Abstract
Heteropteran insects produce a series of volatile compounds from their scent glands that protect them from predators and parasites. These compounds also play roles in chemical communication that elicit aggregation, dispersal, and mating behaviors. Hygia lativentris (Coreidae) adults frequently aggregate on host plants. When disturbed, they quickly disperse with the release of a sour smell, suggesting that these bugs possess an alarm pheromone in their secretions. This adult secretion-induced dispersal has been examined with a laboratory assay. Hexanal, the predominant component of the adult secretion was identified as a component of the alarm pheromone by evaluation of the adult bug’s response time and escape distance from the chemical source. Physicochemical analyses with gas chromatography/mass spectrometry and nuclear magnetic resonance spectroscopy revealed that secretory components differed between nymphs and adults, and also during adult aging. Nymphs produced two unsaturated compounds, (E)-2-hexenal and (E)-4-oxo-2-hexenal, together with hexanal and 1-hexanol, which were found in all developmental stages. In adults, hexyl acetate was the major component of secretions within 3 days of emerging, while the amount of this ester decreased and those of hexanal, hexanoic acid, and hexanal trimer increased with aging. The decomposition of hexyl acetate into hexanal via 1-hexanol was attributed to the presence of esterases and alcohol dehydrogenases specifically found in adult secretory glands. In contrast, the formation of a hexanal trimer may be due to a non-enzymatic reaction under acidic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terrestrial heteropterans produce and release pungent secretions that are mostly composed of short-chain aliphatic aldehydes, alcohols and esters, alkanes, terpenes, and phenolics. These secretions play roles in chemical communication and defense (Aldrich 1988; Millar 2005; Moraes et al. 2008; Noge et al. 2012b). These volatile components normally are stored in the dorsal abdominal glands (DAGs) of nymphs and the metathoracic scent glands (MTGs) of adults. Heteropteran nymphs leave their glandular contents on the exuviae when they metamorphose into adults (Aldrich 1988; Borges and Aldrich 1992). One of the characteristic features before and after this metamorphosis is change in chemical composition between nymphs and adults. Two patterns of nymph-adult chemical polymorphisms have been identified: nymphs and adults possess completely different sets of components (e.g., Prestwich 1976; Prudic et al. 2008), and nymphs and adults have a series of common components with their own life-stage-specific components (e.g., Farine et al. 1993; Fávaro et al. 2012; Noge et al. 2012a). Additionally, in some cases, composition changes have been detected during adult stages in which a higher amount of an ester has been detected only from newly molted adults (Aldrich and Yonke 1975; Aldrich et al. 1978; Prestwich 1976). This phenomenon has been explained by the presence of esterases that degrade the esters in the MTG of Leptoglossus phyllopus together with alcohol dehydrogenases (ADHs) (Aldrich et al. 1978). However, this is the only example that shows the enzymes involved in chemical composition changes.
Some of the chemical components found in heteropteran scent glands function as either aggregation, alarm, and sex pheromones or as defense substances. The identification of heteropteran pheromones is important in order to incorporate pheromones into pest management (e.g., Fávaro et al. 2012; Yasuda and Higuchi 2012; Zarbin et al. 2012). Findings also have contributed to a deeper understanding of the biological aspects of Heteroptera mediated by certain chemical signals. Although advances in chemical analysis techniques have enabled the easier identification of heteropteran secretory compounds, their roles need to be investigated in more detail.
Hygia lativentris (Motschulsky) is a coreid bug that occurs in Japan, Korea, and China. Adult bugs commonly aggregate on the stems of their host plants. Examples are the Japanese knotweed, Fallopia japonica, the giant knotweed, F. sachalinensis, and the Japanese dock, Rumex japonicus, (Polygonaceae) around Akita City, Japan. Hygia lativentris is not an economically important species, but, from an ecological point of view, the aggregation of this bug after mating is unique. When efforts are made to catch aggregating bugs, they drop from the host plants as in another coreid bug, Anoplocnemis dallasiana (Prestwich 1976). They may be able to detect unnatural airflow or aerial vibrations. When disturbed, they also drop to the ground or escape along the plant stem with the release of a sour, apple-like smell. The scent discharge appears to elicit escape behavior. We speculated that the adults of H. lativentris possess an alarm pheromone, and attempted to identify its components and their functions. Although the secretions of H. lativentris have not yet been analyzed, hexanal was identified from the secretions of the congener, H. opaca (Uhler) 50 years ago (Tsuyuki et al. 1965). An ester-like compound also was detected in the secretions of H. opaca, but its structure was not determined. Here, we describe the identification of the alarm pheromone of H. lativentris, together with changes in the composition of this bug’s secretions during its developmental stages. We report the presence of enzymes found in the MTG that may be involved in the changes in composition and pheromone production.
