Abstract
The guava fruit fly, Anastrepha striata, is a pest of several cultivated species of Myrtaceae in the American tropics and subtropics. During calling, A. striata males release numerous volatiles. This study was conducted to identify which of the male volatiles function as the A. striata sex pheromone and to investigate the effects of age and time of day on the emission of pheromone components. Analysis of the volatiles from males collected by solid phase microextraction using gas chromatography coupled to electroantennographic detection (GC-EAD) showed that three volatile compounds elicited repeatable responses from the antennae of females. The EAD-active compounds were identified by GC/mass spectrometry as ethyl hexanoate, linalool, and ethyl octanoate. In two-choice tests using Multilure traps placed in field cages, traps baited with live males, ethyl hexanoate, or the three-component blend captured more females than unbaited traps. However, there was no difference in catches when traps baited with live males were compared against traps baited with ethyl hexanoate. Although traps baited with the three-component blend caught more females than traps baited with live males, the difference was not significant. Analyses of pheromonal components released by A. striata males 8 to 26 days old showed that there was an effect of age on pheromone production and also a significant effect of time of day on pheromone emission. Release of the volatile compounds occurred from 14.00 to 18.00 hr, although traces of linalool were detected from 08.00 hr. Peak emission of pheromone compounds occurred at 14.00 hr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastrepha striata has been reported to attack fruits of several guava species, Psidium spp., and other myrtaceous fruits such as the arrayan, Myrceugenia glaucescens, Surinam cherry, Eugenia uniflora, and rose-apple, Sysygium jambos (Norrbom 2001). This fly has been found in Mexico, Peru, Bolivia, and Brazil, and has been trapped sporadically in the United States (Foote et al. 1993). In Mexico, guava production is growing in importance, but export of this fruit to the USA is restricted because of quarantine against A. striata (Norrbom 2001). In order to develop new strategies for monitoring and management of this pest, our knowledge of the biology, ecology, and behavior of A. striata needs to be increased. For example, relatively little is known about the mating behavior of A. striata, although this is essential for fruit fly reproduction. Mating behavior starts when males vigorously move their wings while emitting volatiles that attract females by everting their rectal epithelium and expanding their abdominal pleura, a behavior hereafter termed ‘calling’ (Aluja et al. 1993; Arita and Kaneshiro 1986). A female then approaches a calling male and initiates a series of circular movements around the male, which stands motionless with its proboscis extended. Successful copulation begins only after several attempts (Aluja et al. 1993, 2000). Behavioral observations with A. striata males conducted in Veracruz, Mexico, showed that the mating of this species extends from morning to afternoon hours (Aluja et al. 1993), while in the coastal area of Chiapas, this species mates mainly during evening hours (unpublished data).
Volatile compounds emitted by Anastrepha suspensa, A. ludens, A. obliqua, A. striata, A. fratecurlus, and A. serpentina males during calling behavior have been identified from male salivary gland extracts (Břízová et al. 2013; Chuman et al. 1988; Gonçalves et al. 2013; Heath et al. 2000; Ibañez-López and Cruz-López 2001; Robacker et al. 2009; Rocca et al. 1992). However, there have been relatively few successful behavioral evaluations of the volatiles identified (López-Guillen et al. 2011; Nation 1975, 1989; Robacker 1988; Robacker et al. 2009). In some same cases, synthetic compounds have failed to attract flies in field tests (Nation 1989) or even in the laboratory (Robacker et al. 2009). In the case of A. striata, Heath et al. (2000) reported that males of this species release a mixture of ethyl hexanoate, ethyl octanoate, and linalool. However, it is not known if these compounds function as sex pheromone components for this species.
Here, by using chemical, electrophysiological and behavioral assays we confirmed the identity of volatile compounds produced by A. striata males and determined which of these function as sex pheromone components attracting females. Additionally, we investigated the effect of age and time of day on the emission of pheromone components.
