Abstract
Soda lime-based CO2 absorbents are safe, but not ideal for reasons of ecology, economy, and dust formation. The Memsorb™ is a novel CO2 removal device that uses cardiopulmonary bypass oxygenator technology instead: a sweep gas passes through semipermeable hollow fibers, adding or removing gas from the circle breathing system. We studied the in vitro performance of a prototype Memsorb™ used with a Zeus IE® anesthesia machine when administering sevoflurane and desflurane in O2/air mixtures. The Zeus IE® equipped with Memsorb™ ventilated a 2L breathing bag with a CO2 inflow port in its tip. CO2 kinetics were studied by using different combinations of CO2 inflow (VCO2), Memsorb™ sweep gas flow, and Zeus IE® fresh gas flow (FGF) and ventilator settings. More specifically, it was determined under what circumstances the inspired CO2 concentration (FICO2) could be kept < 0.5%. O2 kinetics were studied by measuring the inspired O2 concentration (FIO2) resulting from different combinations of Memsorb™ sweep gas flow and O2 concentrations, and Zeus IE® FGFs and O2 concentrations. Memsorb™’s sevoflurane and desflurane waste was determined by measuring their injection rates during target-controlled closed-circuit anesthesia (TCCCA), and were compared to historical controls when using a soda lime absorbent (Draegersorb 800+) under identical conditions. With 160 mL/min VCO2 and 5 L/min minute ventilation (MV), lowering the sweep gas flow at any fixed Zeus IE® FGF increased FICO2 in a non-linear manner. Sweep gas flow adjustments kept FICO2 < 0.5% over the entire Zeus IE® FGF range tested with VCO2 up to 280 mL/min; tidal volume and respiratory rate affected the required sweep gas flow. At 10 L/min MV and low FGF (< 1.5 L/min), even a maximum sweep flow of 43 L/min was unable to keep FICO2 ≤ 0.5%. When the O2 concentration in the Zeus IE® FGF and the Memsorb™ sweep gas flow differed, FIO2 drifted towards the sweep gas O2 concentration, and more so as FGF was lowered; this effect was absent once FGF > minute ventilation. During sevoflurane and desflurane TCCCA, the Zeus IE® FGF remained zero while agent usage per % end-expired agent increased with increasing end-expired target agent concentrations and with a higher target FIO2. Agent waste during target-controlled delivery was higher with Memsorb™ than with the soda lime product, with the difference remaining almost constant over the FGF range studied. With a 5 L/min MV, Memsorb™ successfully removes CO2 with inflow rates up to 240 mL/min if an FICO2 of 0.5% is accepted, but at 10 L/min MV and low FGF (< 1.5 L/min), even a maximum sweep flow of 43 L/min was unable to keep FICO2 ≤ 0.5%. To avoid FIO2 deviating substantially from the O2 concentration in the fresh gas, the O2 concentration in the fresh gas and sweep gas should match. Compared to the use of Ca(OH)2 based CO2 absorbent, inhaled agent waste is increased. The device is most likely to find its use integrated in closed loop systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Whenever an anesthesia circle breathing system is used with fresh gas flows (FGFs) well below minute ventilation (MV), Ca(OH)2 based CO2 absorbents are used to prevent high inspired CO2 concentrations (FICO2). Modern KOH free and NaOH poor or free Ca(OH)2 absorbents are safe over the entire range of FGF, but still have some (minor) shortcomings: their production and disposal are environmentally unfriendly, they need to be replaced frequently, anesthesia providers rarely use them to full capacity, and the dust they generate may accumulate in sensitive parts of the anesthesia machine. In addition, the ubiquitous use of heat and moisture exchangers has made the exothermic and water producing properties of CO2 absorbents less compelling. A modified cardiopulmonary bypass oxygenator, the Memsorb™ (DMF Medical, Halifax, Canada; further referred to as “Memsorb”), addresses the limitations of traditional CO2 absorbents. Based upon the technology of membrane oxygenators, exhaled gases flow through semipermeable hollow fibers and sweep gas creates a gradient for gas exchange (Fig. 1). We determined the in vitro performance of a Memsorb canister used with a Zeus IE® anesthesia machine (Dräger, Lübeck, Germany; further referred to as “Zeus”) to deliver sevoflurane and desflurane in O2/air mixtures. This manuscript is part one of a two-part study determining the in vitro and in vivo performance of Memsorb. The goal of this part is to establish an understanding of its in vitro function to inform the protocol for evaluating in vivo performance.
