Abstract
Cancer-derived exosomes, a subset of extracellular vesicles, carry vital information about tumor progression, metastasis, and drug resistance, making them attractive targets for cancer diagnostics and therapeutics. The identification of these cancer exosomes with high sensitivity and specificity has enormous promise for early diagnosis and prognosis. Nano-mediated biological sensors are establishing themselves as innovative techniques for detecting cancer exosomes based on the distinctive physicochemical attributes of nanomaterials to improve detection sensitivity and specificity. This article presents an overview of the recent developments in nano-mediated biosensors directed particularly toward the detection of cancer exosomes. The development of ultrasensitive sensors has been enhanced by using nanomaterials such as magnetic nanoparticles, quantum dots, and gold nanoparticles. Surface modifications of these nanomaterials by conjugating the cancer-specific antibodies or aptamers facilitate target recognition and binding of cancer exosomes, thus increasing the sensitivity of detection. This review compiles different detection techniques, including SERS, Electrochemical, SPR, Chemiluminescence, and Fluorescence-based biosensor detection, in combination with different nanomaterials that are currently being researched or utilized as biosensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer, also referred to as a malignant tumor or neoplasm, is a complex genetic disorder having genetic or epigenetic roots that impact various different regions of the body. These genetic or epigenetic variations that drive the transformation of normal cells to malignant ones can either be acquired or hereditary. However, the consequences are always severe, often resulting in substantial mortality rates. The World Health Organization (WHO) report of 2022 shed light on the gravity of this issue and claimed Cancer to be one of the leading causes of death in the world, accounting for nearly one in every six deaths worldwide. This claim is also supported by the WHO’s GLOBOCAN 2020 report, which records a staggering 19,292,789 (approximately 19.3 million) incident cases of cancer across the globe, resulting in a mortality rate of 51.61%, equating to 9,958,133 (approximately 10 million) fatalities, including the nonmelanoma skin cancers [1]. This troubling and tragic data emphasizes the need for more advancing research, effective therapeutic options, and early detection of cancer. Early detection is critical in this attempt because it increases survival chances by allowing immediate therapies aimed at slowing or stopping the progression of malignancies. Unfortunately, most cancers at present usually get detected at their advanced or last stage when the prognosis is rather severe, and the treatment options are very limited [2] compared to instances identified in their early stages. Therefore, it’s essential to improve early cancer detection methods to reduce the significant impact of this global problem. Our understanding and knowledge of tumor biology, which can be used to develop new and better ways to diagnose cancer, is a key step in creating an effective early-detection technique. Liquid biopsy is a significant noninvasive sampling strategy that could potentially be used to diagnose cancer early on as it provides useful insight regarding tumor cells. This technique takes advantage of novel biomarkers detected in circulation, among which are circulating tumor cells, DNA, RNA, and exosomes. For example, Yang et al. (2022) reported considerable progress in the early detection of ovarian cancer. They utilized nano-pattern embedded microfluidic chips, versatile electrochemical analysis platforms, and separation by magnetic platforms. These technologies allow for the analysis of extremely-low levels of circulating tumor cell (CTC) biomarkers and exosomes in blood samples from ovarian cancer patients [3].
