Abstract
Prior studies have examined the static effect of intravenous ketamine on the BIS Index for sedation but it remains unknown if the BIS Index is a reliable method to track sedation levels in the presence of ketamine. The major objective of the current investigation was to compare the BIS Vista Index ability to track varying depths of sedation as determined by OASS scores in a standardized anesthetic regimen with and without ketamine. The study was a randomized, double blinded clinical trial. Patients undergoing breast surgery under sedation with propofol were randomized to receive ketamine (1.5 μg kg min−1) or saline. Infusion data was used to estimate propofol plasma concentrations (Cp). The main outcome of interest was the correlation between the BIS Vista Index with the OASS score. Twenty subjects were recruited and fifteen completed the study. Four hundred fifty-five paired data points were included in the analysis. Model performance (Nagelkerke R2) of the multinomial logistic regression model was 0.57 with the c-statistic of 0.87 (95 % CI 0.82–0.91). Compared to awake the odds ratio for BIS values predicting moderate sedation in the saline/propofol group 1.19 (95 % CI 1.12–1.25) but only 1.06 (95 % CI 1.02–1.1) in the ketamine/propofol group (P = 0.001). There was no difference in the odds for BIS values to predict deep sedation between groups (P = 0.14). The BIS monitor can be used to monitor sedation level even when ketamine is used with propofol as part of the sedation regimen. However, ketamine reduces the value of the BIS in predicting moderate sedation levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Surgical procedures performed under sedation encompass a large portion of current anesthesia practice [1–3]. In cases performed under sedation, the reduction in the use of anesthetic agents while still achieving adequate levels of sedation, is highly desirable since drug-induced hypoventilation is the leading cause of morbidity and mortality [4]. The use of depth of anesthesia monitors can result in less consumption of anesthetic agents [5–7]. In addition, recent evidence suggests that the use of depth of anesthesia monitors in sedation cases may reduce the development of respiratory complications [8].
The BIS Index is the most common depth of anesthesia monitor studied in surgical cases with decreasing values as depth of anesthesia increases [9–11]. In contrast to other intravenous anesthetics, ketamine has been reported to increase or not affect BIS values [12, 13]. The BIS algorithm evaluates the suppression of activity, β power and slow synchronized power of the electroencephalograph [14]. Because ketamine has been show to increase θ activity of the EEG [15], when given with hypnotic agents that depress cortical activity such as sevoflurane or propofol, the EEG pattern is driven towards higher frequencies and desynchronization [14]. The effect of ketamine on EEG activity results in an increase in the BIS Index value. Bolus administration of ketamine during sevoflurane and propofol anesthesia has been shown to increase BIS values [14, 16, 17].
Since ketamine can minimize hypoventilation in patients undergoing deep sedation, it is important that depth of anesthesia monitors used during sedation cases are still reliable in the presence of ketamine [18]. Currently, no studies have compared the relationship between the BIS Index values, Observer Alertness of Sedation Scale (OASS) scores and propofol serum concentrations in patients undergoing sedation using a standardized anesthetic regimen with and without ketamine.
The major objective of the current investigation was to compare the BIS Vista Index ability to track varying depths of sedation as determined by OASS scores in a standardized anesthetic regimen with and without ketamine. We hypothesize that the addition of ketamine would result in higher BIS Vista Index values for varying depths of sedation as assessed by OASS scores but it would not distort the BIS ability to track levels of sedation.
2 Methods
The study was a prospective, randomized, double blinded, placebo controlled clinical trial. Clinical trial registration for this study can be found at ClinicalTrial.gov; url:http://www.clinicaltrials.gov; registration identified: NCT01250418. Study approval was obtained from the Northwestern University Institutional Review Board, and written informed consent was obtained from all the study participants. Eligible subjects were healthy female patients undergoing unilateral segmental breast biopsies under monitored anesthesia care and sedation. Patients with a history of drug or alcohol abuse, use of anticonvulsants, cerebrovascular disease and pregnancy were not enrolled. Reasons to drop out after enrollment included conversion to general anesthesia, need for supplemental opioid analgesia and surgeon request.
Subjects were randomized using a computer generated table of random numbers into two groups to receive ketamine 0.25 mg kg−1 bolus followed by an infusion of 1.5 μg kg min−1 or saline. The ketamine dose was similar to the one used by other investigators [19–21]. Group assignments were sealed in sequentially numbered opaque envelopes that were opened by one of the investigators not involved with the patient care or data collection after the subject provided written informed consent. The same investigator prepared syringes labeled with study drug to blind subjects enrolled in the study, anesthesia providers and a research nurse collecting the data.