Methods and Materials
Insects
Nymphs and adults of Hygia lativentris (Motschulsky) (see Online Resource 1) were collected on the stems of F. japonica and F. sachalinensis in Kamishinjyo, Akita City, Japan in May and August, 2012, and May to August, 2013. They were fed on F. japonica or F. sachalinensis stems in the laboratory for the period of the experiments. Most adults were mating when they were collected, and the eggs laid were collected and moved to a new cage. Hatched nymphs were reared in the laboratory, and each nymph stage was collected by using a toothpick without causing disturbance. Young adults were defined as those within 3 days of ecdysis, and adults more than 2 weeks-old after ecdysis were treated as mature adults. The term “mature” refers to old and not sexually mature.
Extraction of H. lativentris Secretions
To identify and quantify the secretory components of each developmental stage, nymphs, young adults, and mature adults were extracted individually in n-hexane containing 20 ng/μl of 1-dodecene (Wako Pure Chemical industries, Osaka, Japan) as an internal standard for 5 min (N = 5 or 6 per developmental stage). The volume of solvent for extraction depended on the size of each stage: 200 μl for 1st, 2nd, and 3rd instars, 500 μl for 4th and 5th instars, and 1 ml for adults. The weights of individual 3rd instar nymphs and adults were measured before extraction. Since the weights of 1st and 2nd instar nymphs were very small, the total weight of 6 bugs was measured and the average weight of individuals was calculated.
To confirm the origin of the nymphal and adult compounds, the DAGs from 5th instars and the MTG complex from adults also were extracted in n-hexane. Nymphs and adults were refrigerated at 4 °C for more than 20 min for anesthesia without the premature discharge of their secretory components. A pair of DAGs with tergum were removed by dissection from the 5th instars, and then extracted in 200 μl of n-hexane for 5 min (N = 3). The DAGs of the 3rd or 5th instar nymphs also were collected from their exuvia within 24 hr after they ecdysed (each N = 3), and were macerated in 200 μl of n-hexane, and extracted for 5 min. The MTG complex was removed from anesthetized adults by dissection, and extracted in 1 ml of n-hexane for 5 min (N = 7 females and 7 males). All extracts (1 μl) were analyzed by gas chromatography linked to mass spectrometry (GC/MS) for chemical identification.
Chemical Analysis
Analyses by GC/MS were carried out using a Clarus 600 GC/MS (PerkinElmer, Shelton, CT, USA) operated at 70 eV using a DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, Inc., Santa Clara, CA, USA) with helium carrier gas at 1.0 ml/min. The oven temperature was programmed from 50 °C for 3 min, then at 10 °C/min to 290 °C and held for 5 min. The injector and detector temperatures were 250 °C.
1H and 13C NMR spectra were acquired on a JNM-ECS400 spectrometer (1H at 400 MHz and 13C at 100 MHz; JEOL, Tokyo, Japan) in CDCl3, using tetramethylsilane as an internal standard.
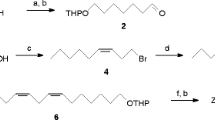
Six compounds were identified by comparing their GC retention times and mass spectra with those of commercial or synthesized standards. Hexanal, 1-hexanol [Tokyo Chemical Industry (TCI), Tokyo, Japan], (E)-2-hexenal (ACROS Organics, Geel, Belgium), and hexanoic acid (Wako Pure Chemical Industries) were commercially available products. 4-Oxo-(E)-2-hexenal (OHE) was synthesized from 2-ethylfuran (TCI) according to Moreira and Millar (2005). Hexyl acetate was synthesized by the acetylation of 1-hexanol according to Sakakura et al. (2007). Structures were confirmed by 1H and 13C NMR analysis.
Compounds 7–9 (Fig. 1) were isolated from the n-hexane extract obtained from 15 adults. After evaporating the solvent, the residue (15.3 mg) was applied to a silica gel column (600 mg), and successively eluted with 6 ml each of n-hexane, 1, 2, 3, 5, 20, and 50 % diethyl ether in n-hexane. The compositions of each elute were monitored by GC/MS. The 2 % diethyl ether in n-hexane fraction containing hexanal and compounds 8 and 9 was evaporated to remove hexanal. The remaining oil (1.2 mg) that contained compound 8 as the main component and compound 9 as the minor component (ratio 20:3) was subjected to NMR analyses (1H and 13C NMR, COSY, DEPT, HMQC). Compound 7 and hexanoic acid were found in 20 and 50 % diethyl ether in n-hexane fractions, respectively. After adding saturated NaHCO3 solution, compound 7 was isolated from the organic layer. Purified compound 7 decomposed easily into hexanal, and thus the identification was not completed.