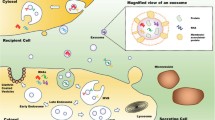
Exosomes are the extracellularly secreted structures, typically called extracellular vesicles (EVs), that function as important biological mediators in intercellular communication and signaling pathways Fig. 1. EVs were first discovered in sheep’s RBC-erythrocyte cancer; they usually get detected by all types of cells and exist in bodily fluids such as blood, saliva, and urine. EVs are nanosized particles, which typically range in size from 30 to 150 nm and encapsulate a variety of proteins, lipids, and nucleic acids [4, 5]. They can potentially be useful in regenerative medicine by directing stem cell development and reprogramming developed cells into stem cell-like states. Furthermore, exosomes are good carriers for medication and gene therapy [6], with benefits such as reduced immunogenicity and increased stability. They are commonly used in diagnostics as biomarkers for illness detection [7, 8], for instance, cancer, which is the focus of this paper. Stromal cells in the complex tumor microenvironment (TME) release exosomes, which have a substantial impact on tumor cell malignancy and stress responses [9]. Research indicates that tumor cells utilize exosome release to create an immunosuppressive environment within the tumor or to prepare a pre-metastatic habitat in distant organs [10]. On the other hand, immune cell-derived exosomes, such as those from dendritic or B cells, are distinguished by unique polarisation characteristics and act in intricate ways in the control of adaptive immunity against viruses or pathogens [11]. Multiple studies have proven that exosomes emanating from cytotoxic lymphocytes and antigen-presenting cell populations (APCs) can boost immune reactions. [12]. Several exosomal constituents serve a purpose for disease evaluation, diagnosis, and prognosis since exosomes are released into the circulation. Cancer diagnosis relies significantly on the quantity and composition of exosomes, which include proteins, mRNA, miRNA, and lncRNA. [13, 14]. Circulating exosomes exhibit elevated levels of exosomal markers (CD63, CD81, CD9) [15, 16], and certain tumor antigens (CEA, CA125) [17, 18], which may hold diagnostic value.
Exosomes have emerged as interesting possibilities in the search for non-invasive early cancer diagnosis. In the past decade, several methods for detecting exosomes have been investigated, including Western blotting [19], mass spectrometry [20], enzyme-linked immunosorbent assay (ELISA) [21], and nanoparticle tracking analysis (NTA). NTA in particular has gained popularity due to its ability to determine both the size distribution and relative content of exosomes in cell culture supernatants and biological fluids [22]. However, it suffers standardization issues, particularly in differentiating phenotypes among polydisperse populations [23]. To overcome this limitation, NTA may be used with other methods like, atomic force microscopy (AFM) and, transmission electron microscopy (TEM) to analyze exosome sizes and shape dispersion [24]. Nevertheless, these microscopic methods lack high-throughput and rapid detection capabilities. As a response to the need for simpler, more sensitive, fast, and high-throughput exosome detection methods, biosensors have gained prominence. In particular, due to their efficiency, high selectivity, ease of operation, and cost-effectiveness, nanomaterial-based biosensors, with their sizes ranging from 1 to 100 nm, are an ideal alternative for exosome detection. Nanomaterials have gotten a lot of interest because of their remarkable optical, electrical, magnetic, and catalytic properties, making them versatile tools in diverse fields, including environmental research, food technology, and medicine [25, 26]. Over the last decade, researchers have employed nanomaterials to enhance the accuracy, sensitivity, and rapidity of exosome screening and detection. Thus, providing important technological assistance for early cancer detection. The amalgamation of nanotechnology with biological detection has enormous promise, aiming to transform the area of exosome analysis and enhance the quest for early cancer detection.
The central objective of this article is to present a comprehensive overview of the most noteworthy recent advances in nanomaterial-based biological sensors for diagnosing cancer. This thorough study will address a wide array of topics, including exosome biology and separation techniques, as well as several biosensor techniques for detection such as optical, chemical, electrical, and others. Additionally, we examine the diverse categories of nanomaterials employed in nanomaterial-based biosensor techniques. This work aims to contribute to our collective understanding of cutting-edge cancer detection research by offering an integrated view of these important components, supporting developments at the convergence of biosensors and nanomaterials.