Subjects were given 2 mg IV of midazolam and transported to the operating room where standard monitors were applied. The BIS Vista (Aspect Medical, Cambridge MA, application version 3.00 platform version 2.03) electrodes were placed on the subject’s forehead in concordance with the manufacturer’s specifications. The signals averaging time for the BIS Vista monitor was set at 15 s. The monitor was covered with an opaque board in order to blind anesthesia providers and personnel responsible for data collection. A single research nurse recorded an Observer Assessment of Sedation Scale (OASS) score prior to the study drug and at 1 min intervals for a minimum of 30 min in all subjects [22, 23]. The OASS is scored: 5 if the patient responds to name spoken in normal tone, 4 if the patient is lethargic but responds to name in normal tone, 3 if the patient responds only after name is called loudly and/or repeatedly, 2 if the patient responds only after mild prodding or shaking, 1 if the patient responds only after painful trapezius squeeze and 0 if the patient does not respond after painful trapezius squeeze.
Before the study infusion was begun, 25 μg of remifentanil was administered to provide analgesia during application of local anesthetic by the surgeon. No additional opioids were allowed to be administered during the study period. The study drug infusion was then started and a propofol infusion was commenced at 125 μg kg min−1. The propofol infusion was titrated up or down in 25 μg kg min−1 increments by the anesthesia provider to maintain clinically adequate procedural sedation. The anesthesia provider was unaware of the study group allocation. The anesthesia care provider was instructed to maintain the infusion dose of propofol for minimum of 5 min prior to an infusion rate adjustment if the patient was tolerating the procedure. BIS Index values and infusion rate changes were recorded electronically for all subjects continuously. The average value of the index collected every 5 s over a 1 min epochs were determined to correspond with OASS values. Other data collected included patients demographic characteristics and surgical duration.
Infusion data was used to estimate propofol plasma concentrations (Cp) using the method of Schnider et al. [24] Propofol plasma concentrations estimates were calculated at 1 min intervals for the duration of the infusion using a 3 compartment model with age, height, weight and lean body mass used as covariates in the estimation of compartmental volumes and clearances. Pharmacokinetic analysis was done using the SAAM II software system (SAAM Institute, Seattle, WA) implemented on a Windows-based computer.
Levels of sedation were defined as awake/light sedation (OASS score of 4 or 5), moderate sedation (OASS score of 2 or 3), and deep sedation (OASS score of 0 or 1). The main outcome of interest for the current manuscript was the accuracy, area under the receiver operating characteristics curve (AUC), for predicting OASS level of sedation by BIS Vista Index value in patients sedated with saline/propofol compared with ketamine/propofol. A post hoc sample size estimation determined that a sample of 8 subjects per group with a minimum of 30 samples per subject would provide 240 samples per group and would achieve 80 % power to detect a difference of 0.1 in the AUC values between groups, assuming the AUC for saline/propofol was 0.8, using a two-sided z test at a significance level of 0.05.
BIS Index values and estimated propofol concentrations among the OASS sedation levels were compared using the Kruskal–Wallis H test. Comparison between the saline/propofol and ketamine/propofol groups within the same sedation group was made using the Mann–Whitney U test corrected for six comparisons using the Bonnferoni method. Median differences and 95 % confidence intervals of the differences were determined using a 10,000 replication bootstrap.
The level of sedation predicted by the BIS Index was modeled using a multinomial logistic regression analysis with ketamine/propofol and saline/propofol groups as a fixed factor and the BIS Index value as a covariate. The sedation agents group and an interaction term of sedation agents group * BIS value were entered into the model. Model performance was assessed using Nagelkerke R2 and the accuracy of the model was evaluated by constructing receiver operator characteristics (ROC) curves for predicted and actual sedation groups and calculating the area under the curve (AUC, c-statistic). AUC’s for BIS predicted OASS sedation groups and actual OASS group were compared between saline/propofol and ketamine/propofol using the method of Delong [25]. The odds ratio and the 95 % confidence interval for predicting moderate and deep sedation with awake/light sedation as the reference category were calculated for the ketamine/propofol and saline/propofol groups. Statistical analysis was performed using R version 3.1.0, release date 4/10/2014 (The R Foundation for Statistical Computing, Vienna, Austria).
