Abstract
Background
Bispectral index (BIS ) monitoring has been used as an objective measurement tool for sedation depth and has been proposed as a guidance to reduce the risk of intraprocedural over-sedation. However, the results of several studies evaluating the benefits of BIS monitoring for gastrointestinal endoscopy were not consistent.
Aims
This meta-analysis aimed to assess the reduction in total consumption of administrated propofol and recovery time under BIS during gastrointestinal endoscopy.
Methods
Electronic databases (MEDLINE, EMBASE, WEB of SCIENCE, and the Cochrane Central Register of Controlled Trials) were searched for articles published through March 2015. After screening, the reviewers extracted information on 11 randomized controlled trials. A total of 1039 patients (526 in BIS and 513 in non-BIS group) were included in this study.
Results
Meta-analyses showed that the total propofol consumption (the pooled standardized mean difference [SMD]: −0.15, 95 % confidence interval [CI]: −0.28 to −0.01) was significantly lower in the BIS group than in the non-BIS group, although mean propofol consumption was not significantly different. Recovery time (the pooled SMD: −0.04 [95 % CI −0.46 to 0.38, P = 0.85]), procedure time (the pooled SMD: 0.13 [95 % CI −0.03 to 0.29, P = 0.11]), adverse events, and satisfaction-related outcomes were not significantly superior in the BIS group when compared with the non-BIS group.
Conclusions
This first meta-analysis showed that BIS monitoring appears to be an effective and safe method for avoiding unnecessary administration of propofol and for providing adequate sedation during endoscopic procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bispectral index (BIS) monitoring has been widely used in clinical anesthesia as an index for monitoring the electrical activity of the cerebral cortex and the sedative ingredients of anesthesia [1, 2]. More specifically, computer-generated BIS scores range from 0 to 100 (0, coma; 40–60, general anesthesia; 60–90, sedated; 100, awake) and reflect the level of sedation regardless of a patient’s clinical characteristics or the type of sedative drug used [3]. In one meta-analysis in anesthesiology, the use of BIS monitoring consistently reduced anesthetic use by approximately 19 % compared with standard monitoring [4]. In clinical practice, the anesthesia guided by BIS could improve anesthetic delivery and postoperative recovery from relatively deep anesthesia. In addition, BIS-guided anesthesia has a significant impact on reduction of the incidence of intraoperative recall in surgical patients with high risk of awareness [5].
As the number of gastroenterologists administering propofol directly for endoscopic sedation is increasing, concern regarding titration of propofol during procedures to allow maximum patient tolerance without inducing cardiopulmonary adverse events has grown. Since the first application of BIS monitoring in the endoscopic field as an adjunctive monitoring device for sedation in 2004 [6], there have been several attempts to examine the efficacy and safety of BIS use in patients undergoing endoscopy.
However, data on the efficacy of BIS monitoring is still limited. First, several issues related to the optimal cut-off value of BIS level for achieving adequate sedation, as well as correlations between BIS levels and modified observer assessments of alertness/sedation (MOAA/S) scores remain unresolved. In previous studies, authors found the BIS value reflecting moderate sedation based on endoscopic sedation to be 80–85 [1, 7–9]. However, those studies did not consider several potentially confounding factors, such as various sedative agents, sedative methods or endoscopic procedures; therefore, it is doubtful whether the BIS values obtained in those studies can be applied to sedation conducted under different conditions. Second, controversies exist regarding the clinical role of BIS monitoring during endoscopic sedation. Many studies have identified a significant reduction in the consumption of sedatives, sedation-induced adverse events and recovery time when the BIS value is used as the primary target for sedation in endoscopic procedures. Additionally, other studies identified a significant association between BIS monitoring and a higher quality of sedation in patients [10–14]. Contrary to the above, some intriguing studies recently found that BIS monitoring did not lead to clinical benefits, such as improved oxygenation or a reduced rate of cardiopulmonary adverse events, and thus the clinical benefit in daily practice may be limited [9, 15–19].