Exosome Role and Signaling in Cancer Progression
Exosomes are tiny extracellular vesicles resembling disc-like spheres, with size ranging from 30 to 150 nm. They have been established as significant facilitators in the propagation of cancer and communications between cells. It has been discovered that many different types of cells, including cancer cells as well as non-cancerous cells, produce exosomes during diseased or normal pathological circumstances. These vesicles have the ability to modify cellular behavior in both autocrine and paracrine ways because they contain an extensive range of bioactive substances, including proteins, lipids, RNAs, and DNAs [25]. Exosome synthesis and biogenesis includes various key pathways and factors, the most famous of which being the ESCRT (Endosomal Sorting Complex Required for Transport) pathway. This pathway comprises a series of complicated and sequential machinery complexes that facilitate the sorting of various substances into exosomes and contribute to their biogenesis [27]. Exosomes are exceptionally resilient and can be detected in body fluids including ascites fluids, plasma, tears, blood, urine, saliva, and CSF (cerebro-spinal fluid) [26]. According to Lowry et al., these tiny vesicles play a major role in cancer cell invasion, metastases, and apoptosis. They may even have an effect on the immune system and drug resistance [27]. Exosomes perform an integral part in the remodeling of the tumor microenvironment in cancer by stimulating angiogenesis, inhibiting immune monitoring, and aiding in the establishment of pre-metastatic conditions. Furthermore, they contribute significantly to drug resistance induction by operating drug efflux pumps and other resistance-associated molecules to recipient cells [28]. Exosomes typically have a lipid bilayer as their envelope membrane. Ceramide, phosphatidylserine (PS), and cholesterol is abundant in this lipid bilayer. These imparts exosomes with unique characteristics to induce different molecular pathways and function as potential biomarkers for early diagnosis. The exosomal biomarkers is usually classified into three categories, namely, Tumor derived exosomes (TEXs), Exosomal nucleic acids and exosomal surface proteins. TEXs, released by cancerous cells, play a crucial role in altering the physiology of both nearby and distant normal cells, thereby facilitating tumor growth, metastasis, and invasiveness [28]. Studies have demonstrated elevated levels of exosomes in the bodily fluid of cancer patients as cancer cells secrete more exosomes compared to normal cells [29]. As a result, TEXs hold promise as potential biomarkers. The exosomal nucleic acids such as mRNA, tRNA, lncRNA, DNA, and miRNA are also detected in the TEX [30]. The detection of the exosomal markers present inside the lipid bilayer of the exosomes is usually performed after the detergent-mediated lysis of the exosomes [29]. An example of such a technique is mentioned in Sect. Electrical Nanobiosensors discussing studies by Taller et al. developing a microfluid chip for RNA detection. Among these, exosomal miRNAs are particularly noteworthy as they are considered the most widespread and readily apparent cancer biomarkers due to their resistance against RNase-dependent degradation [31]. In addition, the membrane consists of membrane-spanning receptors, adhesion proteins, proteases, and tetraspanins, which are in accordance with the exosome’s parent or donor cell. Surface exosomal markers (for diagnostic and therapeutic targets) such as Tetraspanins (such as CD82, GPC1, CD81, GPC3, CD63, and CD9) or lipid rafts (like ceramide, PS, and cholesterol) are often used as target molecules for exosome detection specifying the underlying disease in the design of biosensors [29, 30, 32]. The major benefits of biosensors over instrument-dependent techniques are their exceptional specificity, high degree of sensitivity, affordability, quick reaction time, and combinatorial abilities. The development of biological sensors enabling exosome detection may enhance cancer treatment and help people in resource-constrained areas who don’t have access to sophisticated diagnostic equipment. As a result, it’s possible that exosome biosensors will be used much more often in different point-of-care (POC) healthcare facilities [29].