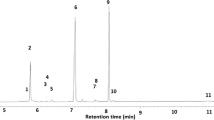
Typical gas chromatograms of secretions of Hygia lativentris a 4th instar nymph, b young adult, and c mature adult (1 hexanal; 2 (E)-2-hexenal; 3 1-hexanol; 4 4-oxo-(E)-2-hexenal (OHE); 5 hexyl acetate; 6 hexanoic acid; 7 unidentified compound; 8 hexanal trimer (cis-isomer); 9 hexanal trimer (trans-isomer)
(2α,4α,6α)-2,4,6-Tripentyl-1,3,5-trioxane (compound 8; cis-isomer) was synthesized according to Augé and Gil (2002) with a slight modification. Briefly, trimethylsilyl chloride (4 μl, 32 mmol; obtained from TCI) was added to hexanal (0.99 g, 9.9 mmol) under nitrogen, and the mixture was stirred overnight at room temperature. After removing trimethylsilyl chloride in vacuo, the resulting oil was purified by silica gel column chromatography by eluting with 2 % diethyl ether in n-hexane to give compound 8 as a colorless oil (0.54 g, 55 % yield). MS m/z (%): 229 (M − C5H11, 0.6), 201 (M − C5H11CO, 28), 101 (98), 99 (29), 83 (100), 82 (12), 72 (12), 71 (17), 57 (44), 56 (52), 55 (76); 1H NMR (CDCl3): δ 4.82 (3H, t, J = 5.4 Hz), 1.64 (6H, m), 1.39 (6H, m), 1.28 (12H, m), 0.87 (9H, t, J = 6.8 Hz); and 13C NMR (CDCl3): δ 101.79, 34.47, 31.66, 23.34, 22.62, 14.09.
Quantitative analysis of the seven identified compounds was performed with selected ion monitoring using the ions m/z 55 for (E)-2-hexenal and 1-dodecene (internal standard), m/z 56 for hexanal, 1-hexanol, and hexyl acetate, m/z 83 for OHE, and m/z 101 for compound 8. The relative ratio of the peak area to the internal standard was calculated, and the quantity of each compound per individual bug was determined by comparing the peak area ratio in the sample with those found in the calibration standard (1–300 ng/μl per compound).
Enzyme Preparation from MTG Complex
In order to determine whether enzymes in the MTG complex were associated with the changes observed in secretory components during adult aging, enzyme activities were detected by electrophoresis and activity staining methods. Six mature adult females of H. lativentris were refrigerated at −20 °C for 10 min in a glass vial to provoke the discharge of their secretory compounds. Each MTG complex was removed by dissection, and six MTG complexes were then homogenized in 45 μl of 40 mM Tris-HCl (pH 8.5) containing 20 % (v/v) of glycerol. The homogenate was centrifuged at 12,000 g for 5 min at 4 °C, and the resulting supernatant was recovered as an enzyme source. After adding a trace of bromophenol blue (BPB) as a marker, 15 μl of the enzyme source (equivalent to two females) were used for subsequent electrophoresis. The midgut of H. lativentris adults also was extracted with the same procedure. The enzyme source from the midgut, equivalent to one female, was used to detect enzyme activities.
Detection of Esterase and Alcohol Dehydrogenase (ADH)
Polyacrylamide vertical slab gel electrophoresis was performed at 4 °C according to the method reported by Noge et al. (2005) using a hand-cast gel (90 × 80 mm, 1 mm in thickness) with slight modifications. Briefly, the separating and stacking gels were made of 9.3 and 3.1 % (w/v) of acrylamide, respectively. The ratio of N,N’-methylenebis (acrylamide) (Wako Pure Chemical Industries) in the acrylamide solution was 2.6 % (w/w) for the separating gel and 20 % (w/w) for the stacking gel. The running buffer was 25 mM Tris and 192 mM glycine. Electrophoresis began at 100 V, and subsequently was performed at 140 V after the BPB entered the separating gel. For detecting esterase activity, the gel was immersed immediately into a solution containing Fast Blue RR (final concentration 2.5 mM; Wako Pure Chemical industries) in 10 mM Tris-HCl (pH 7.5), and then 2-naphthyl acetate (final concentration 2.6 mM; Wako Pure Chemical Industries) dissolved in acetone was added. The gel in the solution was kept at 37 °C. After detecting esterase activity, the gel was washed with distilled water several times and dried to keep as raw data. Alcohol dehydrogenase was stained in a similar manner by nitro blue tetrazolium (final concentration 0.5 mM; Wako Pure Chemical Industries), phenazine methosulfate (final concentration 0.3 mM; Wako Pure Chemical Industries), NADP+ (final concentration 0.6 mM; Oriental Yeast, Tokyo, Japan), and 1-hexanol (final concentration 32 mM) as a substrate in 15 mM Tris-HCl (pH 8.8). The experiment was repeated at least five times.