3 Results
Twenty subjects were recruited and fifteen completed the study (Fig. 1). Four subjects in the saline/propofol and one in the ketamine/propofol group were excluded following allocation. Two subjects in the saline/propofol group and one in the ketamine/propofol group received general anesthesia and two subjects in the saline/propofol group received an additional dose of remifentanil for discomfort during local anesthesia administration by the surgeon. There were no differences in preoperative clinical characteristics in subjects who received ketamine and saline (Table 1).
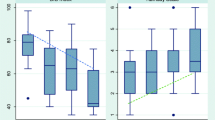
Four hundred fifty-five paired data points were included in the analysis. There were 248 pairs in the saline/propofol group. The OAAS level of sedation was awake/light sedation in 129 (52 %), moderate in 57 (23 %) and deep in 62 (25 %) of the pairs. There were 207 pairs in the ketamine/propofol group. The OAAS level of sedation was awake/light sedation in 99 (48 %), moderate in 57 (27 %) and deep in 51 (25 %) of the pairs. The relationship between OASS sedation level groups and BIS Index values are shown in Fig. 2. Median BIS values were significantly greater at each OASS level of sedation in the ketamine/propofol compared with the saline propofol group (Table 2.)
Boxplot of sedation level values with Bispectral Index (BIS) values. Observer assessment of alertness and sedation score values of 4–5 represent awake/light sedation, values of 2–3 represent moderated sedation and values of 0–1 deep sedation. The median value is represented by a line with the 25th and 75th percentile by the box. The whiskers show the 10th and 90th percentile and the circles the 5th and 95th percentiles. Values are different by level of sedation within each study group (P < 0.001). †Different between saline/propofol and ketamine/propofol at the same level of sedation (P < 0.001)
Model performance (Nagelkerke R2) of the multinomial logistic regression model for BIS predicting OASS level of sedation groups was 0.57 with the c-statistic of 0.87 [95 % CI 0.82–0.91]. The area under the ROC curve (95 % CI) for predicted and actual sedation groups was 0.89 [95 % CI 0.84–0.94] for the saline/propofol group and 0.84 [95 % CI 0.76–0.91] for the ketamine/propofol group. The difference in the area under the ROC curves for saline/propofol and ketamine/propofol was 0.05 [95 % CI −0.06 to 0.14], P = 0.12.
BIS cut-off values for predicting awake/light, moderate and deep sedation were >74, 74–70, <70 for saline/propofol and >75, 75–78 and >78 for ketamine/propofol, respectively. The percentage of correctly predicted pairs based on the multinomial logistic model compared with the observed OAAS sedation groups is shown in Fig. 3. There was no difference in the percentage of correct predicted pairs in the awake/light sedation and deep sedation group in patients that received saline/propofol or ketamine/propofol. For pairs of data in the OAAS moderated category, the model correctly predicted 25 % of the pairs compared to 4 % of the pairs in the ketamine/propofol group, difference 21 % [95 % CI 7–35], P = 0.002. When compared to awake/light sedation the odds ratio for BIS values predicting moderate sedation in the saline/propofol group was 1.19 (95 % CI 1.12–1.25) but the odds of predicting moderate sedation was only 1.06 (95 % CI 1.02–1.1) in the ketamine/propofol group (P = 0.001).
Bar graph of nominal logistic regression model predicted sedation group membership with observer assessment of alertness and sedation score group. There was no difference in the frequency of corrected predicted sedation group membership for awake/light sedation, difference −2 % (95 % CI −11 to 7 %), P = 0.64, or deep sedation, difference 9 % (95 % CI −6 to 24 %), P = 0.24. Moderate sedation group membership was correctly predicted in 25 % of the saline/propofol data pairs, but in only 4 % of the ketamine/propofol pairs, difference 21 % (95 % CI 7–35 %), P = 0.002
Estimated propofol concentrations were lower in the saline/propofol group in the awake/light sedation but not in the moderate sedation and deep sedation groups (Table 2). Linear correlation (r2) between BIS Index values and estimated propofol Cp was greater in subjects receiving ketamine compared with saline, difference 0.22 (95 % CI of the difference 0.02–0.37), P = 0.03.
4 Discussion
The most important finding of the current investigation was that the overall performance of the BIS monitor value to track intraoperative sedation using ketamine/propofol was not substantially lower than when using saline/propofol. The increase in BIS Index values due to the increased EEG activity shifted the BIS Index cut-off values for prediction of OASS by 4–5 points higher compared to those determined for propofol. Model performance was similar for awake/light sedation and deep/sedation, but was substantially reduced for moderate sedation when ketamine was administered.