The results of several studies evaluating the benefit of BIS monitoring for gastrointestinal endoscopy were not consistent [6, 8, 10, 12, 15, 16, 18, 20], and a meta-analysis was not performed comparing BIS and non-BIS in endoscopic sedation. Therefore, the aims of the present meta-analysis were to assess the reduction in total consumption of administrated propofol and recovery time under BIS during gastrointestinal endoscopy, and to examine the effect of BIS monitoring on patient and endoscopist satisfaction.
Materials and Methods
This systematic review was conducted and reported according to the preferred reporting items for systematic review and meta-analyses (PRISMA) statement [21].
Eligibility Criteria and Definitions
Only randomized controlled trials (RCT) in adult patients who underwent endoscopic procedures, published as full articles, were considered. Studies were eligible for inclusion in the meta-analysis if they met the following criteria: (1) studies that examined the efficacy and safety of BIS and standard monitoring for sedation during all endoscopic procedures; (2) studies which were conducted as prospective and randomized methods for comparing BIS and non-BIS; (3) studies in humans; (4) data not duplicated in another manuscript; and (5) studies that reported endpoints including at least one appropriate endoscopic outcome (total propofol dosage, mean propofol dosage, recovery time, or total procedure time), patient or endoscopist satisfaction score, or adverse events (rate of desaturation, hypotension or bradycardia). Inclusion was not restricted by study size or language. Exclusion criteria were: (1) comments, reviews, or guideline articles; (2) studies reporting non-gastrointestinal endoscopy, such as laparoscopic procedures or bronchoscopy; (3) studies providing no data on endoscopic outcomes, satisfaction score, or adverse events; (4) studies dealing with other sedative agents, not propofol-based sedation; and (5) studies repeatedly reported (if so, only the latest reported article was selected).
The widely accepted definition for total procedure time is the interval from first scope insertion until last scope removal. In this study, recovery time was defined as the time from endoscopic withdrawal to recovery of full consciousness with a MOAA/S score 5 or an Aldrete score 9 or above. Adverse events were defined as the following: desaturation as SaO2 < 90 %, hypotension as systolic blood pressure <90 mmHg, and bradycardia as heart rate <50/min.
Information Sources and Search Strategy
A literature search was conducted to identify all relevant studies that compared BIS monitoring with standard monitoring for sedation during endoscopy. A systematic literature search of MEDLINE, EMBASE, and WEB of SCIENCE databases and the Cochrane Central Register of Controlled Trials updated was conducted. The following medical subject heading terms were used: “bispectral index,” “monitoring,” “sedation,” “endoscopic retrograde cholangiopancreatography,” “endoscopic ultrasonography,” “endoscopy,” and “outcomes.” Internet search engines, Google Scholar and Yahoo, were also searched with relevant key words. No language restrictions were imposed. The latest date for updating the search was 15 March 2015.
Study Selection
After removing duplicate studies, the titles and abstracts of studies found by keywords were examined to exclude articles with irrelevant study design. If available, the full text of all selected studies was screened according to the inclusion and exclusion criteria. Selected full-text articles were critically appraised for relevance and validity. Two investigators (S.W.P. and H.L.) independently evaluated the studies for their eligibility and subsequently resolved any disagreements by discussion, together with clinical expert consultation.
Data Extraction and Study Quality Assessment
The data retrieved from each study included the name of the first author, year of publication, country, research design, number of individuals in the BIS and non-BIS groups, type of sedation, target endpoint of sedation in each group, and the primary and secondary outcomes. To avoid bias in the data extraction process, two investigators (S.W.P. and H.L.) independently evaluated each study quality and compared results with one another. In case of disagreement, the third investigator made a determination decision. To minimize the risk of bias in included studies, a formal quality assessment was conducted. The methodological quality of the RCT was assessed by two authors independently (S.W.P. and H.L.) using the scale validated by Jadad et al. [22] and scored from 0 to 5: randomization (0–2 points), blinding (0–2 points), and full accounting of all patients (0–1 point); a higher score indicates better methodological quality.