Alarm Pheromone Bioassay
In order to identify alarm pheromone activity of adult H. lativentris, we performed a behavioral assay using adult secretions and the identified compounds. A circular track was made by joining the ends of a plastic tube (35 cm long, 8 mm o.d.), and this was placed on a tripod stand in the open laboratory (26 °C ± 1 °C). An individual mature adult female or male was placed gently on the tube. The crude extract of adult secretion (0.5 ml, 0.5 bug equivalent) was loaded onto a filter paper disk (8 mm diam) and the disk was then placed 2 mm from the adult’s head. The time taken for the adult bug to change direction and how far it moved away from the starting point within 15 sec were measured as indicators of pheromone activity. If the bug did not respond to the treatment within the experimental period, the time taken to change direction was recorded as 15 sec due to constraints in statistical analyses. Crude extracts of the young and mature adults of both sexes were evaluated (N = 10 females and 10 males per treatment).
For testing individual compounds, a paper disk was loaded with 2 μl of each compound (hexanal, 1-hexanol, hexyl acetate, hexanoic acid, synthesized compound 8, (E)-2-hexenal, or OHE), and used in the behavioral assay described above. n-Hexane was used as a control treatment. The assay was repeated 20 times per compound using 10 females and 10 males. Each bug was tested twice, with a maximum of two different treatments over the course of 1 day. For example, 5 females and 5 males were treated with compound A in the first round and then exposed to compound B in the second round, while the other 5 females and 5 males were treated with compounds A and B in the reverse order. A total of 10 females and 10 males were treated with both compounds. The interval between trials was more than 1 h. No significant differences were observed in the results obtained between the first and second groups of bugs, suggesting that the previous treatment did not affect subsequent behavioral responses. Approximately 20 % of adults did not respond to any treatments, and they were excluded from the results and statistical analyses. Consequently, data obtained from 8 females and 8 males were used, and all data were analyzed with the Tukey-Kramer test using JMP 5.1.2 (SAS Institute 2003).
Compound Recovery Assay from Paper Disks
To evaluate the amounts of the compounds released from filter paper disks in the alarm pheromone assay, the amount of compound remaining on the disk after the experimental period was quantified by GC/MS. A paper disk loaded with 2 μl of each compound was kept for 15 sec in the same manner as that described for the alarm pheromone assay, and then extracted by 1 ml of n-hexane containing 20 ng/μl of 1-dodecene (N = 4 or 5 replicates per compound). A paper disk was extracted immediately after loading each compound as a control (N = 3 replicates per compound). All extracts were diluted 20 fold with n-hexane containing 20 ng/μl of 1-dodecene, and an aliquot of the dilution (1 μl) then was analyzed by GC/MS to quantify the remaining amount of the tested compound. Quantification of compounds was performed as described above.
Results
Chemical Profiles of Nymphal and Adult Secretions of H. lativentris
Analyses of the secretions of H. lativentris revealed that nymphs and adults emitted different compounds (Table 1, Figs. 1 and 2). Nymphs secreted hexanal, 1-hexanol, (E)-2-hexenal, and OHE. Unsaturated aldehydes were specific to nymphal secretions. Components found in nymphal secretions were constant among different instars, but the amount of each component increased with nymphal growth. Hexanal, 1-hexanol, hexyl acetate, and hexanoic acid were found in young and mature adult secretions, whereas three compounds (7–9) were detected only in mature adults. Hexanal was predominant and common in both the nymphs and adults. Components in the adult secretions changed qualitatively and quantitatively with aging (Table 1). The amounts of 1-hexanol and hexyl acetate decreased, while those of hexanoic acid and compound 8 increased with adult aging.