Biogenesis of exosomes (extracellular vesicle): Exosomes are synthesized in the endosomal system and secreted in the ECM, highlighting its amalgamation with the multi-vesicular bodies and later fuse with the cell membrane. Abbreviations: lncRNA (long non-coding RNA), miRNA (microRNA), mRNA (messenger RNA), MVB (Multi-vesicular bodies)
Various Methods of Exosomal Isolation
Exosomes may be isolated from a variety of sources including tumor tissues, cell culture medium, and body fluids sample such as saliva, urine, serum, CSF and blood. The cell culture medium being one of the most common sources of exosomes. In disease diagnosis, bodily fluids are often preferred for exosome samples via liquid biopsies. Once the samples are extracted from the desired source, they are subjected to various isolation method in order to collect exosomes. The isolation methods employed depends on the distinct biochemical and physical properties of the desired exosomes and includes techniques such as differential ultracentrifugation (DUC), size exclusion chromatography (SEC), density gradient fractionation, polymeric precipitation, ultrafiltration, and immunoaffinity isolation [33]. The DUC, that is based on vesicle density differences, is widely used method and yields highly purified exosomes. Another extensively used technique is the chromatography, that relies on particle size differences, and separates exosomes from hybrid proteins. During chromatography elution, the sample tests indicate the exosome sequence first, followed by the hybrid protein particles [34]. In the polymeric precipitation are simple isolation methods with typically high yields but so is the rate of contamination due to the need of overnight optimized incubation [35]. Furthermore, immunoaffinity isolation are used to study specific subgroup since it is based upon the distinct characteristics of surface proteins. The approach is roughly split into two categories: the immuno- enrichment and the immuno- depletion. When antibodies are conjugated with beads to select the desired, the approach is known as immuno-enrichment, whereas when the antibodies are conjugated with beads to capture the unwanted exosomes it is termed as immuno- depletion method [36].
In addition to the traditional methods outlined above, there have been reports on a few new isolation methods. The report of a new technique called ExoCounter, reported by Kabe et al., is one such example. This technique captures the exosomes using a specific antibody-functionalized optic disc that is then further tagged with nano-sized magnetic beads [37]. Another innovative approach involves antibody-conjugated magnetic nanowires, as demonstrated by Lim et al., which improves the capture efficacy when compared with the typical magnetic beads [38]. These advancements signal promising developments in exosomal isolation techniques.
Types of Biosensor Detection Methods for Exosomes
Biosensors stand at the junction of Biology and technology and have come a long way from their early conceptualization to becoming a vital instrument in various scientific fields such as clinical detection [39, 40], cellular physiology [41], environmental studies [42], and many more. The widespread implementation of biosensors in the past few decades has completely changed the field of biological and medical analysis by providing a new standard of quick, precise, and stable analytical techniques [43, 44]. The foundation of biosensors as an advanced analytical tool was laid by Hermann Staudinger’s pioneering research on macromolecules in the 1910s. The term “Biosensor,” however, was later officially coined in the 1960s by Clark and Lyons, who developed the first enzyme-based electrode for glucose detection [45]. Current-day biosensors work on a basic foundation centered on the translation of biological reactions into quantitative data. The biorecognition component, transducer, and detector are the three main components of this process. The biorecognition component, which may consist of enzymes, antibodies, DNA, or cells, gives the biosensor specificity [46]. The transducer translates biological responses into measurable signals by using electrochemical, optical, or piezoelectric methods [47]. Finally, detectors assess the transduced impulses and provide quantitative or qualitative data Fig. 2, concluding the biosensing process. The aforementioned group of elements serves as the foundation for biosensor abilities, allowing for a wide range of applications in measuring and identifying various compounds.
Biosensors are classified into multiple categories, each of which has been developed for specific use. Enzyme-based biosensors, which were among the first, use enzymes as biorecognition elements. The pioneering work of Clark and Lyons laid the groundwork for this category. Immunoassay biosensors are critical in medical diagnostics because they rely on the precise binding of antibodies and antigens. Köhler and Milstein’s 1975 discovery of monoclonal antibodies made an important contribution to the development of immunoassay biosensors [48]. DNA-based biosensors, which emerged from Watson and Crick’s 1953 discovery of the DNA double helix structure [49], transformed genetic diagnostics. Optical biosensors employ light-based sensing technologies and have gained importance thanks to pioneers such as Roger Y. Tsien, who was awarded the Nobel Prize in Chemistry in 2008 for his study on the green fluorescent protein [50].