Methods and Materials
Flies
Non-irradiated pupae of A. striata were provided by Moscamed-Moscafrut mass rearing facility (SAGARPA-IICA) located in Metapa de Domínguez, Chiapas, Mexico. The flies were originally collected from guava fruits in the Soconusco region, Chiapas, Mexico, and had been reared on artificial diet for at least 20 generations at the Moscafrut mass-rearing facilities. The pupae were placed in 30 × 30 × 30 cm glass cages. Males were separated from females one day after adult emergence and placed in similar glass cages. Emerged adults were fed with a mixture of hydrolyzed protein (MP Biomedical, Irvine, CA, USA) and sucrose (1:3); water was provided in test tubes covered with cotton wicks. Adults were kept at 27 ± 1 °C, 70 ± 5 % RH and a photoperiod of 12 : 12 hr L:D.
Volatile Collection
Volatile compounds released by A. striata males were sampled by solid phase microextraction (SPME). In general, 10 males were placed in a 25 ml vial (5 cm diam × 10 cm long) with PTFE/silicon septum (Supelco, Toluca, Mexico), and volatiles were captured with a polydimethyl siloxane/divinylbenzene SPME fiber (film thickness 65 μm, Supelco, Toluca, Mexico). Sampling was performed by inserting the SPME needle through the septum into the headspace of the glass vial, and volatiles were collected for 5 min. After the sampling period, the fiber was removed and inserted into the injector of a gas chromatograph coupled to a mass spectrometer (GC/MS). The samples were desorbed for 5 min in the GC injector for analysis. SPME volatiles to study the effect of age were collected from virgin males 8, 10, 12, 14, 16, 18, 20, 22, 24, and 26 days old; volatiles were sampled from 16.00 to 18.00 hr. The effect of time of day on emission of volatiles was studied using virgin males 8 to 12 days old; volatiles were sampled every 2 hr between 08.00 to 20.00 hr. For this experiment, only three compounds were quantified. Five replicates were done for each experiment. Following the same methodology, volatiles from females at 10 day old also were sampled. Five replicates were performed.
Instrumental Methods
SPME samples were analyzed by GC/MS using Varian model CP-3800 gas chromatograph coupled with a Varian Saturn 2200 mass spectrometer (Varian, Palo Alto, CA, USA) fitted with a non-polar fused silica capillary column (30 m × 0.25 mm) coated with SPB-1 (Supelco, Toluca, Mexico). The oven temperature was programmed at 50 °C for 2 min then at 15 °C/min to 280 °C. The carrier gas was helium and the injector port temperature was held at 200 °C. The samples were ionized by electronic impact to 70 eV. The compounds were identified by comparing their retention times and mass spectra with those of synthetic standards.
Volatiles sampled by SPME also were analyzed by gas chromatographic-electroantennographic detection (GC-EAD) (Arn et al. 1975), in a gas chromatograph (Varian 3600, Palo Alto, CA, USA) coupled to an electroantennogram apparatus (Syntech, Hilversum, The Netherlands). The capillary column, carrier gas, oven program, and injection conditions were the same as used for GC/MS. One replicate was made with one fly antenna, and 10 antennae of females were used in this test.
Chemicals
Synthetic chemicals were obtained from Sigma/Aldrich, and purities were 97 % according to the supplier.
Behavioral Tests
All tests were performed in two-choice tests in a semi-natural condition using cylindrical, clear nylon screen field cages (2.85 m diam × 2 m high). Two potted mango trees were placed at the center inside each cage. Two Multilure traps (Better World Manufacturing Inc., Fresno, CA, USA) were hung 10 cm from the top of cage with 185 cm between each trap, and water was used to retain the flies in traps. For live baits, 20 males were placed in a small cage made of plastic netting that was then placed inside the trap. Synthetic compounds (100 μg) were loaded onto rubber septa as dispensers (11 mm sleeve stopper natural red rubber; Wheaton, NJ, USA). Traps were hung at 14.00 hr, and the lures were placed in traps 15 min before female flies were released into the cage. Approximately 50 females were released in the center of the cage in the late afternoon, and the number of insects caught by each trap was counted at 07.00 hr the following day. Trap positions were randomized daily to reduce positional effects. During the test period, temperature ranged from 23 to 31 °C and relative humidity from 60 to 95 %.
