Abstract
Low flow anesthesia increases the use of CO2 absorbents, but independent data that compare canister life of the newest CO2 absorbents are scarce. Seven different pre-packed CO2 canisters were tested in vitro: Amsorb Plus, Spherasorb, LoFloSorb, Medisorb, Medisorb EF, LithoLyme, and SpiraLith. CO2 (160 mL min−1) flowed into the tip of a 2 L breathing bag that was ventilated with a tidal volume of 500 mL, a respiratory rate of 10/min, and an I:E ratio of 1:1 using the controlled mechanical ventilation mode of the Aisys® (GE, Madison, WI, USA). In part I, canister life of each brand (all of the same lot) was tested with 12 different fresh gas flows (FGF) ranging from 0.25 to 4 L min−1. In part II, canister life of six canisters each of two different lots of each brand were tested with a 350 mL min−1 FGF. Canister life is presented as “FCU”, fractional canister usage, the fraction of a canister used per hour, and is defined for the inspired CO2 concentration (FICO2) that denotes exhaustion. In part III, canister life per 100 g fresh granule content was calculated. FCU decreased linearly with increasing FGF. The relative position of the FCU–FGF curves of the different brands depends on the FICO2 threshold because the exhaustion rate (the rate of rise once FICO2 starts to increase) differs among the brands. Intra-lot variability was 18 % or less. The different prepacks can be ranked according their efficiency (least to most efficient) as follows: Amsorb Plus = Medisorb EF < LoFloSorb < Medisorb = Spherasorb = LithoLyme < SpiraLith (all for an FICO2 threshold = 0.5 %). Canister life per 100 g fresh granule content is almost twice as long when LiOH is used as the primary absorbent. The most important factors that determine canister life of prepacks in a circle breathing system are the chemical composition of the canister, the absolute amount of absorbent present in the canister, and the FICO2 replacement threshold. The use of the fractional canister usage allows cost comparisons among different prepacks. Results should not be extrapolated to prepacks that fit onto other anesthesia machines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Conventional low flow anesthesia is increasingly used to decrease waste and thus cost of and pollution by anesthetic agents. In addition, already three anesthesia machines use automated low flow, target controlled agent and carrier gas delivery with minimal user intervention, making anesthesia with fresh gas flows (FGFs) well below 1 L.min−1 the routine rather than the exception: the Aisys® (GE, Madison, WI, USA) and FLOW-i (Maquet, Solna, Sweden) use a maintenance FGF of 500 and 300 mL min−1, respectively, and the Zeus (Draeger, Lubeck, Germany) is fully closed. These low FGFs will increase CO2 absorbent usage though, because more CO2 will pass through them. The composition of the newest CO2 absorbents has changed (e.g., KOH has been eliminated to reduce anesthetic degradation), and the canister life of these absorbents has been incompletely studied and compared. This paucity of data is reflected in the small number of references quoted in this manuscript [1].
The aim of this study was to determine canister life of prefilled, disposable canisters (“prepacks”) over a wide range of FGFs with the Aisys® under conditions that resemble in vivo conditions. Prepacks have a low volume to shorten the circle system’s time constant, can be easily replaced without opening the circle system, and may ensure a more consistent humidity in the circle system [2]. Prepacks with different chemical composition of different manufacturers readily available to the authors were studied under standardized conditions with the same anesthesia machine, with FGF ranging from 0.25 to 5 L.min−1. We examined the effect of canister brand, fresh gas flow (FGF), and FICO2 replacement threshold on canister life. In addition, we examined intra- and inter-lot variability. This study does not address the effects of granular size and shape, canister shape, void space and other factors that affect canister performance.
2 Materials and methods
The patient’s CO2 production (VCO2) was simulated by flowing CO2 into the tip of a 2 L breathing bag that was attached to the Y-piece of a circle breathing system (Fig. 1). The breathing bag was ventilated with a tidal volume of 500 mL, a respiratory rate of 10/min, and an I:E ratio of 1:1 using the controlled mechanical ventilation mode of the Aisys® (GE, Madison, WI, USA). We used a CO2 inflow of 160 mL min−1: endogenous CO2 production during general anesthesia in adults is approximately 130 mL min−1 [3, 4], and applying a CO2 pneumoperitoneum increases alveolar CO2 washout by approximately 30 mL min−1 [5–7]. With a minute ventilation of 5 L.min−1, the in vitro end-expired CO2 was approximately 4.5 %, resembling in vivo conditions. We avoided the use of higher CO2 flows, so-called “accelerated” testing because they cause higher temperature gradients within the canister, which may cause decreased CO2 absorption by the formation of water deposits on the granules due to cooling by the colder temperature of the operating room [8].