At present, with the rapid growth of nanotechnology, the domain of biosensors has been influenced, and as a result, taking advantage of various types of nanomaterial to build a new generation of biosensors known as nano biosensors with higher detecting sensitivity and accuracy has been developed [51, 52]. Nano biosensors include a wide range of nanomaterials that come from various sources with variable shapes, sizes, and compositions. The diversity of nano biosensors is a huge step forward, opening up new avenues for biosensor development [51, 53]. In recent years, research into biosensors, notably nano biosensors, has expanded to the identification and quantification of exosomes. Multiple studies have shown that optical, electrochemical, and electrical mechanisms are the primary sensing methods used in the development of exosome biosensors [54, 55]. Particularly, nanomaterials have played an important role in these studies, allowing for higher accuracy and sensitivity for recognizing low concentrations of exosomes [56]. In the following section ahead, we are going into each method of biosensor detection and will address their different systems, functioning mechanisms, and material compositions, as well as specific advantages and disadvantages.
Optical Nano Biosensors
Liquid biopsies, as a non-invasive sample technique, have been the subject of significant interest in clinical medicine in recent years due to their potential for detailed tumor analysis, disease assessment, and early diagnosis. Given the great potential of exosomes detection in biological samples due to their ability to exhibit significant cancer biomarkers, it has become crucial to thoroughly investigate proper detection methodologies that have a low limit of detection (LOD), require minimal sample volume, support real-time analysis and are appropriate for high throughput screening [57]. Optical techniques, at present, have demonstrated excellent stability and accuracy when assessing biological targets. Moreover, recently, with the development of optical biosensors that utilize nanomaterials for detecting targets is a new and ongoing trend in analytical diagnostics [58]. Various nanomaterials with peculiar optical properties, such as graphene [59], carbon nanotubes (CNT) [60], gold (Au) and silver (Ag) nanoparticles [61], and quantum dots [62], have been widely used in the production of optical substrates. The purpose of these substrates is to improve target detection sensitivity in a range of applications. The optical detection of exosomes involves various techniques such as Fluorescence, Colorimetry, Surface plasmon resonance (SPR), Raman scattering, Chemiluminescence, and Electrochemiluminescence. In the following section, we are going to discuss these various techniques briefly.
Fluorescence-Based Biosensor Detection
A potent technique in exosome investigation for cancer detection is fluorescence biosensor detection, which depends on the unique fluorescence characteristics associated with particular molecules. Fluorescence biosensors offer remarkable sensitivity and specificity, enabling the detection of even low concentrations of exosomes, which is crucial for early cancer diagnosis. Fluorescence biosensors use the fluorescent phenomenon to monitor the concentration, position, and dynamics of biomolecules. This phenomenon occurs when electromagnetic radiation is absorbed by fluorophores or fluorescently labeled molecules and reemitted as fluorescence. Fluorescence biosensors are often composed of three components: a photodetector that gauges the fluorescence intensity and spectrum, an excitation light source (such as lasers or LEDs), and fluorophore molecules that label the target biomolecules. In which, Techniques such as (1) FRET (Fluorescence resonance energy transfer), (2) FLIM (fluorescence lifetime imaging), (3) FCS (fluorescence correlation spectroscopy), and (4) FI (changes in fluorescence intensity) are often used to bio-recognize the excitation signal [63]. The efficiency of the approach is further enhanced by its capacity to offer real-time results, which enables dynamic monitoring of changes connected to exosomes. A number of advanced laboratory-based methods for the identification of exosomes have made significant advances by utilizing the principle of fluorescence. Among these methods, there has been major progress in techniques such as fluorescence spectroscopy, real-time polymerase chain reaction (rt-PCR), as well as flow cytometry [64]. Over the past few years, there has been a growing utilization of nanomaterials, encompassing 2D nanomaterials, QDs, CNTs, metallic nanoparticles (MNPs), and various others, in the development of biosensors employing fluorescence resonance energy transfer (FRET), which is mainly due to the fact that all these materials possess distinctive optical properties [65, 66]. Numerous attempts are underway to develop more sophisticated and compact fluorescence-based biological sensors for identifying exosomes. Table 1 provides an overview and analysis of the development of these investigations.