In the first experiment, the attraction of A. striata females to the following three pairs of traps was evaluated: live A. striata males against a trap without bait (control) (N = 10); ethyl hexanoate alone vs. control (N = 10); and a three-component blend of ethyl hexanoate, linalool and ethyl octanoate at the relative proportions of 22 : 42 : 36 as produced by male flies versus control (N = 10).
The second experiment compared the attractiveness of live A. striata males against ethyl hexanoate alone (N = 5) or against the above three-component blend (N = 8).
Data Analysis
The number of A. striata females caught in the field cage tests was analyzed using a t-test. The effect of age and the time of day on pheromone release was analyzed using a multivariate analysis of variance (MANOVA) on the relative abundance (GC peak area) of each compound. In the MANOVA, F values were calculated from Pillai test (age) or Wilk’s lambda test (time of day). Data on time of day were transformed previously by natural log to normalize distributions before MANOVA. Means were separated with α = 0.05. The analyses were done using R statistical software.
Results
Volatiles Emitted by A. striata Males and Females
GC/MS analyses of the volatile compounds produced by A. striata males and sampled by SPME showed the presence of eleven compounds (Fig. 1). The most abundant compounds were identified as ethyl hexanoate (2), linalool (6), and ethyl octanoate (9). Minor compounds were myrcene (1), limonene (3), cis-(β)-ocimene (4), trans-(β)-ocimene (5), (Z)-3-nonenol (7), (Z,Z)-3,6-nonadien-1-ol (8), terpineol (10), and (Z,E)-α-farnesene (11) In contrast, females sampled by SPME under the same conditions did not release significant amounts of any volatile compounds.
GC/MS Chromatogram of volatiles emitted by calling Anastrepha striata males collected by SPME. Myrcene (1), ethyl hexanoate (2), limonene (3), cis-β-ocimene (4), trans-β-ocimene (5), linalool (6), (Z)-3-nonenol (7), (Z,Z)-3,6-nonadienol (8), ethyl octanoate (9), terpineol (10), (Z,E)-α-farnesene (11)
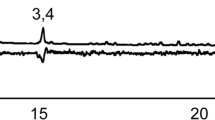
GC-EAD analyses of the antennal response of A. striata females to the volatiles from males obtained by SPME showed three repeatable antennal responses (Fig. 2a). The corresponding EAD-active compounds were identified as ethyl hexanoate (2), linalool (6), and ethyl octanoate (9). These results were confirmed by injecting 100 ng of the synthetic compounds and consistent female EAD responses to the three synthetic compounds were elicited (Fig. 2b).
Behavioral Tests
Traps baited with live males (t = 4.65, df = 9, P = 0.001) (Fig. 3a), ethyl hexanoate (t = 5.7, df = 7, P < 0.001) (Fig. 3b), and the three-component blend (t = 3.2, df = 9, P = 0.01) captured more females than control traps (Fig. 3c). There was no difference when traps baited with live males were compared against traps baited with ethyl hexanoate (t = −0.64, df = 4, P = 0.55) (Fig. 4a). Although traps baited with the three-component blend caught more females than traps baited with live males, this difference was not significant (t = −1.2, df = 9, P = 0.25) (Fig. 4b).