Evaluation Criteria for Endpoints
The primary end point was total propofol dosage during various endoscopic procedures. Secondary end points were: (1) mean propofol dosage; (2) recovery time; (3) total procedure time; (4) desaturation rate; (5) hypotension rate; (6) bradycardia rate; (7) patient satisfaction score; and (8) endoscopist satisfaction score.
Statistical Analysis
The meta-analysis was carried out with Review Manager 5.3 software (provided by the Cochrane Collaboration). For outcome data regarding total propofol dosage, mean propofol dosage, recovery time, total procedure time, patient satisfaction score, and endoscopist satisfaction score, we calculated the standardized mean difference (SMD), and for desaturation, hypotension, and bradycardia rates, we calculated the odds ratio (OR) as a summary statistic. All differences calculated were expressed as 95 % confidence intervals (CI). Heterogeneity among the studies was assessed using the Chi-square test. P value <0.05 was considered to suggest significant heterogeneity. At the same time, I 2 was also used to assess heterogeneity. I 2 more than 50 % was considered to indicate statistical significance.
Results
Study Selection and Assessment
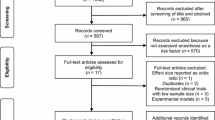
Our search strategy yielded a total of 62 potential studies for the meta-analysis (Fig. 1). After searching the titles and abstracts, we excluded 11 repeated articles. Then, based on the inclusion and exclusion criteria, 35 articles were excluded: nine studies were unrelated to the present meta-analysis, 21 studies dealt with only non-gastrointestinal endoscopy, such as laparoscopy or bronchoscopy, and five studies dealt with other, non-propofol-based sedative agents. Among the remaining 16 papers, an additional five articles were excluded due to inappropriate study design (non-randomized single-arm cohort studies). Finally, 11 studies were found to be appropriate for meta-analysis [9–18]. One of the 11 studies was performed in two phases (47 patients in phase 1 and 55 patients in phase 2) and we considered it two studies, a phase 1 study and a phase 2 study [15]. These studies described a total of 1039 patients: 526 patients in the BIS group and 513 patients in the non-BIS group. All studies were published in the past 10 years, from 2005 to 2015, and all were prospective randomized controlled studies (one [11] of the 11 studies was a randomized crossover study). Of the 11 studies, four were conducted in Korea [9, 11, 13, 17], three in the USA [15, 18], and one each in Germany [10], Greece [12], Japan [16] and Bahrain [14]. All studies were reported as full-text articles in English. The baseline characteristics of the studies included in the meta-analysis are summarized in Table 1. Quality assessment indicated that all 11 trials had a Jadad score of three or more, which suggested a good study design and a high-quality study.
Comparison of Sedation-Related Outcomes
Propofol Consumption
Of the 11 studies, eight measured propofol consumption during various endoscopic procedures. One study dealt with mean propofol consumption (mg/kg/h) instead of total propofol consumption. Therefore, total propofol consumption during endoscopic procedures was examined in seven studies; three studies evaluated endoscopic retrograde cholangiopancreatography (ERCP), two evaluated endoscopic submucosal dissection (ESD), one evaluated endoscopic ultrasound (EUS) only and one evaluated outpatient colonoscopies. Our meta-analysis revealed that the pooled standardized mean difference (SMD) in total propofol consumption was −0.15 (95 % CI −0.28 to −0.01, P = 0.03), showing decreased total propofol consumption under BIS. There was no heterogeneity among the studies with a fixed-effects model (P = 0.22; I 2 = 28 %) (Fig. 2). The subgroup analysis of four studies that dealt with mean propofol consumption (mg/kg/h) showed similar results with no statistically significant difference in total propofol consumption (the pooled SMD: −0.92 [95 % CI −2.36 to 0.52, P = 0.21]). The χ 2 and I 2 were 127.27 (P < 0.001) and 98 %, respectively, indicating significant heterogeneity among the studies with respect to mean propofol consumption (Fig. 3).
