Fly, scathophaga stercoraria. Funct Ecol 9:683–688
Paczkowski S, Maibaum F, Paczkowska M, Schutz S (2012) Decaying mouse volatiles perceived by calliphora vicina Rob.-desv. J Forensic Sci 57:1497–1506
Papaj DR (2000) Ovarian dynamics and host use. Ann Rev Entomol 45:423–448
Papaj DR (2005) Ovarian dynamics in relation to host quality in the walnut-infesting Fly, rhagoletis juglandis. Funct Ecol 19:396–404
Parker GA (1968) The sexual behavior of the blowfly, protophormia terrae-novae R-D. Behavior 32:291–308
Parker GA (1970a) The reproductive behavior and the nature of sexual selection in Scatophaga stercoraria L. (Diptera: Scatophagidae): I. Diurnal and seasonal changes in population density around the site of mating and oviposition. J Anim Ecol 39:185–204
Parker GA (1970b) The reproductive behavior and the nature of sexual selection in scatophaga stercoraria L. (diptera: scatophagidae): II. The fertilization rate and the spatial and temporal relationships of each sex around the site of mating and oviposition. J Anim Ecol 39:205–228
Parker GA (1974) The reproductive behavior and the nature of sexual selection in scatophaga stercoraria L. (diptera: scatophagidae). IX. Spatial distribution of fertilization rates and evolution of male search strategy within the reproductive area. Evolution 28:93–108
Pfeil RM, Mumma RO (1993) Bioassassay for evaluating attraction of the phorid fly, Megaselia halterata to compost colonized by the commercial mushroom, Agaricus bisporus and to 1-octen-3-ol and 3-octanone. Entomol Exp Appl 69:137–144
Podskalská H, Růžička J, Hoskovec M, Šálek M (2009) Use of infochemicals to attract carrion beetles into pitfall traps. Entomol Exp Appl 132:59–64
Pollock JN (1971) An experimentally tested model of the cumulative mating frequency curve for Lucilia sericata (Mg.) (Diptera: Calliphridae). Bull Entomol Res 60:671–682
Ponnusamy L, Xu N, Nojima S, Wesson DM, Schal C, Apperson CS (2008) Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proceed Nal Acad Sci 105:9262–9267
Robacker DC, Lauzon CR (2002) Purine metabolizing capability of Enterobacter agglomerans affects volatiles production and attractiveness to Mexican fruit fly. J Chem Ecol 28:1549–1563
Robacker DC, Lauzon CR, He X (2004) Volatiles production and attractiveness to the Mexican fruit fly of Enterobacter agglomerans isolated from apple maggot and Mexican fruit flies. J Chem Ecol 30:1329–1347
Schoefield S, Cork A, Brady J (1995) Electroantennogram responses of the stable fly, Stomoxys calcitrans, to components of host odor. Physiol Entomol 20:273–280
Schuhmann H (1965) Merkblaetter ueber angewandte parasirtenkunde und schaedlingsbekaempfung. Merkblatt Nr. 11 Die schmeissfliegengattung calliphora. Angew Parasitol 6:1–17 [Suppliment]
Schuhmann H (1971) Merkblaetter ueber angewandte parasirtenkund und schaedlingsbekaempfung. Merkblatt Nr. 18, Die gattung Lucilia (goldfliegen). Angew Parasitol 12:1–20 [Suppliment]
Shorey HH, Bartell RJ, Browe LBB (1969) Sexual stimulation of males of Lucilia cuprina (Calliphoridae) and Drosophila melanogaster (Drosophilidae) by the odors of aggregation sites. Ann Entomol Soc Am 62:1419–1421
Siegel S, Castellan NJ (1988) Nonparametric statistics for the behavioral sciences. McGraw-Hill, New York
Simopoulos AP, Salem N (1992) Egg yolk as a source of long-chain polyunsaturated fatty acids in infant feeding. Jr Am J Clin Nutr 55:411–414
Sivinski J (1988) Unusual female-aggregated mating systems in phorid flies. J Ins Behav 1:123–128
Spike BP, Wright RJ, Danielson SD, Stanley-Samuelson DW (1991) The fatty acid compositions of phospholipids and triacylglycerols from two chinch bug species Blissus leucopterus leucopterus and B. iowensis (Insecta: Hemiptera; Lygaeidae) are similar to the characteristic dipteran pattern. Comp Biochem Physiol B 99:799–802
Staedler E (2002) Chemoecology of insect eggs and Egg deposition, plant chemical cues important for egg deposition in herbivorous insects. In: Hilker M, Meiners T (eds) Chemoecology of insects, 7th edn. Blackwell Verlag GmbH, Berlin – Vienna
Stanley-Samuelson DW, Jurenka RA, Cripps C, Blomquist GJ, de Renobales M (1988) Fatty acids in insects: composition, metabolism and biological significance. Arch Insect Biochem Physiol 9:1–33