The Memsorb. A and B The Memsorb consists of a constellation of parallel hollow fibers, the walls of which have a different permeability for different gases. Gas from the circle breathing system (CO2 and anesthetic in this example) flow between the hollow fibers. Sweep gas (in this example O2) is directed into the lumen of these fibers. Gas exchange occurs across the walls of the hollow fibers. Ideally, only CO2 should be removed by the device. C Gas from the circle breathing system enters via the central hole (purple) on top of the Memsorb canister. These gases then pass through a circular “wall” of horizontally mounted hollow fibers, after which they exit the canister via the space between the wall of fibers and the outer portion of the canister (red). D The bottom of the device contains a water drainage port (yellow arrow), an entree port for the sweep gas (black tubing/white arrow), and an exit port (red arrow) for the mixture of gas removed from the circle breathing system plus whatever remains from the sweep gas (plus the water drainage port, yellow arrow)
2 Materials and methods
2.1 The Memsorb system (Fig. 1)
A prototype Memsorb unit was provided by DMF Medical at no cost. The unit was designed to fit onto the Zeus CLIC adaptor® that connects CO2 absorbers to the Zeus. Circle system gas enters and exits via the top of the device, while sweep gas enters and leaves the hollow fibers via an inlet and outlet port at the bottom. Gas exchange between circle system gas flowing between the hollow fibers and sweep gas flowing in the lumen of the hollow fibers occurs across the walls of these hollow fibers. The device also has a drainage channel for any fluid that might accumulate.
Gas exchange depends on factors such as the properties of the fiber walls and the manner in which they are organized, concentration gradients, bulk movement of gas in the circle system (which depends in a complex manner on FGF, MV, and circle system configuration), sweep gas flow and pressure, and possibly a host of other factors, some proprietary. Because the walls of the hollow fibers are less permeable (and ideally would be impermeable) to potent inhaled agents than they are to other components of the anesthetic gas mixture, they offer the intriguing possibility of filtering out CO2 yet retaining (most of) the potent inhaled agent and possibly other desired gases (O2, N2, N2O, and H2O) in the anesthetic gas mixture, but details have not been published.
2.2 Sweep gas delivery (Fig. 2)
An O2/air blender (Sechrist, 3500 low flow air-O2 mixer, AR-MED, Egham, United Kingdom) with visible flow indicators (floating balls) was used to deliver the O2/air sweep gas to the Memsorb canister. The tubing connecting the blender to the Memsorb inlet contained a gas sampling port and a pressure line monitor just distal to the blender (not shown). A calibrated multi-gas analyzer (M-CAiOV compact module, GE, Madison, WI, USA) continuously measured the sweep gas O2 concentration. Because the blender’s floating ball flow display was limited to 10 L/min yet preliminary testing had indicated higher sweep gas flows were needed, higher flows were volumetrically calibrated to the pressure inside the tubing. This calibration curve was constructed by adjusting the rotameter to attain a certain line pressure (Pressure Monitoring Set, Edwards LifesciencesTM, Irvine, CA), followed by volumetrically measuring the corresponding flow (time to fill a 6L breathing bag) and repeating this over a wide range of settings. Pressure was related to flow by linear regression: sweep gas flow = 71.1–71.5*e−sweep pressure/48.8 (R2 = 0.9959), with flow in L/min and pressure in mm Hg. Only sweep flows are reported. This relationship was not affected by connecting the Memsorb to the Zeus.