Recovery Time
Five studies reported recovery time after sedation for endoscopic procedures under BIS monitoring or standard monitoring. Three of these evaluated outpatient colonoscopies, one evaluated ERCP and another evaluated EUS. The pooled analysis did not show any significant differences between the two groups in SMD of recovery time (the pooled SMD: −0.04 [95 % CI −0.46 to 0.38, P = 0.85]) (Fig. 4). The χ2 and I 2 were 16.87 (P = 0.002) and 76 %, respectively, which indicated significant heterogeneity among studies with respect to recovery time.
Comparison of Procedure-Related Outcomes
Procedure Time
Seven studies reported total procedure time during endoscopic procedures under BIS monitoring or standard monitoring. One evaluated outpatient colonoscopies, four evaluated ERCP, one evaluated outpatient EUS and one evaluated ESD. In the meta-analysis with a fixed-effects model, the pooled SMD in the BIS group for total procedure time tended to be higher than that in the non-BIS group, though not statistically significant (the pooled SMD: 0.13 [95 % CI −0.03 to 0.29, P = 0.11]) (Fig. 5). No heterogeneity was identified between the studies (P = 0.13, I 2 = 39 %).
Comparison of Adverse Events
Desaturation
The desaturation rate was reported in six studies. Pooled analysis using a fixed-effects model demonstrated no significant differences between both groups regarding desaturation rate (BIS group [41/371, 11.05 %] vs. non-BIS group [49/364, 13.46 %]: OR 0.79; 95 % CI 0.51–1.24) (Fig. 6). There was no heterogeneity across the studies (P = 0.72, I 2 = 0 %).
Hypotension
Five studies reported the hypotension rate, including 345 patients in the BIS group and 340 patients in the non-BIS group. In a pooled analysis with a fixed-effects model, the hypotension risk was slightly higher in the non-BIS group (22/340, 6.47 %) than in the BIS group (21/345, 6.09 %), but this difference was not statistically significant (OR 0.95; 95 % CI 0.51–1.77) (Fig. 7). There was no heterogeneity across the studies (P = 0.67, I 2 = 0 %).
Bradycardia
Four studies reported the bradycardia rate, including 267 patients in the BIS group and 262 patients in the non-BIS group. In a pooled analysis with a fixed-effects model, the bradycardia risk was slightly higher in the non-BIS group (10/262, 3.82 %) than in the BIS group (3/267, 1.12 %), but this difference was not statistically significant (OR 0.31; 95 % CI 0.09–1.06) (Fig. 8). There was no heterogeneity across the studies (P = 0.99, I 2 = 0 %).
Comparison of Satisfaction-Related Outcomes
Patient Satisfaction Scores
Of the ten studies, five measured the satisfaction scores of patients after various endoscopic procedures. In the meta-analysis with a random-effects model, the pooled SMD for patient satisfaction scores between both groups was not significantly different (the pooled SMD: 0.03 [95 % CI −0.23 to 0.29, P = 0.83]) (Fig. 9). The χ 2 and I 2 were 10.89 (P = 0.03) and 63 %, respectively, which indicated significant heterogeneity among the studies with respect to the satisfaction scores of patients.
Endoscopist Satisfaction Scores
Of the ten studies, five measured the satisfaction scores of endoscopists after various endoscopic procedures. In the meta-analysis with a random-effects model, the pooled SMD for endoscopist satisfaction scores between both groups was not significantly different (the pooled SMD: 0.19 [95 % CI −0.18 to 0.55, P = 0.31]) (Fig. 10). The χ 2 and I 2 were 20.79 and 81 %, respectively, which indicated significant heterogeneity among the studies with respect to endoscopist satisfaction scores.