Colorimetry-Based Biosensor Detection
Colorimetric biosensors operate on the basis of absorption and color alterations within a reaction media. As the outcomes of this analytical approach can be effortlessly noticed with the naked eye and no additional sophisticated equipment is needed, it turns out to be an extremely simple procedure. [90, 91]. The colorimetric biosensor has shown potential as a tool for detecting exosomal biomarkers owing to its high extinction coefficient, which allows for quick analysis. The basis for colorimetry-based biosensor detection is the measurement of the wavelength or intensity of color changes brought on by biological activity. Colorimetric biosensors often employ carbon nanomaterials, gold nanoparticles, and metal oxide nanomaterials. Owing to remarkable characteristics such as high stability, low cost, and large-scale production capabilities, carbon nanomaterials have garnered significant attention for the development of colorimetric biosensors. A notable example of such can be found in the studies conducted by Wang et al., where they investigated the increase of the inherent peroxidase-like activity on graphite-phase carbon nitride nanosheets (g-C3N4 NSs) utilizing the capabilities of single-stranded DNA (ssDNA). By integrating a specific aptamer ssDNA targeting the exosome transmembrane protein CD63 in the breast cancer cell line (MCF-7) with g-C3N4, they observed a substantial boost in its intrinsic peroxidase activity, leading to the generation of a dark blue product (Fig. 3) [92]. Moreover, colorimetric biosensors based on nanomaterials can be broadly classified into two groups depending on their distinct material properties: those with innate optical properties, such as colloidal gold nanoparticles (AuNPs), and those with catalytic properties, such as iron oxide nanoparticles (Fe3O4 NPs) [93].
AuNPs have an exceptionally high extinction coefficient, making them a good choice for solution colorimetric analyses. Moreover, changes in the distance between AuNPs cause the surface plasmon resonance effect, which causes fluctuations in the ultraviolet absorption intensity of AuNPs or a noticeable shift in the color of the solution [94]. Additionally, AuNPs act like tiny enzymes or nanozymes, at the nanoscale, imitating the functions of natural enzymes [95]. This characteristic makes them suitable for use in color-based biosensors for detecting exosomes. These biosensors can be applied to analyze the proteins in exosomes, assisting in the diagnosis of cancer. This property of AuNPs was explored in the studies of Jiang et al., where they formulated an AuNP-based colorimetric biosensor capable of assessing exosome surface proteins at an efficient rate [96]. AuNPs were conjugated using exosomal protein aptamers (CD63) in this assay. The linking of these aptamers kept the particles apart under a high concentration of salt (NaCl). When exosomes were present, the connection between the aptamers and AuNPs broke, causing the particles to collide and, as a result, change color. Within minutes, this approach could identify numerous surface proteins on exosomes such as CD63, PTK7, PDGF, PSMA, and EpCAM from distinct cell lines, for instance, PC-3, CEM, HeLa, and Ramos [93, 96]. The catalytic characteristics are used in another type of colorimetric biosensor. A notable example is the work of Chen and colleagues, who developed a visible, label-free, and simple approach for detecting PC3-derived exosomes in blood [97] using aptamer-functionalized magnetite nanoparticles (Fe3O4 NPs). This novel approach makes use of the catalytic capabilities of Fe3O4 NPs to provide an efficient and user-friendly detection process. Given below is the table (Table 2) that provides an overview of various applications of colorimetric sensors for the detection of exosomes.