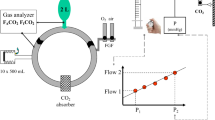
In vitro set-up. A 160 mL min−1 CO2 flow enters the tip of a 2 L breathing bag that is attached to the Y-piece of a circle breathing system. The bag is ventilated with a tidal volume of 500 mL, a respiratory rate of 10/min, and an I:E ratio of 1:1 using the controlled mechanical ventilation mode of the Aisys® (GE, Madison, WI, USA). Gases sampled by the gas analyzer (approximately 200 mL min−1) are redirected towards the expiratory limb of the circle breathing system. The experiment continues until the FICO2 has reached at least 1.0 % (see text and Fig. 2 for details). CO2 from the wall outlet is titrated via a rotameter towards a line pressure distal to the flow meter that corresponds with a 160 mL min−1 CO2 flow. Four resistors between the CO2 rotameter and the tip of the balloon attenuated the backpressure of ventilation on the CO2 flow. A pressure-flow calibration curve is constructed by dialing at least 6 different (arbitrary) rotameter settings, recording the corresponding line pressure, and measuring the corresponding CO2 flow volumetrically. The line pressure was measured between the second and third resistor (Pressure Monitoring Set, Edwards Lifesciences™, Irvine, CA); the corresponding CO2 flow was measured volumetrically by measuring the time to fill a 250 mL glass syringe (Popper and Sons, Inc. New Hyde Park, NY, USA) after switching a three way stopcock placed between the last resistor and the tip of the balloon. The calibration procedure was repeated before testing each new canister. A 1 mm Hg pressure difference corresponded to a 3.4 mL min−1 CO2 flow difference (obtained from the linear regression curve)
A CO2 flow rotameter (MEDEC®, Aalst, Belgium) was plugged into the CO2 wall outlet of the operating room. Because the resolution of the flow meter (50 mL min−1) was inadequate for the purpose of the study, CO2 flow was derived from the line pressure in the line distal to the flow meter (Fig. 1). Four resistors between the CO2 rotameter and the tip of the balloon attenuated the backpressure of ventilation on the CO2 flow. A pressure—flow calibration curve was constructed by dialing at least six different (arbitrary) rotameter settings, recording the corresponding line pressure, and measuring the corresponding CO2 flow volumetrically. The line pressure was measured between the second and third resistor (Pressure Monitoring Set, Edwards Lifesciences™, Irvine, CA); the corresponding CO2 flow was measured volumetrically by measuring the time to fill a 250 mL glass syringe (Popper and Sons, Inc. New Hyde Park, NY, USA) after switching a three way stopcock placed between the last resistor and the tip of the balloon. The calibration procedure was repeated before testing each new canister. A 1 mm Hg pressure difference corresponded to a 3.4 mL min−1 CO2 flow difference (obtained from the linear regression curve).
The CO2 flows used to test the different canisters were compared using ANOVA (followed by the Holm-Sidak to test for intergroup differences) to make sure that minor differences in CO2 inflow could be ruled out as a factor contributing to any possible differences in canister life of the different prepacks.
Inspired and expired gases were sampled and analyzed by a M-CAiOV module (GE, Madison, WI), which was calibrated according to the manufacturer’s specifications. Before each experiment, we ensured the CO2 waveform had a consistent morphology, without any oscillations or other artifact. To eliminate artifacts on the capnogram from gas entrainment from the inspiratory limb of the breathing system during the expiratory phase, two heat and moisture exchangers (Ref 352/5877, Covidien™, Mansfield, MA) were placed in between the bag and the Y-piece. The gas was sampled from the filter closest to the breathing bag; sampled gases were redirected to the circuit via a connector between the expiratory limb and its connection to the Aisys®.