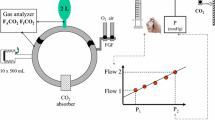
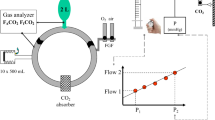
The Zeus equipped with the Memsorb. O2 (green), air (yellow), and N2O (blue) flow from the wall outlet into the breathing circuit. During manual agent delivery, the agent is injected into the delivered gas (β) with both carrier gas and agent entering distally to the Memsorb, while during automated delivery agent is injected directly into the circle system via a separate channel (α), with both carrier gas and agent entering proximally to the Memsorb. An electronically controlled turbine mixes the gas in the system (shortening response times) and ventilates the patient. An O2/air blender controls the flow and O2 concentration of the sweep gas to the Memsorb; flow meter setting is titrated using the line pressure which can be converted into flow via a calibration curve (see text for details)
2.3 The Zeus (Fig. 2)
Details of the Zeus have been published elsewhere [1]. The heart of the system is a turbine that functions as the ventilator and as a mixing tool. Agent is injected as a liquid, either directly into the circle breathing system proximal to the Memsorb and separate from carrier gas delivery during target-controlled agent and gas delivery (Fig. 2α) or into the carrier gas stream and distal to the Memsorb during conventional “manual” agent and carrier gas delivery (Fig. 2β).
The different experiments described below used different agent and carrier gas delivery modes (conventional versus target-controlled) and different FGF. In target-control mode (brand name “auto control mode”), the anesthesia provider enters the inspired O2 (FIO2) and end-expired agent concentration (FET) targets. A FGF target also has to be entered, for which two options exist. The first target control delivery FGF option is to use the default setting, target-controlled closed-circuit anesthesia (TCCCA) mode. In this mode, virtually no gas leaves the exhaust when O2/air mixtures are used with the Zeus equipped with a conventional Ca(OH)2 based CO2 absorbent, and therefore the FGF (displayed on the screen) is the sum of uptake by the patient and leaks [2]. Because leaks have to be less than 100 mL/min at 25 to 30 mbar, the TCCCA mode becomes an excellent tool to study the performance of the Memsorb: any loss of gas with the Zeus–Memsorb combination in excess of that when the Zeus is using with a conventional Ca(OH)2 absorber can only be due to the use of the Memsorb. The second target control delivery FGF option is to select a specific FGF (“target FGF”) that can range from 0.5 to 18 L/min. Regardless of which of the two target-controlled delivery options is selected, the actual FGF that is being delivered by the Zeus at any time is the result of a trade off the control algorithm makes between rapidly achieving the desired targets (high FGF) and simultaneously minimizing anesthetic agent waste (low FGF).
2.4 In vitro tests
2.4.1 Basic setup (Fig. 2)
The following set up was used in all experiments unless described differently. A 2 L breathing bag (Armstrong Medical Limited, Coleraine, Northern Ireland) was ventilated via an 8.0 mm endotracheal tube (Covidien, Mansfield, MA, USA) by a Zeus anesthesia machine in controlled mechanical ventilation mode, with the following settings: tidal volume 500 mL, respiratory rate 10/min, I:E ratio 1:1, and 5 cm H2O PEEP. The endotracheal tube was connected to a spirometer (D-lite, GE, Madison, WI, USA) that also contains a gas sampling port for the gas analyzer incorporated in the Zeus. An HME filter (Ref 352/5877, Covidien) was placed between the D-lite and the circle breathing Y-piece (DAR Adult Breathing Circuit, Covidien) because this was empirically found to help ensure a stable, slightly upsloping FETCO2 plateau. CO2 production (VCO2) was simulated by feeding 160 mL/min of CO2 from a wall mounted modified CO2 flow meter (MEDEC, Aalst, Belgium; accuracy of 1.5 mL/min) into the tip of the breathing bag [3]. The Memsorb canister was latched onto the CLIC adaptor® of the Zeus.