Steidle JLM, Schöller M (1997) Olfactory host location and learning in the granary weevil parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). J Ins Behav 10:331–342
Stensmyr MC, Urru I, Collu I, Celander M, Hansson BS, Angioy A-M (2002) Pollination: rotting smell of dead-horse arum florets. Nature 420:625–626
Stevens J, Wall R (1997) The evolution of ectoparasitism in the genus Lucilia (Diptera: Calliphoridae). Int J Parasitol 27:51–59
Stoffalano JG, Bartley MM, Yin C-M (1990) Male and female phormia Regina (diptera: calliphoridae) trapped at two different baits in the field. Ann Entomol Soc Am 83:603–606
Stoffolano JG, Li M-F, Sutton JA, Yin C-M (1995) Feces feeding by adult phormia Regina (diptera: calliphoridae): impact on reproduction. Med Vet Entomol 9:388–392
Theunissen J (1973) Egg chamber development in the onion fly, hylemya antiqua (meigen) (diptera: anthomyiidae). Int J Insect Morph Embryol 2:87–99
Urech R, Green PE, Rice MJ, Brown GW, Duncalfe F, Webb P (2004) Composition of chemical attractants affects trap catches of the Australian sheep blowfly, Lucilia cuprina, and other blowflies. Jo Chem Ecol 30:851–866
Urech R, Green PE, Rice MJ, Brown GW, Webb P, Jordan D, Wingett M, Mayer DG, Butler L, Joshua E, Evans I, Toohey L, Dadour IR (2009) Suppression of populations of Australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), with a novel blowfly trap. Austr J Entomol 48:182–188
Van der Niet T, Hansen DM, Johnson SD (2011) Carrion mimicry in a South African orchid: flowers attract a narrow subset of the fly assemblage on animal carcasses. Ann Bot 107:981–992
Vass AA, Smith RR, Thompson CV, Burnett MN, Wolf DA, Synstelien JA, Dulgerien N, Eckenrode BA (2004) Decompositional odor analysis database. J Forensic Sci 49:760–769
Vogt WG, Woodburn TL, Tyndale-Biscoe M (1974) A method of age determination in Lucilia cuprina using cyclic changes in the female reproductive system. Bull Entomol Res 64:365–370
Wahl HG, Hoffmann A, Luft D, Liebich HM (1999) Analysis of volatile organic compounds in human urine by headspace gas chromatography–mass spectrometry with a multipurpose sampler. J Chromatogr 847:117–125
Wall R, Fisher P (2001) Visual and olfactory cue interaction in resource- location by the blowfly, Lucilia sericata. Physiol Entomol 26:212–218
Wall R, Warnes ML (1994) Responses of the sheep blowfly Lucilia sericata to carrion odour and carbon dioxide. Entomol Exp Appl 3:239–246
Woodard CBS (2006) Odour masking of a vertebrate carcass by a burying beetle (Nicrophorus marginatus). - A thesis in biology for the Degree of Master of Science, Faculty of Texas Tech University
Woodburn TL, Vogt WG (1982) Attractiveness of merino sheep before and after death to adults of Lucilia cuprina (wiedemann) (diptera: calliphoridae). J Aust Entomol Soc 21:131–134
Yin C-M, Zou B-X, Li M-F, Stoffolano JG Jr (1993) Discovery of a midgut peptide hormone which activates the endocrine cascade leading to oogenesis in Phormia regina (Meigen). J Ins Physiol 39:165–171
Zhu JWJ, Chaudhury MF, Tangtrakulwanich K, Skoda SR (2013) Identification of oviposition attractants of the secondary screwworm, Cochliomyia macellaria (F.) released from rotten chicken liver. J Chem Ecol 39:1407–1414
Zou BX, Yin CM, Stoffolano JG, Tobe SS (1989) Juvenile hormone biosynthesis and release during oocyte development in phormia Regina meigen. Physiol Entomol 14:233–239
Acknowledgments
We are very grateful to M. Hilker and J. Ruther for providing the synthetic chemicals and advice for the set up. In particular we thank C. Timm for his contribution to illustrations and for essential comments on this article. We also thank the anonymous reviewer for comments to an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasper, J., Hartley, S., Schatkowski, S. et al. The Influence of the Physiological Stage of Lucilia Caesar (L.) (Diptera: Calliphoridae) Females on the Attraction of Carrion Odor. J Insect Behav 28, 183–201 (2015). https://doi.org/10.1007/s10905-015-9491-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-015-9491-7