Our findings are clinically important since current evidence suggests that sedation guided by Bispectral Index leads to lower respiratory adverse events than guided by sedation scores [7]. In addition, the use of ketamine has also been demonstrated to reduce hypoventilation and apnea in patients undergoing surgical procedures under propofol sedation [18]. Clinical practitioners that intent to reduce adverse respiratory events by using a BIS-guided sedation regimen with ketamine should target greater BIS values (4–5 points greater) to achieve the desirable level of sedation.
Another important finding of the current investigation was the preservation of a linear relationship between the BIS values and depths of sedation when ketamine was used. In contrast, prior studies utilizing the BIS monitor with an anesthetic protocol inclusive of ketamine did not result in predictable BIS values due to different levels of hypnosis [26, 27]. Other studies have evaluated the BIS monitor in sedation cases with ketamine. However, no prior study have tested the ability of the BIS monitor to predict levels of sedation in a dynamic anesthetic regimen inclusive of propofol. Some studies have only limited to evaluate the static change in BIS values when a ketamine bolus was given and still have generated conflicting results [12, 28, 29]. Nevertheless, to the best of our knowledge, this is the first study to evaluate the dynamic relationship between depth of sedation monitoring and the use of ketamine for sedation cases.
It was also interesting to note that we observed similar estimated plasma concentrations of propofol in both study groups. Prior studies evaluating interactions between ketamine and BIS values did not control for differences in concentrations of other anesthetics, which mistakenly attributed the distortion in the BIS values only to the use of ketamine [12, 26, 27]. Future studies evaluating depth of anesthetic monitoring should, therefore, control for variations in other anesthetics concomitantly administered.
Our study should only be interpreted within the context of its limitations. Our sample sized was inadequate to draw definite conclusion on the relationship of the BIS value and level of sedation. Although we included over 400 pairs of data, the number of data pairs was inadequate to model all OAAS sedation levels, especially at levels of moderate sedation. We did not draw blood samples to verify plasma levels of propofol but rather estimated them from the infusion pump information. It is possible that the initial bolus of remifentanil required to provide adequate clinical care could have affected the initial sedation assessments. We utilized a small and single remifentanil bolus in order to minimize the potential confounding effect. In addition, a sensitive analysis performed to exclude the 5-min initial data did not change any of the current findings.
In summary, the BIS monitor can be useful to monitor sedation even when ketamine is used with propofol as part of the sedation regimen. Because the BIS Index values are most sensitive to changes in consciousness is not surprising that the values have high predictive value for the awake/light sedation and deep sedation levels. Even without ketamine BIS Index values alone are not highly predictive of moderate levels of sedation. However, higher BIS values correlates with lower sedation scores. The use of BIS guided sedation is still a valid strategy in patient receiving propofol and ketamine.
References
Gharaei B, Jafari A, Aghamohammadi H, Kamranmanesh M, Poorzamani M, Elyassi H, Rostamian B, Salimi A. Opioid-sparing effect of preemptive bolus low-dose ketamine for moderate sedation in opioid abusers undergoing extracorporeal shock wave lithotripsy: a randomized clinical trial. Anesth Analg. 2013;116:75–80.
Smith MC, Williamson J, Yaster M, Boyd GJ, Heitmiller ES. Off-label use of medications in children undergoing sedation and anesthesia. Anesth Analg. 2012;115:1148–54.
Webb ST, Hunter DN II. Is sedation by non-anaesthetists really safe? Br J Anaesth. 2013;111:136–8.
Robbertze R, Posner KL, Domino KB. Closed claims review of anesthesia for procedures outside the operating room. Curr Opin Anaesthesiol. 2006;19:436–42.
Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110:i98–105.
Adams R, Brown GT, Davidson M, Fisher E, Mathisen J, Thomson G, Webster NR. Efficacy of dexmedetomidine compared with midazolam for sedation in adult intensive care patients: a systematic review. Br J Anaesth. 2013;111:703–10.
Kertai MD, Whitlock EL, Avidan MS. Brain monitoring with electroencephalography and the electroencephalogram-derived bispectral index during cardiac surgery. Anesth Analg. 2012;114:533–46.