2.1 Part I: canister life across a 0.25–4.0 L min−1 FGF range with the same lot
The characteristics of the different prepacks that were tested can be found in Table 1. Twelve canisters of the same lot of each brand were tested with one of the 12 following O2/air fresh gas flows (L.min−1): 0.25; 0.3; 0.35; 0.5; 0.65; 0.75; 1.0; 1.2; 1.5; 2.0; 3.0; and 4.0. The delivered O2 concentration was 55 %, except in those instances where the hypoxic guard imposed the delivery of higher O2 concentrations. The weight of fresh CO2 absorbent was obtained by subtracting the weight of the plastic encasement from the initial total weight using a high precision weighing scale (XP10002, Mettler-Toledo, Columbus, Ohio).
Prior to inserting each canister into the breathing system, the CO2 rotameter dial was adjusted to obtain a stable line pressure that corresponded to a CO2 inflow of 160 mL min−1 (derived from the calibration procedure described above). After the canister was inserted, inspired and expired CO2 concentrations as well as line pressure were downloaded every 2 min into a spreadsheet. The canister was left in place until the inspired CO2 fraction (FICO2) had reached at least 1.0 %.
Canister life was expressed as the time (in hours) from canister insertion until FICO2 had reached 0.1 % FICO2 and each additional 0.1 % increment up to 1.0 % (Fig. 2). We will further refer to these 0.1 % increments as “FICO2 thresholds” because they refer to the FICO2 concentration at which the anesthesia providers replace the canister.
Expressing canister performance as fractional canister usage (FCU). Canister life was expressed as the time (in hours) from canister insertion until FICO2 had reached 0.1 % FICO2 and each additional 0.1 % increment up to 1.0 %. The upper left and right graph describe the FICO2 course (thick gray line) with a 0.35 and 3 L min−1 FGF, respectively. The 0.1, 0.5, and 1.0 % thresholds are represented by the blue, black, and orange line, respectively. In the blue shaded box, canister life for each FICO2 threshold is converted into fractional canister usage (FCU) as 100/hours till reaching threshold. The FCU values for the different FGF are then plotted, and a linear regression is applied to those values pertaining to one specific FICO2 threshold
The inverse of canister life, 1/(canister life in hours until a certain FICO2 threshold was reached), was defined as “fractional canister usage” (FCU), the fraction of the canister that is used in 1 h. A certain FCU pertains to the combination of 1 specific FICO2 threshold, canister brand, and FGF. The FCU with the 0.1, 0.2 …,1.0 % threshold to replace the canister is abbreviated as FCU0.1 FCU0.2,…, FCU1.0, respectively. For example, FCU0.1 represents the fraction of the canister that is used in 1 h in the clinical situation where the canister is replaced once the FICO2 rises to 0.1 %. The higher the FICO2 threshold, the longer the canister life and thus the smaller the FCU will be. FCU can be presented as a fraction (0–1) or a percentage (0–100 %). For example, a canister with a FCU0.5 of 0.125 or 12.5 % at 1 L min−1 FGF means that when the canister is used with the Aisys with a 5 L.min−1 ventilation, a CO2 inflow of 160 mL min−1, and a 1 L.min−1 FGF, 1/8 of the canister will be used per hour (or the canister will last 8 h) when it is replaced once FICO2 = 0.5 %.
Because visual inspection revealed the FGF-FCU relationship for each FICO2 threshold to be linear, a linear curve fit was applied to the FGF-FCU data (Fig. 2). The intersection with the X-axis (=FGF) was forced through 5 L.min−1 because there is no rebreathing and thus no CO2 being absorbed (FCU = 0) when FGF is equal or higher than minute ventilation (5 L.min−1). Note that because the FGF-FCU fit was forced through 5 L.min−1, the slope and the Y-axis intercept of the linear fit provide the same information.
The relative performance of the different type of canisters for each FICO2 threshold was determined by dividing the slope of a particular brand by that of the respective slope intercept of the most efficient canister (note: use of the Y-axis intercept would have given exactly the same results). The canister that was most efficient for an FICO2 threshold of 0.5 % was arbitrarily assigned the value “100 %”.