Discussion
We investigated 11 studies to determine whether BIS monitoring for gastrointestinal endoscopy reduces total consumption of administered propofol and recovery time. Although BIS monitoring has been found to be safe and effective for sedation in gastrointestinal endoscopy [10, 12, 14, 16], there are no consistent results as to whether BIS monitoring improves clinically practical outcomes [9, 11, 13, 15, 17, 18], and no studies with large sample sizes have compared BIS and non-BIS monitoring in endoscopic sedation. To date, BIS processed with electroencephalogram signals has typically been used as a monitor for depth of general anesthesia [23]. Furthermore, BIS-guided anesthesia has a significant impact on reduction of the incidence of intraoperative recall in surgical patients with high risk of awareness [5]. In anesthesiology, Liu’s review [4] showed that the use of BIS monitoring modestly to marginally reduced anesthetic consumption, the risk of side effects, and post-anesthesia care unit time, in spite of its higher cost relative to standard monitoring. In gastroenterology, therefore, our review is meaningful because it is the first to compare the results among actual patients treated with gastrointestinal endoscopy under BIS and non-BIS monitoring for sedation.
In our review, the total consumption of propofol under BIS monitoring was significantly lower than under non-BIS, while there was no difference between the two groups in mean propofol consumption. This result was similar to the results from a previous meta-analysis [5] demonstrating that the application of BIS within the standard practice of anesthesia can reduce the consumption of anesthetic agents and recovery times from anesthesia in surgical patients undergoing general anesthesia. Regarding the consumption of anesthetic agents, the pooled data from this review [5] involving 662 participants indicated a significant reduction in propofol consumption under BIS monitoring, with an overall decrease of 1.44 mg/kg/h (95 % CI −1.95 to −0.93; I 2 = 79 %). Similarly, one multiphase clinical trial [24] for pediatric outpatients undergoing painful procedures demonstrated that BIS monitoring can be a useful guide for the titration of propofol by physicians to achieve deep sedation in their patients. Another study [25] demonstrated that excessively deep sedation in the standard practice group (or non-BIS group in our review) might be attributed to anesthesiologists’ tendency to use more adjuvants or analgesic to manage signs of insufficient sedation. Therefore, the authors concluded that BIS-guided sedation could be helpful in optimizing the amount of adjuvants (hypnotics) or analgesics, as well as main sedative agents, and the anesthetic-sparing effect of BIS monitoring resulted in a shorter recovery time and improved quality of recovery from the patient’s perspective.
In terms of recovery time, our pooled data indicated that there were no significant differences between the BIS and non-BIS groups, although only one study among five reported that the recovery times under BIS monitoring were shorter than with standard monitoring alone. As mentioned earlier, Punjasawadwong et al. [5] reported contrary results that anesthesia guided by BIS within the recommended range could shorten postoperative recovery from relatively deep anesthesia. Our lack of significant results between the BIS and non-BIS groups can be attributed to several factors: not all studies provided an explicit definition of recovery times, therefore, the results may be influenced to a greater or lesser extent; also, there may be a discrepancy between the sedation scales (one study [9] used the modified Aldrete score and other two studies [10, 18] used the MOAA/S). Hence, it can be difficult to provide a precise assessment of the depth of sedation level after a procedure. In addition, the recovery times were not defined in the last study [15]. Additionally, our review revealed that procedure times in the BIS group tended to be longer than those in the non-BIS group, though not at a level reaching significance, similar to the differences between both groups regarding recovery times. The time to endoscope withdrawal and recovery are direct indicators of the performance of sedation/recovery, whereas procedure time is influenced by additional factors, such as the type of intervention, endoscopic findings, level of difficulty, need for therapeutic intervention, and the experience of the endoscopist.