2.4.2 Experiments
For experiments A and B described below, conventional (“manual”) delivery of carrier gas and agent was used, implying liquid agent (see β in Fig. 2) was injected into the carrier gas inflow before it entered the circle breathing system, and distal to the Memsorb. For experiment C, automated delivery was used, implying liquid agent was injected directly into the circle breathing system proximal to the Memsorb and independent from the carrier gas stream (see α in Fig. 2).
2.5 CO2 kinetics
2.5.1 Effect of sweep gas flow on FICO2 with different FGFs (conventional gas delivery) (Fig. 3A)
The effect of different sweep gas flows (from 5.2 to 23.5 L/min) on FICO2 was tested with 5 different FGFs (0.25; 0.5; 1.0; 1.5; and 2 L/min). The same O2 concentration (100%) was used in both the sweep gas and Zeus carrier gas, because the use of different O2 and N2 concentrations in these two gas streams was found early on to cause gas transfer between the Zeus and Memsorb that would interfere with the experiment.
CO2 kinetics. A Effect of sweep gas flow (100% O2) on the inspired CO2 concentration (FICO2) with different FGFs (100% O2) with VCO2 = 160 mL/min and MV = 5 L/min. B The inverse of the sweep gas flow that maintained FICO2 = 0.5% was linearly related with the FGF (VCO2 = 160 mL/min and MV = 5 L/min). C Sweep gas flows that maintained FICO2 = 0.5% with MV = 5 L/min over a wide range of FGF with different VCO2. D Sweep gas flows that maintained FICO2 = 0.5% over a wide range of FGF with VCO2 = 240 mL/min and MV = 10 L/min composed of 3 different combinations of tidal volume (mL) and respiratory rate (breaths per min, bpm): 1000 mL * 10 bpm; 666 mL * 15 bpm; and 500 mL * 20 bpm. When 240 mL/min VCO2 was combined with a 10 L/min MV, even a sweep gas flow up to maximum 43 L/min failed to maintain FICO2 ≤ 0.5% once the FGF decreased below 1.5 L/min (**)
2.5.2 Effect of different VCO2 on sweep gas flows required to maintain 0.5% FICO2 over a wide FGF range during constant MV (conventional gas delivery) (Fig. 3B, C)
We determined the sweep gas flows that maintained 0.5% FICO2 with VCO2 of 80, 120, 160, 200, 240, and 280 mL/min over a 0.25 to 6 L/min FGF range (0.25; 0.5; 1,0; 1.5; 2; 3; 4; 5; and 6 L/min) with a MV of 5 L/min. The O2 concentration in both the carrier gas and the sweep gas was 100%.
2.5.3 Effect of tidal volume and respiratory rate on sweep gas flow required to maintain 0.5% FICO2 during hyperventilation with increased VCO2 (conventional delivery) (Fig. 3D)
Sweep gas flows to maintain 0.5% FICO2 were determined with a CO2 inflow of 240 mL/min and a total MV = 10 L/min composed of 3 different combinations of tidal volume (ml) and respiratory rate (breaths per min, bpm): 1000 mL * 10 bpm; 666 mL * 15 bpm; and 500 mL * 20 bpm. The O2 concentration in the O2/air mixture of both the carrier gas and the sweep gas was set at 100%.
2.6 O2 kinetics
2.6.1 Effect of sweep gas O2 concentration on FIO2 with different FGF with varying O2 concentrations (conventional delivery) (Fig. 4)
While using the lowest sweep gas flow that maintained FICO2 ≤ 0.5%, the sweep gas O2 concentration was increased stepwise from 21 to 100% (21, 30, 40, 60, 80, and 100%) and the resulting FIO2, measured at the D-lite, was recorded. This sequence was repeated with 5 different FGFs (1.0; 1.5; 2.0; 4.0; and 6.0 L/min), each with 4 different FGF O2 concentrations (30, 50, 70, and 100%). O2 kinetics with FGF of 0.25 and 0.5 L/min were not studied because of limits imposed by the hypoxic guard.