2.2 Part II: intra-lot and inter-lot canister life variability (0.35 L min−1 FGF)
The canisters used to define the FGF-FCU relationship of the individual brands all had an identical lot number. To examine whether the results are more widely applicable, we tested intra-lot and inter-lot variability in two different lots. For each brand, intra-lot and inter-lot FCU variability was tested with an extra 12 canisters (6 each of a different lot) with a 350 mL min−1 FGF. Initial fresh absorbent content, CO2 inflow, and FCU for a 0.5 % FICO2 threshold between the 2 lots were compared with a t test, with p < 0.05 denoting a statistically significant difference. Intra-lot variability was assessed with the coefficient of variation (standard deviation/mean, expressed in %). We also compared the different brands by combining the data of the 12 canisters of the 2 lots with regard to initial fresh absorbent content, CO2 inflow, and FCU for a 0.5 % FICO2 using ANOVA, with p < 0.05 denoting statistical significance, followed by pairwise comparisons with the Holm-Sidak method.
To present intra-lot and inter-lot canister life variability in an intuitive, visual manner, we also plot the results with time (hours) time to 0.1 % on the X-axis, and rise time from 0.1 to 0.5 % on the Y-axis because the absolute and relative contribution of canister life until FICO2 reaches 0.5 % differs among the brands of canisters. If 0.5 % FICO2 is used as the canister replacement threshold (see discussion), total canister life will be the sum of the time till FICO2 reaches 0.1 % plus the time it will take for FICO2 to rise from 0.1 to 0.5 %.
The relative performance of the different type of canisters for each FICO2 threshold was determined by dividing canister life of the type of canister under consideration by that of the canister that was most efficient for an FICO2 threshold of 0.5 %, and expressed as %.
2.3 Part III: canister efficiency on a per product weight basis with a 0.35 L min−1 FGF
To compare the efficiency of the canisters on a per product weight basis, canister life was normalized to fresh granule content by calculating how long 100 g of product lasts with each FICO2 threshold. Both absolute canister life (min/100 g of fresh CO2 absorbent) and relative canister life (relative to that of the most efficient canister, which was assigned the value of 100 %) are presented for FICO2 thresholds ranging from 0.1 to 1.0 %. These data were calculated separately for the data for part I and II. For the data from part I, canister life for the 0.35 L min−1 is derived from the fitted parameters of the FCU fit (see part I). The canister data of part II for the different brands were compared using ANOVA, with p < 0.05 denoting statistical significance, followed by pairwise comparisons with the Holm-Sidak method.
3 Results
In part I, average CO2 flow did not differ between any of the tests of the different canister brands. Canister life and FCU are presented in Table 2. While canister life (expressed in hours until a certain threshold was reached) increased with FGF in a non-linear manner (Fig. 3a,c), FCU decreased linearly with FGF (Fig. 3b, d). The relative position of the FCU–FGF curves of the different brands depends on the FICO2 threshold. The efficiency relative to the most efficient canister for each FICO2 threshold is presented in Table 3 (upper pane).
Canister life (in hours, left panes a, b) and fractional canister usage (FCU, right panes c, d) of the different prepacks for an FICO2 threshold of 0.1 % (upper panes a, c) and 0.5 % (lower panes b, d), respectively. Lines on the left panes are added for clarity, lines in the right panes are based on the fitting parameters from Table 2. Color code: Amsorb Plus = blue triangle, Spherasorb = red circle, LoFloSorb = white circle/connecting black line, Medisorb = yellow diamond, Medisorb EF = grey square, LithoLyme = purple diamond, and SpiraLith = green circle
The results of part II, intra- and inter-lot variability, are presented in Table 4 and Fig. 4. Average CO2 flow did statistically differ between lots for the Medisorb and Amsorb Plus, but this constitutes <2.5 % from the target 160 mL min−1 FGF.
Relationship between time until start of canister exhaustion (FICO2 = 0.1 %) and rate of rise of FICO2 from 0.1 to 0.5 %. Small symbols denote individual canister data, with two different symbols denoting different lots of the same brand, with “#1” = first lot and “#2” = second lot. Large symbols denote average values of the two lots, with “Total av 1 + 2” denoting the average of all canisters of both lots. Color code: AS = Amsorb Plus, blue triangle; SS = Spherasorb, red circle; LFS = LoFloSorb, white circle/connecting black line; MS = Medisorb, yellow diamond; EF = Medisorb EF, grey square; LL = LithoLyme, purple diamond; and SL = SpiraLith = green circle
Also in part II, canister life (for a 0.5 % FICO2 replacement threshold) was found to differ significantly between the different brands, except between Amsorb Plus and Medisorb EF, and between LithoLyme, Spherasorb, and Medisorb (Table 5). The amount of fresh CO2 absorbent differed between all brands, but CO2 flow did not (Table 5).