Compounds 8 and 9 had similar mass spectra, indicating that they were stereoisomers. Compound 8 was identified as (2α,4α,6α)-2,4,6-tripentyl-1,3,5-trioxane, a trimer of hexanal, by comparing its GC retention time and mass spectrum with those of the synthesized standard (Fig. 2). Although the fraction containing compound 8 from the adult secretion contained a small amount of compound 9, two-dimensional NMR analyses of the mixture identified the signals derived from each compound. The mass and NMR spectra of purified compound 8 from adult secretions were summarized as follows: MS m/z (%): 201 (M − C5H11CO, 23), 101 (96), 99 (26), 83 (100), 82 (16), 72 (14), 71 (18), 67 (13), 57 (51), 56 (59), 55 (80); 1H NMR (CDCl3): δ 4.82 (3H, t, J = 5.6 Hz), 1.64 (6H, m), 1.39 (6H, m), 1.28 (12H, m), 0.87 (9H, t, J = 6.8 Hz); and 13C NMR (CDCl3): δ 101.79, 34.47, 31.66, 23.34, 22.17, 14.09. Based on the NMR spectra of the mixture of compounds 8 and 9, compound 9 was tentatively identified as a stereoisomer of compound 8, (2α,4α,6β)-2,4,6-tripentyl-1,3,5-trioxane: MS m/z (%): 201 (M − C5H11CO, 8), 101 (73), 99 (4), 83 (100), 82 (10), 72 (10), 71 (7), 67 (10), 57 (49), 56 (65), 55 (74); 1H NMR (CDCl3): δ 5.17 (1H, t, J = 6.8 Hz), 5.12 (2H, t, J = 5.4 Hz), 1.64 (6H, m), 1.39 (6H, m), 1.28 (12H, m), 0.87 (9H, t, J = 6.8 Hz). The molecular ions of compounds 8 and 9 were confirmed as m/z 300 by GC/MS operated at 14 eV (data not shown). Compound 7 was not identified due to its low yield and instability. 2-Butyl-2-octenal was identified previously from the coreid bug, Amblypelta nitida (Baker et al. 1972). We synthesized this compound using an aldol condensation reaction of hexanal, and found that its GC retention time and mass spectrum were not consistent with those of compound 7.
Esterase and ADH in the MTG Complex of H. lativentris
Four esterases and two ADHs were found in the MTG of H. lativentris adults (Fig. 3). Esterase activities also were detected in the midgut, but did not correspond to those found in the MTG. No ADH activity was detected in the midgut.
Alarm Pheromone of Adult H. lativentris
Adult bugs exhibited an escape response to the crude extracts of adult secretions, and not to the control treatment (Fig. 4). Since no significant difference was observed in the escape responses of males and females, these results were combined. Adults quickly responded to each crude extract and turned around. Adults escaped significantly further away from the chemical source.
Behavioral responses of adult Hygia lativentris to adult secretions. a Time required to start an escape response (means ± SD sec; N = 16) and b changes in the distance to the chemical source (means ± SD cm; N = 16) after exposure to each crude extract. Bars with the same letter are not significantly different (P > 0.05)
Among the components identified in the adult secretions, hexanal exhibited strong alarm pheromone activity in H. lativentris (Fig. 5). Significant differences were observed between the response times of 1-hexanol, hexyl acetate, and hexanoic acid and that of the control treatment, while adult bugs did not escape after these treatments. Compound 8 was inactive in both the response time and escaping distance. Two nymph-specific compounds, (E)-2-hexenal and OHE, were significantly active as a signal for dispersal behavior compared to the control treatment (Fig. 5). No bugs dropped off the assay apparatus after the odor treatments.
Alarm pheromone activities of individual secretory components identified in Hygia lativentris. a Time required to start an escape response (means ± SD sec; N = 16) and b changes in the distance to the chemical source (means ± SD cm; N = 16) after exposure to each compound (2 μl). Bars with the same letter are not significantly different (P > 0.05). Hexanal trimer used in this assay was a synthetic cis-isomer
No significant differences were observed in the response times to the three different amounts of hexanal. However, the escaping distances elicited by 0.5 and 1 μl of hexanal were slightly less than that elicited by 2 μl of hexanal (Fig. 6).
The amount of volatiles that evaporated from the paper disk during the experimental period of the bioassay was different for each compound. The recovery of hexanal from the paper disk after 15 sec of the bioassay was 85.8 ± 3.3 % (mean ± SD). This indicated that the amount of hexanal volatilized during the bioassay was 220.2 ± 50.6 μg i.e., 14.2 ± 3.3 % of the amount loaded. The following amounts of compounds were volatilized during the experimental period: 1-hexanol (253.1 ± 63.2 μg; 15.5 ± 3.9 % of the amount loaded), hexyl acetate (250.2 ± 36.9 μg; 13.2 ± 1.9 %), hexanoic acid (214.8 ± 123.0 μg; 10.5 ± 6.0 %), cis-hexanal trimer (113.8 ± 88.8 μg; 5.2 ± 4.1 %), (E)-2-hexenal (118.4 ± 47.1 μg; 8.1 ± 3.2 %), and OHE (68.6 ± 44.4 μg; 6.8 ± 5.4 %).