Another concern when using BIS monitoring to titrate propofol is the possibility of intraprocedural adverse events. Among the RCTs analyzed in our review, sedatives were induced by anesthesiologists, gastroenterologists, additional physicians, and trained registered nurses; we did not find any differences in the occurrence of adverse events in either the BIS or non-BIS groups in trials, which were conducted with advanced procedures and carry a relatively high risk of adverse events. Cardiopulmonary adverse events analyzed in our review, such as desaturation, hypotension, and bradycardia, are less of a concern with BIS monitoring in propofol sedation because BIS monitoring minimized cardiopulmonary adverse events more so than non-BIS monitoring with general anesthesia. Although BIS monitoring could be helpful in reducing the consumption of propofol, this effect may not lead to a reduction in the risk of cardiopulmonary adverse events; this observation is attributed to the substantial time lag between the decrease of the BIS level below a specific level and the respective clinical findings, which is indicative of a deeper sedation state [18]. Insufficient sensitivity of BIS monitoring to predict the clinically determined consciousness of patients was also observed in another study [20]. Consequently, standard monitoring could be a more reliable method for monitoring cardiopulmonary parameters (e.g., vital signs and oxygen saturation measured by pulse oximetry) and clinical signs (e.g., coughing, cyanosis, and limb movement) in spite of the insufficiency of monitoring methods for assessing the depth of consciousness and evaluating the brain status of anesthetized patients [26].
Although we included relevant studies in our review, several limitations of this study should be noted. Significant heterogeneity among studies was detected in the current meta-analysis, which may distort the outcome of the overall analysis. First, different populations may contribute to heterogeneity. Therefore, these results should be interpreted with caution, as the population from each country was not uniform. Another limitation is that the included studies varied in the method of propofol administration (in three studies, propofol was administered by midazolam-based balanced propofol sedation, in two studies by mainly midazolam-based sedation, and in others by mainly propofol-based sedation). The included studies also evaluated different endoscopic procedures and this may also contribute to the heterogeneity among study results. Third, one of the main goals of our study was to demonstrate the reduction of propofol consumption, but the available studies had limited information regarding average propofol consumption beyond total propofol consumption during a procedure. Finally, not all of the studies provided an explicit definition for variables, especially for the definition of recovery time, procedure time, desaturation, hypotension, or bradycardia; therefore, the results may be influenced to a more or lesser extent.
In conclusion, this first meta-analysis exploring BIS monitoring during endoscopic procedures showed a significant reduction in total propofol consumption, although the recovery times under BIS monitoring did not vary significantly compared with standard monitoring. Endoscopists should be aware that BIS monitoring appears to be an effective and safe method for avoiding unnecessary administration of propofol and for providing adequate sedation during endoscopic procedures. Further studies are needed to conduct a full economic evaluation in terms of the costs and benefits of BIS monitoring.
References
Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847.
Yang KS, Habib AS, Lu M, et al. A prospective evaluation of the incidence of adverse events in nurse-administered moderate sedation guided by sedation scores or Bispectral Index. Anesth Analg. 2014;119:43–48.
Kertai MD, Whitlock EL, Avidan MS. Brain monitoring with electroencephalography and the electroencephalogram-derived bispectral index during cardiac surgery. Anesth Analg. 2012;114:533–546.
Liu SS. Effects of Bispectral Index monitoring on ambulatory anesthesia: a meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology. 2004;101:311–315.
Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Datab Syst Rev. 2007:CD003843.
Chen SC, Rex DK. An initial investigation of bispectral monitoring as an adjunct to nurse-administered propofol sedation for colonoscopy. Am J Gastroenterol. 2004;99:1081–1086.
Billard V, Gambus PL, Chamoun N, Stanski DR, Shafer SL. A comparison of spectral edge, delta power, and bispectral index as EEG measures of alfentanil, propofol, and midazolam drug effect. Clin Pharmacol Ther. 1997;61:45–58.
Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc. 2000;52:192–196.