The efficiency relative to the most efficient canister for each FICO2 threshold is presented in Table 3 (lower pane).
In part III, canister life per 100 g fresh granule content was found to be almost twice as long when LiOH is used as the primary absorbent instead of Ca(OH)2, a consistent finding in canisters tested in part I and II (Table 6).
4 Discussion
Meaningful comparisons between different CO2 absorbents require that the conditions in which they are being tested are standardized and represent real-life conditions. By standardizing the experimental set-up, we attempted to eliminate the variability of CO2 production, degree of rebreathing, and rebreathing pattern (anesthesia machine configuration).
The relationship between FGF and canister life (hours) is curvilinear (Fig. 3). While absolute canister life may be more intuitive to use, the use of FCU has the advantage to have a linear relationship with FGF (Fig. 3). This facilitates comparisons among canisters as well as cost. Note this linear relationship may not hold for other anesthesia machines because the properties of the circle breathing system and the manner in which gases are handled during the different phases of the respiratory cycle will affect this relationship [9]. This is corroborated by preliminary data comparing canister life of prepacks of different brands with the Aisys, the Zeus, and the FLOW-i (Maquet, Solna, Sweden). Therefore, our data for prepacks fitting on the Aisys should not be extrapolated to those fitting onto other anesthesia machines. In addition, the absolute and relative weight of fresh absorbent is different for canisters that fit onto different machines.
Because the anesthesia machine, ventilation, and CO2 inflow were the same for all the tests, differences in canister life were only determined by the chemical composition of the CO2 absorbent, FICO2 replacement threshold, and properties of the canister content. The latter include—but are not limited to—the weight of fresh CO2 absorbent, granular size and shape, canister shape, and void space. Let us consider some of these.
The effect of chemical composition was assessed by plotting total canister life of the different brands per 100 g of fresh canister content versus the FICO2. CO2 absorption capacity on a weight basis was higher with LiOH than with Ca(OH)2 as the primary absorbent. Within the Ca(OH)2 canister group, the presence of a catalyst (NaOH or LiCl) increased canister life for the lower FICO2 thresholds, an effect that faded if a higher FICO2 replacement threshold was accepted. Because the prepacks of the different brands contain different amounts of CO2 absorbent, differences in efficiency on a per weight CO2 absorbent basis cannot be directly translated into differences in canister life. From a clinical and economical point of view, canister life is what we are interested in if we use prepacks. Because of the many factors that affect CO2 absorbent efficiency, care has to be taken to extrapolate the weight-based results to bulk CO2 absorbent for refillable canisters.
Because the rate of rise of FICO2 above 0.1 % differs among the prepack brands, the FICO2 threshold that the clinician uses to replace the canister has a profound impact on the absolute and relative performance of the different brands. The rise is slowest with the Ca(OH)2 based canisters that have no or little catalyst (Fig. 4), and thus most canister life can be gained by accepting a higher FICO2 threshold with these absorbents (Fig. 4). On the contrary, less is gained by waiting for FICO2 to reach 0.5 % before replacing a SpiraLith canister. The FICO2 can be allowed to rise until it affects the end-expired CO2 concentration (FACO2) of the patient up to a point where it becomes clinically unacceptable: FACO2 is the physiologically important variable for the patient, not FICO2. According to the alveolar gas equation, the effect of FACO2 on FICO2 is additive, FACO2 = FICO2 + (VCO2/alveolar ventilation). In most of our patients, the FACO2 (35 mmHg or 4.5 %) can be allowed to increase by 4 mmHg or 0.5 % without causing any harm. Therefore, the FICO2 can be allowed to rise to 4 mmHg or 0.5 % in most of our patients.
The use of “fractional canister usage” allows canister costs to be calculated by multiplying the FCU (expressed in fraction) with the cost of 1 canister, and this for any FGF. While the exact conditions in real life do differ from the in vitro conditions of this study, the ratios of the product of FCU and the cost of 1 canister of the different brands can be used to make fair cost comparisons among canisters.