Mean number (± SE) of Anastrepha striata females captured in Multilure traps baited with (a) 20 live males or control; (b) ethyl hexanoate or control; (c) a three-component blend of ethyl hexanoate, linalool and ethyl octanoate (22 : 42 : 36) or control. Differences between paired bars (t test) indicated by *, P < 0.001. (N = 10)
Effects of Age and Time of Day on Pheromone Emission
MANOVA analyses showed that there was a significant effect of age on pheromone emission (F = 3.72; df = 9,27; P < 0.001). Peak emission of volatiles occurred at 14 days age. Ethyl octanoate showed the most important effect followed by linalool and ethyl hexanoate (Fig. 5). There also was a significant effect of time of day on pheromone emission (F = 6.79; df = 2, 22; P < 0.001). Peak emission of pheromone compounds occurred at 1400 hr (Fig. 6). Release of the three volatile compounds occurred from 14:00–18:00 hr. However, a trace of linalool was detected as of 08:00 hr.
Discussion
In this study, we report the composition of the volatiles emitted by A. striata males during calling. Of the 11 compounds identified, only three elicited antennal responses from females – ethyl hexanoate, linalool, and ethyl octanoate. These compounds were as attractive to females as live males when tested together, and can, therefore, be considered components of the male-produced sex pheromone of this fruit fly species. The conclusion is supported by the fact that females did not release any volatile compounds when they reached sexual maturity. A previous study reported the presence of these compounds in the effluvia of A. striata males but their biological significance was not established (Heath et al. 2000).
Along with these three compounds, A. striata males released eight other compounds, but whether these compounds are relevant in the sexual behavior of this fruit fly species remains to be investigated. Usually, Anastrepha fruit fly males emit several compounds during calling behavior, but not all of them function as sex pheromones (Robacker 1988; Robacker et al. 2009; Rocca et al. 1992). For example, Robacker et al. (2009) identified two major volatile components from males of A. serpentina, 2,5-dimethylpyrazine (1) and 3,6-dihydro-2,5-dimethylpyrazine (2), and a minor component identified as 2,3,5-trimethylpyrazine (3). Compounds 1 and 3 elicited antennal responses in GC-EAD analyses, but no response was observed for compound 2. Nevertheless, females were attracted in cage-top laboratory bioassays to a blend of compounds 1 and 2 (Robacker et al. 2009). Anastrepha ludens males release nine volatile compounds during calling but only (Z)-3-nonenol, (Z,Z)-3, 6-nonadienol, and anastrephin elicit strong behavioral responses in a wind-tunnel bioassay, including attraction, increased searching rate, changes in searching strategy, and agonism (Robacker 1988).
Comparing the volatiles emitted by A. striata males with those released by males of other Anastrepha species, we can see that the pattern of A. striata volatiles differs from that of A. suspensa, A. obliqua, A. ludens, A. fraterculus, and A. serpentine, except for trans-β-ocimene, (Z)-3-nonenol, (Z,Z)-3,6-nonadienol, and (Z,E)-α-farnesene, which are shared by A. suspensa, A. ludens, A. obliqua (Heath et al. 2000), and A. fraterculus (Břízová et al. 2013), while limonene has been detected in A. ludens (Rocca et al. 1992), A. fraterculus (Břízová et al. 2013), and A. obliqua (Gonçalves et al. 2013). Recently, however, ethyl hexanoate, ethyl octanoate, and linalool were found in the effluvia of two wild Brazilian populations of A. obliqua collected from mangoes and star fruits (Gonçalves et al. 2013), but whether these compounds are part of the sex pheromone of this species remains unknown. Mexican populations of A. obliqua seem not to produce/release these compounds (López-Guillen et al. 2011). Norrbom et al. (2000), using morphological characters, placed A. striata in a group different from A. suspensa, A. obliqua, A. ludens, and A. fraterculus.