Discussion
The results of the present study showed that the chemical profile of nymphal secretions differs from those of adult secretions in H. lativentris. The adults contain only saturated compounds, while the nymphs store both saturated and unsaturated compounds in the DAGs. This is common in the family Coreidae. Hexanal and hexyl acetate appear to be universally distributed in adult coreid bugs (Aldrich and Yonke 1975; Blatt et al. 1998; Tsuyuki et al. 1965; Waterhouse and Gilby 1964). Nymph-specific (E)-2-hexenal and OHE also have been detected in most of the nymphs belonging to this family (Aldrich and Yonke 1975; Eliyahu et al. 2012; Prestwich 1976). (E)-2-Hexenal is known to be a repellent against predators (Blum 1961; Eisner et al. 2005; Noge et al. 2012b), and OHE has been shown to exhibit deterrent activity and toxicity against mantids and ants, as well as antibacterial activity (Eliyahu et al. 2012; Noge et al. 2012a; Prudic et al. 2008). This finding suggests that the production of (E)-2-hexenal and OHE is beneficial in the nymphal chemical defense system. Recently, Soldi et al. (2012) reported that in Phthia picta the typical coreid compounds were present in the MTG reservoirs of both sexes, and the male-produced sex pheromone was detected only in the lateral accessory glands of the male MTG complex. However, in the present study, no qualitative differences were found between adult males and females, which is consistent with previous findings (Aldrich and Yonke 1975; Prestwich 1976). The adult bugs of H. lativentris commonly aggregate on host plants and cannot fly for dispersal. Thus, they may not require a sex-specific chemical attractant to find a mate from a distance.
The decreases observed in the amounts of hexyl acetate and 1-hexanol during adult aging were attributed to the presence of the esterases and ADHs specifically found in the adult MTG complex. Similar enzymes have been reported in another coreid bug, Leptoglossus phyllopus (Aldrich et al. 1978). De-esterification of hexyl acetate and oxidation of the resulting alcohol yields hexanal. This route may represent a supply source of the alarm pheromone in H. lativentris. One plausible explanation for this composition change is that the ester is a reservoir of the reactive agent, hexanal, for newly emerged adults, as discussed by Aldrich et al. (1978).
In contrast, hexanoic acid and compounds 8 and 9 (hexanal trimers), found mainly in mature adults, may be produced by non-enzymatic reactions. The amount of hexanoic acid frequently was found to increase, while that of hexanal decreased when H. lativentris adults died (personal observation by KN), suggesting that hexanoic acid is produced by autoxidation of hexanal in the scent gland. A hexanal trimer previously was identified as an autoxidizing product of methyl linoleate. In this reaction, formic acid, which is also produced by the autoxidation of methyl linoleate, catalyzes the trimerization of hexanal (Horvat et al. 1966). A small amount of hexanal trimers (compounds 8 and 9) and an unidentified compound 7 were produced when hexanal was mixed with hexanoic acid [100:1 (v/v)] for 1 week at 25 °C (data not shown). A hexanal trimer also was detected in the 10-week-old adults of L. phyllopus together with hexanoic acid (Aldrich et al. 1978).
Among the adult secretory components identified in the present study, only hexanal elicited an adult dispersal response. Hexanal previously was found to be the alarm pheromone in other heteropterans, for example, Dysdercus intermedius (Calam and Youdeowei 1968), L. zonatus (Leal et al. 1994), L. occidentalis (Blatt et al. 1998), and Thasus neocalifornicus (Prudic et al. 2008). Although adults also responded to 1-hexanol, hexyl acetate, and hexanoic acid, and turned away from the chemical source, these compounds did not elicit the dispersal response, thus suggesting that the induction of escape behavior requires hexanal as the specific signal. Future research should address how this switching of the two different behaviors (turning or dispersal) is regulated. Our results showed that adult bugs responded to the nymph-specific components, (E)-2-hexenal and OHE, and may reflect their life cycle in which nymphs are sometimes found with gregarious adult bugs. Alternatively, aldehydes with six carbons may be essential for eliciting the adult escaping response in this species. A nymph-specific component may also elicit the adult dispersal response. The adult bugs of L. zonatus were previously reported to respond to (E)-2-hexenal, which is not found in adult secretions (Leal et al. 1994).