The canisters used to define the FGF-FCU relationship all had an identical lot number. Are these results applicable to other lot numbers? Inter-lot differences were only statistically significant for the 2 longest lasting canisters, SpiraLith and LithoLyme-12 and 18 % respectively. Intra-lot variability was small, with the notable exception of the Medisorb canister. SpiraLith canisters have both the steepest FICO2 rise and the least intra-lot variability, a consistency we hypothesize is due to the preformed channels that leave little room for the more random channeling that is likely to occur with canisters containing the Ca(OH)2 granules. If the results of the 2 lots are combined, the different prepacks can be ranked according their efficiency (least to most efficient) as follows: Amsorb Plus = Medisorb EF < LoFloSorb < Medisorb = Spherasorb = LithoLyme < SpiraLith (all for an FICO2 threshold = 0.5 %).
The use of a consistent, replicable model allows canisters to be compared and allows for external verification. Still, real life conditions may differ from in vitro conditions: CO2 loads in real life vary, both in time and quantity. No canister is used for more than 24 h straight. It is unclear how—and whether—changing FGFs and CO2 loading conditions with prolonged periods of non-use (at night, during the weekends) affect canister performance, both absolute and relative, from one brand to another. In addition, O2 was not removed from the system, while in real life patients do remove O2. Therefore, in real life, the degree of rebreathing might be slightly different from that in the experimental setup. However, the effect on canister life is likely to be very small, and certainly will not affect relative canister life. Ongoing clinical studies will help address the issues raised in this paragraph.
To summarize, canister life of CO2 absorber prepacks were determined over a wide range of FGFs with the Aisys® (GE, Helsinki, Finland) under identical CO2 load conditions. The most important factors that determine canister life of prepacks in a circle breathing system are the chemical composition of the canister, the absolute absorbent content of the canister, and the FICO2 replacement threshold. The use of the fractional canister usage allows for cost comparisons among different prepacks. Results should not be extrapolated to prepacks that fit onto other anesthesia machines.
References
Higuchi H, Adachi Y, Arimura S, Kanno M, Satoh T. The carbon dioxide absorption capacity of Amsorb is half that of soda lime. Anesth Analg. 2001;93:221–5.
Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 108–10.
Robinson GJI, Peyton PJ, Terry D, Malekzadeh S, Thompson B. Continuous measurement of gas uptake and elimination in anesthetized patients using an extractable marker gas. J Appl Physiol. 1985;97:960–6.
Stuart-Andrews C, Peyton P, Humphries C, Robinson G, Lithgow B. Continuous measurement of multiple inert and respiratory gas exchange in an anaesthetic breathing system by continuous indirect calorimetry. J Clin Monit Comput. 2009;23:41–9.
Blobner M, Felber AR, Gögler S, Feussner H, Weigl EM, Jelen G, Jelen-Esselborn S. The resorption of carbon dioxide from the pneumoperitoneum in laparoscopic cholecystectomy. Anaesthesist. 1993;42:288–94.
Kazama TI, Ikeda K, Sanjo Y. Comparative carbon dioxide output through injured and noninjured peritoneum during laparoscopic procedures. J Clin Monit Comput. 1998;14:171–6.
Tan PLI, Lee TL, Tweed WA. Carbon dioxide absorption and gas exchange during pelvic laparoscopy. Can J Anaesth. 1992;39:677–8.
Hirabayashi GI, Uchino H, Nakajima T, Ogihara Y, Ishii N. Effects of temperature gradient reduction in three different carbon dioxide absorbents. Eur J Anaesthesiol. 2009;26:469–74.
Harper M, Eger EI 2nd. A comparison of the efficiency of three anesthesia circle systems. Anesth Analg. 1976;55:724–9.
Acknowledgments
The study has not been funded. Jan Hendrickx has received lecture support, travel reimbursements, equipment loans, consulting fees and/or meeting organizational support from a number of companies involved with inhaled agent delivery (alphabetically): AbbVie, Acertys, Air Liquide, Allied Healthcare, Armstrong Medical, Baxter, Draeger, GE, Hospithera, Heinen und Lowensein, Intersurgical, Maquet, MDMS, MEDEC, Micropore, Molecular, NWS, Philips, Quantum Medical.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hendrickx, J.F.A., De Ridder, S.P.A.J., Dehouwer, A. et al. In vitro performance of prefilled CO2 absorbers with the Aisys® . J Clin Monit Comput 30, 193–202 (2016). https://doi.org/10.1007/s10877-015-9699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9699-2