Furthermore, the pheromone components of A. striata are constituents of host fruit volatiles of Anastrepha spp. (Cruz-López et al. 2006; Malo et al. 2005). For instance, ethyl hexanoate and ethyl octanoate have been isolated from guava fruits and these compounds attract A. ludens (Malo et al. 2005). Moreover, a recent study has identified guava and sweet orange volatiles that are attractive for A. striata females (Díaz Santiz 2014). Interestingly, the attractive compounds include, among others, ethyl hexanoate and ethyl octanoate from guava, and linalool from sweet orange. The latter make our results intriguing. It is difficult to understand how A. striata females detect male volatiles in a field inundated with host volatiles with composition similar to that of male volatiles. The fact that male pheromone and fruit volatiles share similar compounds that attract females suggests that they may use the same sensory mechanisms during sexual behavior and host plant location. Further research is necessary to understand the function of these volatiles on the behavior of A. striata females.
We found that A. striata started to release pheromone compounds in the afternoon, when calling behavior also occurs. The latter observation contrasts with A. striata populations from Veracruz, which have a more extended calling behavior (Aluja et al. 2000). These differences between populations seem to be common in the sexual behavior of fruit flies. For example, calling and mating of Mexican populations of A. obliqua occur in the early morning hours (Aluja and Birke 1993), whereas these sexual activities in Brazilian populations of this species are restricted to the afternoon period (Henning and Matioli 2006). Mexican and Brazilian populations of A. obliqua also seem to produce a different pattern of volatiles (Gonçalves et al. 2013; López-Guillen et al. 2011). We do not know if the two populations of A. striata differ in the chemical composition of their male volatiles.
In summary, we found that that three of the eleven identified compounds emitted by calling A. striata males function as the sex pheromone of this fruit fly species. We also found that age and time of day affect the production/release of the sex pheromone in A. striata. Further studies will optimise the attraction of A. striata to the sex pheromone blend in field trapping tests.
References
Aluja M, Birke A (1993) Habitat use by adults of Anastrepha obliqua (Diptera: Tephritidae) in a mixed mango and tropical plum orchard. Ann Entomol Soc Am 86:799–812
Aluja M, Jacome I, Birke A, Lozada N, Quintero G (1993) Basic patterns of behavior in wild Anastrepha striata (Diptera:Tephitidae) flies under field-cage conditions. Ann Entomol Soc Am 86:776–793
Aluja M, Piñero J, Jácome I, Díaz-Fleischer F, Sivinski J (2000) Behavior of flies in the genus: Anastrepha (Trypetinae: Toxotrypanini). In: Aluja M, Norrbom AL (eds) Fruit flies (Tephritidae): Phylogeny and evolution of behavior. CRC Press LLC, Boca Raton, pp 375–406
Arita LH, Kaneshiro KY (1986) Structure and function of the rectal epithelium and anal glands during mating behavior in the Mediterranean fruit fly. Proc Hawaiian Entomol Soc 26:27–30
Arn H, Städler E, Rauscher S (1975) The electroantennographic detector a selective and sensitive tool in the gas chromatographic analysis of insect pheromones. Z Naturforsch 30c:722–725
Břízová R, Mendonça AL, Vanícková L, Mendonça AL, Da Silva CE, Tómala A, Paranhos BA, Dias VS, Joachim-Bravo IS, Hoskovec M, Kalinová B, Do Nascimento RR (2013) Pheromone analyses of the Anastrepha fraterculus (Diptera: Tephritidae) cryptic species complex. Fla Entomol 96:1107–1115
Chuman T, Siviniski J, Heath RR, Calkins CO, Tumilson JH, Batiste MA, Wydra RL, Strekowski L, Nation JL (1988) Suspensolide a new macrolide component of male Caribbean fruit fly (Anastrepha suspensa) volatiles. Tetrahedron Lett 29:6561–6564