2.7 Anesthetic agent kinetics (Fig. 5)
2.7.1 Desflurane and sevoflurane use during TCCCA with different FIO2 targets
Desflurane and sevoflurane liquid injection rates (mL/h) and air and O2 FGF of the Zeus were measured over a range of target FET of desflurane (FETdes) (3, 6, 9, and 12%) and sevoflurane (FETsevo) (1, 2, 3.5, and 5%) (Fig. 5A). Because preliminary testing had indicated that the target FIO2 could affect the kinetics of a concomitantly administered inhaled agent, these experiments were performed with 40 and 80% target FIO2. The sweep gas O2 concentration matched the target FIO2. The sweep gas flow was adjusted to an FICO2 of 0.5%. Results are presented as the injection rate per 1% FETagent.
Inhaled agent kinetics. A Desflurane (blue) and sevoflurane (yellow) liquid injection rate (mL/min) expressed per % of target concentration with 40% target FIO2 (black triangle) or 80% target FIO2 (dark green diamond) during target-controlled closed circuit anesthesia. FGF remained zero in all instances. B Liquid agent injection rate (Vsevo and Vdes) when the Zeus is used with Memsorb or Draegersorb 800+ with different FGF targets (target-controlled delivery) starting at 500 mL/min and up. Target end-expired concentration of desflurane (FETdes) was 6%, and target end-expired concentration of sevoflurane (FETsevo) was 2%. Linear fits of Vagent and FGF data are: Vsevo MS = 2.5 + FGF * 11.4 (r2 = 0.99); Vsevo 800 + = FGF * 11.4 (forced fit through 0—see Sect. 2); Vdes MS = 11.1 + FGF * 23.0 (r2 = 0.99); Vdes 800 + = FGF * 24.7 (forced fit through 0—see Sect. 2)
2.7.2 Agent usage with Memsorb or Draegersorb 800 + soda lime (Fig. 5B)
After priming the circuit with the chosen agent, the liquid agent injection rate during target-controlled delivery with 2% FETsevo or 6% FETdes in O2/air with two different FIO2 target (40 and 80%) was compared between the Zeus–Memsorb and Zeus–soda lime Drägersorb 800+ combination over a range of FGF targets (0.5; 0.7; 1.0; 1.5; 2.0; 3.0; 4.0; 5.0; 6.0 L/min). Linear fitting was used to examine whether any difference in agent usage (liquid agent injection rate in mL/h) between the two would remain constant over the tested FGF range. The fit with the Zeus–soda lime Drägersorb 800+ combination was forced through zero because the injection rate is zero at zero FGF.
2.8 Data collection and analysis
All relevant data from the Zeus and the Memsorb blender system were collected and stored onto a PC using Rugloop© (Demed, Temse, Belgium) software. No statistical analysis was required.
3 Results
3.1 CO2 kinetics
With a combination of VCO2 of 160 mL/min, 5 L/min MV, and any fixed FGF, lowering the sweep gas flow increased FICO2 in a non-linear manner (Fig. 3A). The relationship between the sweep gas flow that maintained the FICO2 at 0.5% and the FGF was inverse: sweep gas flow−1 (min/L) = FGF*0.051 + 0.049 (r2 = 0.997) (Fig. 3B). Increasing the sweep gas flow succeeded in lowering FICO2 below 0.5% over the entire FGF range tested (0.25–2 L/min) (Fig. 3A).
When VCO2 was progressively increased up to 280 mL/min while MV remained 5 L/min, increasing the sweep gas flows up to 28 L/min maintained 0.5% FICO2 (Fig. 3C).