Yang KS, Habib AS, Lu M, Branch MS, Muir H, Manberg P, Sigl JC, Gan TJ. A prospective evaluation of the incidence of adverse events in nurse-administered moderate sedation guided by sedation scores or bispectral index. Anesth Analg. 2014;119:43–8.
Villafranca A, Thomson IA, Grocott HP, Avidan MS, Kahn S, Jacobsohn E. The impact of bispectral index versus end-tidal anesthetic concentration-guided anesthesia on time to tracheal extubation in fast-track cardiac surgery. Anesth Analg. 2013;116:541–8.
Möller Petrun A, Kamenik M. Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013;110:388–96.
Choi WJ, Kim SH, Koh WU, Hwang DI, Cho SK, Park PH, Han SM, Shin JW. Effect of pre-exposure to sevoflurane on the bispectral index in women undergoing Caesarean delivery under general anaesthesia. Br J Anaesth. 2012;108:990–7.
Vereecke HE, Struys MM, Mortier EP. A comparison of bispectral index and ARX derived auditory evoked potential index in measuring the clinical interaction between ketamine and propofol anaesthesia. Anaesthesia. 2003;58:957–61.
Baxter I. Mortality, BIS and the ketamine conundrum. Anaesthesia. 2012;67:800–1.
Hans P, Dewandre PY, Brichant JF, Bonhomme V. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–40.
Corssen G, Domino EF, Bree RL. Electroencephalographic effects of ketamine anesthesia in children. Anesth Analg. 1969;48:141–7.
Hirota K, Kubota T, Ishihara H, Matsuki A. The effects of nitrous oxide and ketamine on the bispectral index and 95 % spectral edge frequency during propofol–fentanyl anaesthesia. Eur J Anaesthesiol. 1999;16:779–83.
Vereecke HE, Struys MM, Mortier EP. A comparison of bispectral index and ARX-derived auditory evoked potential index in measuring the clinical interaction between ketamine and propofol anaesthesia. Anaesthesia. 2003;58:957–61.
De Oliveira GS, Fitzgerald PC Jr, Hansen N, Ahmad S, McCarthy RJ. The effect of ketamine on hypoventilation during deep sedation with midazolam and propofol: a randomised, double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2014;31:654–62.
Niesters M, Aarts L, Sarton E, Dahan A. Influence of ketamine and morphine on descending pain modulation in chronic pain patients: a randomized placebo-controlled cross-over proof-of-concept study. Br J Anaesth. 2013;110:1010–6.
Gharaei B, Jafari A, Aghamohammadi H, Kamranmanesh M, Poorzamani M, Elyassi H, Rostamian B, Salimi A. Opioid-sparing effect of preemptive bolus low-dose ketamine for moderate sedation in opioid abusers undergoing extracorporeal shock wave lithotripsy: a randomized clinical trial. Anesth Analg. 2013;116:75–80.
Grady MV, Mascha E, Sessler DI, Kurz A. The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy. Anesth Analg. 2012;115:1078–84.
Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62.
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–51.
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, Youngs EJ. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–82.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Faraoni D, Salengros JC, Engelman E, Ickx B, Barvais L. Ketamine has no effect on bispectral index during stable propofol–remifentanil anaesthesia. Br J Anaesth. 2009;102:336–9.
Kurehara K, Asano N, Iwata T, Yamaguchi A, Kawano Y, Furuya H. The influence of ketamine on the bispectral index, the spectral edge frequency 90 and the frequency bands power during propofol anesthesia. Masui. 1999;48:611–6.
Phillips W, Anderson A, Rosengreen M, Johnson J, Halpin J. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother. 2010;24:349–55.
Morse Z, Kaizu M, Sano K, Kanri T. BIS monitoring during midazolam and midazolam–ketamine conscious intravenous sedation for oral surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:420–4.
Acknowledgments
Author receives Grants from “IRB# Northwestern University STU00031783”.
Funding
Department of Anesthesiology, Northwestern University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosure
The Department of Anesthesiology at Northwestern University sponsored the research. The authors are employed by the Department of Anesthesiology at Northwestern University.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
De Oliveira, G.S., Kendall, M.C., Marcus, RJ. et al. The relationship between the Bispectral Index (BIS) and the Observer Alertness of Sedation Scale (OASS) scores during propofol sedation with and without ketamine: a randomized, double blinded, placebo controlled clinical trial. J Clin Monit Comput 30, 495–501 (2016). https://doi.org/10.1007/s10877-015-9745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9745-0