Cruz-López L, Malo EA, Toledo J, Virgen A, del Mazo A, Rojas JC (2006) A new potential attractant for Anastrepha obliqua from Spondias mombin fruits. J Chem Ecol 32:351–365
Díaz Santis E (2014). Respuesta olfativa de Anastrepha striata Schiner (Diptera:Tephritidae) a volátiles de frutos Psidium guajava y Citrus sinensis, El Colegio de la Frontera Sur, Tapachula, Chiapas, Mexico. Thesis
Foote RH, Blanc FL, Norrbom AL (1993) Handbook of the fruit flies (Diptera: Tephritidae) of America north of Mexico. Comstock, USA
Gonçalves GB, Silva CE, De Lima MA, Vaníčková L, Tomčala A, Do Nascimento RR (2013) Pheromone communication in Anastrepha obliqua (Diptera: Tephritidae): a comparison of the volatiles and salivary gland extracts of two wild populations. Fla Entomol 96:1365–1374
Heath RR, Landolt PJ, Robacker DC, Dueben BD, Epsky N (2000) Sexual pheromones of tephritid flies: clues to unravel phylogeny and behavior. In: Aluja M, Norrbom AL (eds) Fruit flies (Tephritidae): Phylogeny and evolution of behavior. CRC Press LLC, Boca Raton, pp 793–809
Henning F, Matioli SR (2006) Mating time of the West Indian fruit fly Anastrepha obliqua (Macquart) (Diptera: Tephritidae) under laboratory conditions. Neotrop Entomol 35:145–148
Ibáñez-López A, Cruz-López L (2001) Glándulas salivales de Anastrepha obliqua (Macquart) (Diptera: Tephritidae): Análisis químico y morfológico, y actividad biológica de los componentes volátiles. Folia Entomol Mex 40:221–231
López-Guillen G, Malo EA, Rojas JC (2011) Olfactory responses of Anastrepha obliqua (Diptera: Tephritidae) to volatiles emitted by calling males. Fla Entomol 94:874–880
Malo EA, Cruz-López L, Toledo J, Del Mazo A, Virgen A, Rojas JC (2005) Behavioral and electrophysiological responses of the Mexican fruit fly (Diptera: Tephritidae) to guava volatiles. Fla Entomol 88:364–371
Nation JL (1975) The sex pheromone blend of Caribbean fruit fly males: isolation biological activity, and partial chemical characterization. Environ Entomol 4:27–30
Nation JL (1989) The role of pheromones in the mating system of Anastrepha fruit flies. In: Robinson AS, Hooper G (eds) World Crop Pests, Vol. 3A: Fruit flies, their biology, natural enemies and control. Elsevier, Amsterdam, pp 189–205
Norrbom AL (2001) Anastrepha striata Schiner. The Diptera site.http://www.sel.barc.usda.gov/diptera/tephriti/Anastrep/striata.htm (January 19, 2002)
Norrbom AL, Zucchi RA, Hernández-Ortiz V (2000) Phylogeny of the genera Anastrepha and Toxtrypana (Trypetinae: Toxotrypanini) based on morphology. In: Aluja M, Norrbom AL (eds) Fruit flies (Tephritidae): Phylogeny and evolution of behavior. CRC Press, Boca Raton, pp 299–342
Robacker DC (1988) Behavioral responses of female Mexican fruit flies, Anastrepha ludens, to components of male-produced sex pheromone. J Chem Ecol 14:1715–1726
Robacker DC, Aluja M, Cosse AA, Sacchetti P (2009) Sex pheromone investigation of Anastrepha serpentina (Diptera: Tephritidae). Ann Entomol Soc Am 102:560–566
Rocca JR, Nation JL, Strekowski L, Battiste MA (1992) Comparison of volatiles emitted by male Caribbean and Mexican fruit flies. J Chem Ecol 18:223–244
Acknowledgments
We are grateful for the statistical advice of Javier Valle-Mora (ECOSUR). We thank E. Hernández-Ortiz (Subdirección de Desarrollo de Métodos. Moscamed-Moscafrut, Mexico) for providing the flies used in this study, and Antonio Santiesteban and Armando Virgen for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruz-López, L., Malo, E.A. & Rojas, J.C. Sex Pheromone of Anastrepha striata . J Chem Ecol 41, 458–464 (2015). https://doi.org/10.1007/s10886-015-0581-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0581-y