A schematic representation of exosomal colorimetric biosensors illustrating the high inherent peroxidase-like activity of ssDNA and g-C3N4 NS, integrating CD63, a DNA aptamer in breast cancer cell line. Abbreviations: g-C3N4 NS (graphite-phase carbon nitride nanosheets); TMB (3,3′,5,5′- tetramethylbenzidine). [Adapted with permission Wang et al. 2017 Copyright 2017 American Chemical Society] [92]
Surface Plasmon Resonance (SPR) Based Biosensor Detection
Surface Plasmon Resonance (SPR) has emerged as a highly effective and reliable analytical technique in the clinical detection studies regarding the interaction among biological molecules [104, 105]. This optical phenomenon enables real-time monitoring of these interactions in their natural state. Because of its excellent optical confinement, surface plasmon resonance (SPR) is particularly beneficial in the visible and near-infrared wavelength range [106, 107]. The detection mechanism involves attaching a bioreceptor to the surface of a biosensor, which is then followed by introducing a solution containing another biomolecule that can interact with the bioreceptor [108]. In recent years, the SPR detection technique has seamlessly integrated into the ongoing nanotechnology trend, giving rise to SPR-based nanosensors or nanoplasmonic biosensors. This development has attracted significant attention because of their capability for real-time analysis without the need for sample labeling [109, 110]. The SPR-based nano biosensors technique has been employed in the detection of exosomes as well. Given that exosomes have an average size of around 100 nm and that SPR can typically detect signal changes within a range of 200 nm from the gold surface, using SPR for exosome quantification combines the strengths of both exosomes and SPR at the same time. This method’s exceptional sensitivity is also attributed to the significant molecular weight of exosomes [111]. We have provided a capsulation of the various exosome detection studies using SPR based biosensors in the Table 3, below.
Surface Enhanced Raman Scattering SERS-Based Biosensor Detection
In the field of biological studies and biomedical analysis, SERS (surface-enhanced Raman scattering) is becoming known as a potent plasmonic technology with a broad range of applications. This innovative technique has proven itself useful for monitoring environmental conditions, enabling cellular imaging, and detecting biomolecules [118, 119]. Currently the SERS has been smoothly integrated with the booming field of nanotechnology, particularly in conjunction with metallic nanoparticles, which has garnered a great deal of attention as SERS substrates because of their small dimensions, large specific-surface area, and distinctive electrical, optical, and physicochemical features [108]. Specifically, combination of AuNPs with SERS technology, because AuNPs are the most popular signal amplifying materials. The fundamental mechanism behind the enhancement of Raman intensity in SERS is the localized SPR, which generates an intense electromagnetic field in close proximity to metallic nanoparticles (for instance, AuNPs). This phenomenon enables the extraction of unique fingerprint data using Raman spectra, resulting in great sensitivity and stability in various analytical settings [120, 121]. Another combination offering a compelling solution is the Au@Ag core shell colloids, broadening the excitation spectrum and enabling adjustable surface plasmon resonance (SPR) characteristics. Combining the distinct properties of gold and silver metals results in enhanced stability and exceptional enhancement capabilities for SERS. While Au nanostructures exhibit superior SERS enhancement, shape and size control are difficult to achieve; in contrast, Au nanostructures are easier to create and maintain stability. The incorporation of benefits of both of their qualities into a core shell structure can aid in achieving highly enhanced SERS. Ma et al. demonstrated this concept by developing stable SERS probes, Au@R6G@AgAu nanoparticles (ARANPs), which showed significant sensitivity improvements [122]. Another research study conducted by Pang et al. utilized Au@Ag nanorods with DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) as the SERS signal probe along with Fe3O4@Ag nanomaterials and a signal amplification technique for quantitative detection of miRNA-10b in exosomes and residual plasma (Fig. 4) [123]. Integrated SERS improvements and recirculation signal amplification resulted in an impressive detection limit of 1 aM, allowing for direct and quantitative detection of exosomal miRNA in chronic pancreatitis, pancreatic ductal adenocarcinoma (PDAC), and healthy control plasma samples. At present, because of the high speed, multiplexing capabilities and rapid speed, the SERS nanomaterial-based biological sensors have been successfully applied for the detection of exosomal biosensors. Contrasted with SPR or fluorescence techniques, SERS biosensors can recognize distinct spectral signals in intricate and variable biological surroundings [124]. Table 4 summarizes the SERS biosensors application for the identification of exosome concentration has been provided.