Yu YH, Han DS, Kim HS, et al. Efficacy of bispectral index monitoring during balanced propofol sedation for colonoscopy: a prospective, randomized controlled trial. Dig Dis Sci. 2013;58:3576–3583.
von Delius S, Salletmaier H, Meining A, et al. Bispectral index monitoring of midazolam and propofol sedation during endoscopic retrograde cholangiopancreatography: a randomized clinical trial (the EndoBIS study). Endoscopy. 2012;44:258–264.
Jang SY, Park HG, Jung MK, et al. Bispectral index monitoring as an adjunct to nurse-administered combined sedation during endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:6284–6289.
Paspatis GA, Chainaki I, Manolaraki MM, et al. Efficacy of bispectral index monitoring as an adjunct to propofol deep sedation for ERCP: a randomized controlled trial. Endoscopy. 2009;41:1046–1051.
Park WY, Shin YS, Lee SK, Kim SY, Lee TK, Choi YS. Bispectral index monitoring during anesthesiologist-directed propofol and remifentanil sedation for endoscopic submucosal dissection: a prospective randomized controlled trial. Yonsei Med J. 2014;55:1421–1429.
Al-Sammak Z, Al-Falaki MM, Gamal HM. Predictor of sedation during endoscopic retrograde cholangiopancreatography–bispectral index vs clinical assessment. Middle East J Anaesthesiol. 2005;18:141–148.
Drake LM, Chen SC, Rex DK. Efficacy of bispectral monitoring as an adjunct to nurse-administered propofol sedation for colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2006;101:2003–2007.
Imagawa A, Fujiki S, Kawahara Y, et al. Satisfaction with bispectral index monitoring of propofol-mediated sedation during endoscopic submucosal dissection: a prospective, randomized study. Endoscopy. 2008;40:905–909.
Kang KJ, Min BH, Lee MJ, et al. Efficacy of bispectral index monitoring for midazolam and meperidine induced sedation during endoscopic submucosal dissection: a prospective, randomized controlled study. Gut Liver. 2011;5:160–164.
DeWitt JM. Bispectral index monitoring for nurse-administered propofol sedation during upper endoscopic ultrasound: a prospective, randomized controlled trial. Dig Dis Sci. 2008;53:2739–2745.
Lehmann A, Boldt J, Thaler E, Piper S, Weisse U. Bispectral index in patients with target-controlled or manually-controlled infusion of propofol. Anesth Analg. 2002;95:639–644.
Qadeer MA, Vargo JJ, Patel S, et al. Bispectral index monitoring of conscious sedation with the combination of meperidine and midazolam during endoscopy. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2008;6:102–108.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Drummond JC. Monitoring depth of anesthesia: with emphasis on the application of the bispectral index and the middle latency auditory evoked response to the prevention of recall. Anesthesiology. 2000;93:876–882.
Powers KS, Nazarian EB, Tapyrik SA, et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics. 2005;115:1666–1674.
Recart A, Gasanova I, White PF, et al. The effect of cerebral monitoring on recovery after general anesthesia: a comparison of the auditory evoked potential and bispectral index devices with standard clinical practice. Anesth Analg. 2003;97:1667–1674.
Health Quality O. Bispectral index monitor: an evidence-based analysis. Ontario Health Technol Assess Ser. 2004;4:1–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest or financial ties to disclose. Furthermore, this report is a meta-analysis. The author states that the report includes every item in the PRISMA checklist for meta-analysis clinical studies. Also, this manuscript was screened for plagiarism using Turnitin (www.turnitin.com).
Rights and permissions
About this article
Cite this article
Park, S.W., Lee, H. & Ahn, H. Bispectral Index Versus Standard Monitoring in Sedation for Endoscopic Procedures: A Systematic Review and Meta-Analysis. Dig Dis Sci 61, 814–824 (2016). https://doi.org/10.1007/s10620-015-3945-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3945-9