The actual amount of hexanal evaporated during the experimental period was calculated to be 14.2 ± 3.3 % of the amount loaded onto the filter paper disk. When 2 μl of hexanal were used in the behavioral assay, the actual evaporated amount (220.2 ± 50.6 μg) was almost the same or less than the amount stored in one individual adult bug (Table 1). The adult escaping responses elicited by the smaller amount of hexanal (0.5 and 1.0 μl; Fig. 6) suggests that these experimental conditions reflected the bug’s behavior in nature. The behavioral assay was performed in an open space; thus, the bugs may have perceived a markedly lower amount of hexanal on their antennae. On the other hand, since the amounts of the different test compounds actually volatilized differed markedly (5.2–15.5 %), the results of our experiments with the same dose at source for each compound did not provide a reliable comparison of their relative alarm pheromone activities. However, the bugs would have been exposed to large amounts of all the components, but only hexanal caused a behavioral effect, suggesting that the other compounds were not components of the alarm pheromone.
In some cases, the role of the pheromone in aggregation and dispersal may change in a concentration-dependent manner in Heteroptera (Ishiwatari 1976; Lockwood and Story 1985) and other arthropods (Nishimura et al. 2002). In those cases, the compound functions as an aggregation pheromone at a low concentration and as an alarm pheromone at a high concentration. A previous study reported that aggregation pheromone activity was sometimes masked by other components in the secretions (Leal et al. 1994; Mizoguchi et al. 2003). The adults of H. lativentris frequently aggregate in their natural habitat, suggesting the existence of an aggregation signal. In this paper, we identified the alarm pheromone of H. lativentris, which enables us to investigate further whether the lower amount of hexanal or blends including hexanal may be involved in the bug’s aggregation, or whether there may be different compounds causing the aggregation.
References
Aldrich JR (1988) Chemical ecology of the Heteroptera. Annu Rev Entomol 33:211–238
Aldrich JR, Yonke TR (1975) Natural products of abdominal and metathoracic scent glands of coreid bugs. Ann Entomol Soc Am 68:955–960
Aldrich JR, Blum MS, Hefetz A, Fales HM, Lloyd HA, Roller P (1978) Proteins in a nonvenomous defensive secretion: biosynthetic significance. Science 201:452–454
Augé J, Gil R (2002) A convenient solvent-free preparation of 1,3,5-trioxanes. Tetrahedron Lett 43:7919–7920
Baker JT, Blake JD, MacLeod JK, Ironside DA, Johnson IC (1972) The volatile constituents of the scent gland reservoir of the fruit-spotting bug, Amblypelta nitida. Aust J Chem 25:393–400
Blatt SE, Borden JH, Pierce HD Jr, Gries R, Gries G (1998) Alarm pheromone system of the western conifer seed bug, Leptoglossus occidentalis. J Chem Ecol 24:1013–1031
Blum MS (1961) The presence of 2-hexenal in the scent gland of the pentatomid Brochymena quadripustulata. Ann Entomol Soc Am 54:410–412
Borges M, Aldrich JR (1992) Instar-specific defensive secretions of stink bugs (Heteroptera: Pentatomidae). Experientia 48:893–896
Calam DH, Youdeowei A (1968) Identification and functions of secretion from the posterior scent gland of fifth instar larva of the bug Dysdercus intermedius. J Insect Physiol 14:1147–1158
Eisner T, Eisner M, Siegler M (2005) Secret weapons. Defenses of insects, spiders, scorpions, and other many-legged creatures. Belknap Press of Harvard University Press, Cambridge
Eliyahu D, Ceballos RA, Saeidi V, Becerra JX (2012) Synergy versus potency in the defensive secretions from nymphs of two pentatomomorphan families (Hemiptera: Coreidae and Pentatomidae). J Chem Ecol 38:1358–1365
Farine J-P, Everaerts C, Brossut, R, Le Quére J-L (1993) Defensive secretions of nymphs and adults of five species of Pyrrhocoridae (Insecta: Heteroptera). Biochem Syst Evol 21:363–371
Fávaro CF, Santos TB, Zarbin PHG (2012) Defensive compounds and male-produced sex pheromone of the stink bug, Agroecus griseus. J Chem Ecol 38:1124–1132