When 240 mL/min VCO2 was combined with a 10 L/min MV, even a sweep gas flow up to maximum 43 L/min failed to maintain FICO2 ≤ 0.5% once the FGF decreased below 1.5 L/min (marked with ** in Fig. 3D). With a FGF ≥ 2 L/min, the sweep gas flow that maintained FICO2 ≤ 0.5% had to be higher when tidal volume was smaller and respiratory rate was higher (Fig. 3D).
3.2 O2 kinetics (Fig. 4)
When the O2 concentration in the Zeus carrier gas and the Memsorb sweep gas differed, the FIO2 drifted towards the sweep gas O2 concentration, except when FGF (6 L/min) exceeded MV (5 L/min). The lower the FGF, the more FIO2 drifted towards the sweep gas O2 concentration.
3.3 Anesthetic agent kinetics
During TCCCA with sevoflurane and desflurane with the Memsorb the FGF always remained zero. Agent delivery per % FETagent increased with increasing target FETagent and with a higher target FIO2 (Fig. 5A).
During TCCCA with a 2% FETsevo (Fig. 5B) and after the circuit had been primed, the liquid injection rate was 6 mL/h with the Memsorb and 0 mL/h with the soda lime Draegersorb 800 + ; with a 6% FETdes target, it was 13 mL/h with the Memsorb and 0 mL/h with the soda lime Draegersorb 800 + ; in all instances, FGF remained zero. When the target FGF was increased to 500 mL/min and up, the difference in agent consumption between these CO2 removal devices remained almost constant over the FGF range studied for both agents. Because the Zeus displays injection rates above 100 mL/h only as “injection rates > 100 mL/h” (i.e. without the actual injection rate), no values above 100 mL/h are reported, which was the case for desflurane when the FGF was higher than 3 L/min.
4 Discussion
In this study, the incorporation of the novel CO2 removal device Memsorb into a circle breathing system was found to have complex effects on the kinetics of the different components of the anesthetic gas mixture. We sequentially addressed management of CO2, O2, and volatile anesthetics.
In our experimental setup, VCO2, sweep gas flow, FGF, tidal volume and respiratory rate all affected FICO2. While most often relatively stable intraoperatively, the amount of exhaled CO2 (VCO2, mimicked in this study by the CO2 inflow in to a 2L breathing bag) can change, e.g., after applying a CO2 pneumoperitoneum. The sweep gas flow was the primary tool used to decrease FICO2 when the Memsorb was used. At MV = 5 L/min, a sweep gas flow ≥ 20 L/min was able to keep FICO2 ≤ 0.5% irrespective of FGF and VCO2 except in extreme situations where VCO2 was 280 mL/min with FGF ≤ 0.5 L/min (Fig. 3A, C). Lower sweep gas flows were adequate in situations where VCO2 was lower and/or FGF was higher. Higher sweep gas flows were successful with VCO2 of 280 mL/min, the high end of what may be encountered in clinical practice (the rare exception being hypermetabolic crises). “Sufficient” CO2 removal does not require that FICO2 is zero: some CO2 rebreathing may be present, as was most often the case with the Memsorb. An FICO2 of ≤ 0.5% is acceptable: its effect on FETCO2 can be easily overcome by a modest increase of MV, and 0.5% is widely considered as a safe replacement threshold of an exhausting Ca(OH)2 CO2 absorber. Higher sweep gas flows become progressively less efficient at reducing FICO2. While our results indicate that FICO2 can also be manipulated by changing the respiratory rate and/or tidal volume while maintaining the same MV, its effect is small and likely of limited use in clinical practice. If VCO2 would prove to be excessive for the Memsorb and cause FICO2 to rise too much, the FGF could be increased, but this would come at the expense of more inhaled agent waste. Increasing MV will reduce the FETCO2–FICO2 difference (because the same amount of CO2 will be distributed over a larger volume), but this will also increase rebreathing and thus FICO2.
The clinically relevant parameter is not so much FICO2 but rather FETCO2 because FETCO2 reflects the arterial FCO2. It is therefore important to understand how VCO2, MV and FGF affect the FETCO2–FICO2 relationship, which can be explained by considering CO2 mass balances in the lung and the cicrle breathing system.
In the lungs, the amount of exhaled CO2 is the sum of the amount of inhaled CO2 and VCO2. The amount of exhaled CO2 is the sum of the amount contained in the apparatus and anatomical dead space with concentration FICO2 plus the part of exhaled MV that does take place in gas exchange with concentration FETCO2. The amount of inhaled CO2 is the product of MV and FICO2. If we assume that in- and expired MV are the same and if we define fVD as apparatus and anatomical dead space (expressed as fraction), mass balances can be mathematically expressed as:
This equation explains how FETCO2, FICO2,VCO2 and MV interrelate when one of these factors is adjusted while using the Memsorb.
The second relevant concept is the fraction of rebreathing in a circle system. The amount of rebreathed gas can be approximated by the difference between exhaled MV and FGF: amount rebreathed gas ≈ MV–FGF, which after dividing both sides of the equation by MV yields the fraction of rebreathing, the ratio of rebreathed gas over MV, which can be approximated as:
When a circle breathing system without a fully effective CO2 absorber is used with a FGF below MV, increasing MV will raise FICO2 because the fraction of rebreathing increases (Eq. 2), but at the same time [FETCO2–FICO2] will decrease because the same amount of CO2 is distributed over more or larger tidal volumes over time (Eq. 1). Vice versa, lowering MV will decrease FICO2 because the fraction of rebreathing decreases (Eq. 2), but at the same time [FETCO2–FICO2] will increase because the same amount of exhaled CO2 is distributed over less or smaller tidal volumes (Eq. 1). For the user to control all these variables, a rational, staged approach is needed. Such an algorithm is derived to manage gas kinetics during clinical testing in the accompanying paper [4].
Managing FIO2 is straightforward once some basic principles are taken into account. The FIO2 can be very different from the fresh gas O2 concentration because of the effect of the Memsorb sweep gas flow and its O2 concentration. Balancing these different O2 concentration/flow combinations could be challenging. Matching the O2 concentration in the anesthesia machine fresh gas and Memsorb sweep gas ensures FIO2 will remain virtually the same and becomes independent of the FGF. Note that we did not study the effect of O2 consumption, which was considered zero in this in vitro study. The effect of the sweep gas O2 concentration on the FIO2 is the single most important reason why we believe the device should be part of a closed-loop delivery system—and is the major reason why the device will be tested in patients with a closed-loop automated delivery system.
During TCCCA (Fig. 5A), the agent injection rate did change as FETagent was changed, but the FGF always remained zero, which is possible because carrier gas and agent are injected and controlled separately by the Zeus (Fig. 2, α). We cannot readily explain why the injection rate per percent FET of the agent increases disproportionally as FETagent is increased, indicating that gas mass balances are complex.
In target control, reaching and maintaining the desired anesthetic agent concentration with the Zeus–Memsorb combination will not differ a lot from that with the Zeus–Ca(OH)2 absorber combination from the clinician’s perspective because the combination of a liquid injector and a blower that serves as a mixer allows very fast control. During manual agent delivery with a fixed FGF however, the vaporizer setting will have to be slightly higher to attain the same FETagent with the Memsorb than with the Zeus–Ca(OH)2 absorber combination because of the modest extra agent use, an effect that will be more pronounced at low FGF because losses seem to remain more or less constant over a wide FGF range. The device will be tested in humans using an automated delivery system because this will allow the FETagent to be well controlled while using it under the best of circumstances to minimize anesthetic waste (closed-circuit anesthesia), thus testing it at its performance limits.