Horvat RJ, Mcfadden WH, Ng H, Lane WG, Shepherd AD (1966) Identification of 2,4,6-trialkyl-1,3,5-trioxanes from autoxidized methyl linoleate by mass spectrometry. J Am Oil Chem Soc 43:350–351
Ishiwatari T (1976) Studies on the scent of stink bugs (Hemiptera: Pentatomidae) II. Aggregation pheromone activity. Appl Entomol Zool 11:38–44
Leal WS, Panizzi AR, Niva CC (1994) Alarm pheromone system of leaf-footed bug Leptoglossus zonatus (Heteroptera: Coreidae). J Chem Ecol 20:1209–1216
Lockwood JA, Story RN (1985) Bifunctional pheromone in the first instar of the southern green stink bug, Nezara viridula (L.) (Hemiptera: Pentatomidae): its characterization and interaction with other stimuli. Ann Entomol Soc Am 78:474–479
Millar JG (2005) Pheromones of true bugs. Top Curr Chem 240:37–84
Mizoguchi A, Mori N, Nishida R, Kuwahara Y (2003) α-Acaridial a female sex pheromone from an alarm pheromone emitting mite Rhizoglyphus robini. J Chem Ecol 29:1681–1690
Moraes MCB, Pareja M, Laumann RA, Borges M (2008) The chemical volatiles (semiochemicals) produced by Neotropical stink bugs (Hemiptera: Pentatomidae). Neotropical Entomol 37:489–505
Moreira JA, Millar JG (2005) Short and simple synthesis of 4-oxo-(E)-2-hexenal and homologs: pheromone components and defensive compounds of Hemiptera. J Chem Ecol 31:965–968
Nishimura K, Shimizu N, Mori N, Kuwahara Y (2002) Chemical ecology of astigmatid mites. LXIV. The alarm pheromone neral functions as an attractant in Schwiebea elongata (Banks) (Acari: Acaridae). Appl Entomol Zool 37:13–18
Noge K, Kato M, Iguchi T, Mori N, Nishida R, Kuwahara Y (2005) Biosynthesis of neral in Carpoglyphus lactis (Acari: Carpoglyphidae) and detection of its key enzyme, geraniol dehydrogenase, by electrophoresis. J Acarological Soc Jpn 14:75–81
Noge K, Kimura H, Abe M, Becerra JX, Tamogami S (2012a) Antibacterial activity of 4-oxo-(E)-2-hexenal from adults and nymphs of the heteropteran, Dolycoris baccarum (Heteroptera: Pentatomidae). Biosci Biotechnol Biochem 76:1975–1978
Noge K, Prudic KL, Becerra JX (2012b) Defensive roles of (E)-2-alkenals and related compounds in Heteroptera. J Chem Ecol 38:1050–1056
Prestwich GD (1976) Composition of the scents of eight East African hemipterans. Nymph-adult chemical polymorphism in coreids. Ann Entomol Soc Am 69:812–814
Prudic KL, Noge K, Becerra JX (2008) Adults and nymphs do not smell the same: the different defensive compounds of the giant mesquite bug (Thasus neocalifornicus: Coreidae). J Chem Ecol 34:734–741
Sakakura A, Kawajiri K, Ohkubo T, Kosugi Y, Ishihara K (2007) Widely useful DMAP-catalyzed esterification under auxiliary base- and solvent-free conditions. J Am Chem Soc 129:14775–14779
SAS Institute Inc. (2003) JMP 5.1, Cary, NC, USA
Soldi RA, Rodrigues MACM, Aldrich JR, Zarbin PHG (2012) The male-produced sex pheromone of the true bug, Phthia picta, is an unusual hydrocarbon. J Chem Ecol 38:814–824
Tsuyuki T, Ogata Y, Yamamoto I, Shimi K (1965) Stink bug aldehydes. Agric Biol Chem 29:419–427
Waterhouse DF, Gilby AR (1964) The adult scent glands and scent of nine bugs of the superfamily Coreoidea. J Insect Physiol 10:977–987
Yasuda T, Higuchi H (2012) Sex pheromones of Stenotus rubrovittatus and Trigonotylus caelestialium, two mirid bugs causing pecky rice, and their application to insect monitoring in Japan. Psyche 2012: Article ID 435640
Zarbin PHG, Fávaro CF, Vidal DM, Rodrigues MACM (2012) Male-produced sex pheromone of the stink bug Edessa meditabunda. J Chem Ecol 38:825–835
Acknowledgments
We thank Narumi Akahira and Kouhei Miura (Akita Prefectural University) for insect-collecting assistance. This work was supported by a Grant from the President-Sponsored Research Projects of Akita Prefectural University to K. N. The JNM-ECS400 spectrometer used in this study is a commonly shared instrument at Akita Prefectural University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noge, K., Kakuda, T., Abe, M. et al. Identification of the Alarm Pheromone of Hygia lativentris and Changes in Composition during Development. J Chem Ecol 41, 757–765 (2015). https://doi.org/10.1007/s10886-015-0607-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0607-5