Many aspects remain to be studied. The effect of VCO2 above 280 mL/min remains unknown. The use of N2O may be difficult because it is likely to be washed out unless high O2/N2O sweep gas flows are used. The experiment was not set up to examine humidity conditions. Even though a drainage channel for H2O has been provided, no H2O accumulation was noticed. Because sweep gas is dry, H2O will probably be removed from the circle breathing gas, but the use of HME filters will likely make this humidity issue moot for the patient. Bulk gas transfer between the Memsorb and Zeus and factors affecting it need to be further quantified. Memsorb’s interactions with other anesthesia machines may be different from those with the Zeus, which may affect anesthetic agent and carrier gas management. Even within the Zeus itself, different agent delivery modes may affect agent loss because the site of liquid injection relative to the absorber differs between the two modes. Different anesthesia machines may also require different sweep gas flows: the company’s “recommended" sweep gas flow is 15 L/min, which does not suffice to control FICO2 for all combinations used in this study. It also remains unknown how the device would manage any viral and bacterial challenges, or how its ecologic footprint compares to conventional soda lime absorbers. A review of and comparison with other CO2 scavenging techniques is beyond the scope of this work.
Summarized, at a 5 L/min minute ventilation, the Memsorb could remove CO2 with CO2 production up to 280 mL/min when an inspired CO2 concentration of 0.5% is accepted, but at 10 L/min MV and low FGF (< 1.5 L/min), even a maximum sweep flow of 43 L/min was unable to maintain FICO2 ≤ 0.5%. The O2 concentration in the fresh gas and sweep gas should match, otherwise the inspired O2 concentration will deviate substantially from the delivered O2 concentration. Compared to the use of Ca(OH)2 based CO2 absorbent, inhaled agent usage is increased. The environmental cost of the use of inhaled anesthetics at reduced FGF with the Memsorb could be reduced by capturing and recycling anesthetic gases that still escape from both the anesthesia workstation and the Memsorb canister. The device is most likely to find its use integrated in a closed loop system. Increasing the selectivity of the membrane permeability for different gases in the anesthetic mixture could improve performance and is an area for future research and development.
References
Hendrickx JFA, De Wolf AM. The anesthesia workstation: Quo Vadis? Anesth Analg. 2018;127:671–5. https://doi.org/10.1213/ANE.0000000000002688.
De Cooman S, Lecain A, Sosnowski M, De Wolf AM, Hendrickx JFA. Desflurane consumption with the Zeus® during automated closed circuit versus low flow anesthesia. Acta Anaesthesiol Belg. 2009;60:35–7.
Hendrickx JFA, De Ridder SP, Dehouwer A, Carette R, De Cooman S, De Wolf AM. In vitro performance of prefilled CO2 absorbers with the Aisys®. J Clin Monit Comput. 2016;30:193–202. https://doi.org/10.1007/s10877-015-9699-2.
Bashraheel MK, Eerlings SA, De Wolf AM, Neyrinck A, Van de Velde M, Vandenbroucke G, Carette R, Feldman J, Hendrickx JFA. Memsorb™, a novel CO2 removal device. Part II: in vivo performance with the Zeus IE®.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jan Hendrickx has received lecture support, travel reimbursements, equipment loans, consulting fees and/or meeting organizational support from a number of companies involved with inhaled agent delivery (alphabetically): AbbVie, Acertys, Air Liquide, Allied Healthcare, Armstrong Medical, Baxter, Dräger, GE, Hospithera, Heinen und Löwenstein, Intersurgical, Maquet, MDMS, MEDEC, Micropore, Molecular, NWS, Philips, Quantum Medical. The other authors declare that they have no conflict of interest. None of the authors has been involved with the development of the Memsorb or financially rewarded in any way by DMF Medical, Halifax, Canada.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bashraheel, M.K., Eerlings, S.A., De Wolf, A.M. et al. Memsorb™, a novel CO2 removal device part I: in vitro performance with the Zeus IE®. J Clin Monit Comput 36, 1591–1600 (2022). https://doi.org/10.1007/s10877-021-00802-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00802-0