SERS biosensor: Illustration of a SERS-based detection approach for tumor-derived exosomes employing SERS Au@Ag nanorod nanoprobes with exosome-specific antibodies and magnetic nanobeads. Abbreviations: DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)); Au@Ag NR (gold core – silver shell nanorods). [Adapted from Zong et al. 2016] [125]
Chemiluminescence-Based Biosensor Detection
Chemiluminescence (CL), an influential optical technique, relies on the electromagnetic radiation emitted during an exothermic chemical reaction, eliminating the need for external light sources or optics [132, 133]. This distinguishing feature, combined with high sensitivity, broad linear dynamic range, simple apparatus, rapid analysis, and minimal background interference, has led to the widespread use of chemiluminescence in optical biosensors [134]. Despite its efficacy, only a few chemiluminescence-based techniques coupled with nanomaterials have been reported to detect exosomal biomarkers. This suggests a potential area for investigation and innovation in improving the capabilities of CL-based approaches for exosome identification. To address the above-mentioned constraints, scientists have turned to metal oxide nanomaterials, including iron oxide and CuS nanoparticles [108]. Notably, Wang et al. presented a novel (CL) chemiluminescence-based biosensor that uses superparamagnetic iron oxide particles (SIOPs) to quickly and quantitatively identify tumor-derived exosomes (TEXs) from biofluids [123, 135]. The technique used a double-antibody sandwich design, with anti-CD63 antibody-conjugated SIOPs and anti-CD9 antibodies tagged with acridinium ester. Exosomes isolated from a pancreatic tumor cell line culture medium through ultracentrifugation were subjected to analysis. The antibody-modified SIOPs revealed the capacity to precisely detect TEXs while acting as a capture probe. Resulting in the establishment of a biosensing device for a simplified one-step test. This CL-based biosensor’s limit of detection (LOD) was calculated to be 2.63 × 105 particles/mL. This development represents a noteworthy advancement in the field of exosome detection, offering a swift and quantitative approach for TEX analysis from complex biofluids. Another study, done by Jiang et al., revealed a novel approach for fast detection of extracellular vesicles based on CuS-encased microgels [136].
Electrochemiluminescence-Based Biosensor Detection
Electrochemiluminescence (ECL) is a very sensitive and adaptable technology that has found widespread use in biosensor detection. This approach generates light by an electrochemical reaction, combining elements of electrochemistry and chemiluminescence [108]. The key components of ECL-based biosensors are a working electrode, a counter electrode, and a reference electrode. Immobilized recognition components, such as antibodies, enzymes, or aptamers, that selectively interact with the target analyte are often carried by the working electrode. When a voltage is applied, the electrochemical reaction at the electrode surface produces reactive species, which causes light to be emitted. Low background noise, great sensitivity, and a wide dynamic range are all advantages of ECL-based biosensors. This approach has been used successfully to identify diverse biomolecules such as proteins, nucleic acids, and particular cells, making it a vital tool in biomedical research and diagnostics. For their analytical applications, various nanomaterials with outstanding ECL capabilities, such as CNTs, QDs, metallic nanoparticles, silica nanoparticles (SiNPs), and graphene-based C3N4 nanosheets [137], have been explored. However, research on the identification of exosome’s biomarkers utilizing these nanomaterials in the context of ECL as of yet remains restricted.
Electrochemical Nanobiosensors
Electrochemical biosensors are one of the most sought-after biosensor techniques because of the advantages such as highly sensitive, cost effective and time efficient procedures as well as requirement of small sample quantity [138]. With the flexibility of integrating electrochemical methods with various types of platforms for developing sample manipulation chips [139] on top the above-mentioned advantages of the methods makes electrochemical techniques one of the best candidates for detection and quantification of exosomes in medical samples [140]. The electrochemical method is an umbrella term that includes various techniques such as amperometric and voltametric methods among others. In the section ahead we are briefly going to talk about these various methods for the detection of exosomes.
Amperometric Methods
Amperometric methods are important in the field of electrochemical biosensors because they provide a sensitive and quantitative method of detecting analytes. In amperometric measurements, the sensor maintains a constant potential at the working electrode while monitoring the current, which is associated with analyte concentration [141]. Because of its excellent sensitivity and selectivity, this method is especially beneficial for biosensors. Amperometric methods integrated into electrochemical biosensors have found uses in a variety of disciplines, including medical diagnostics, environmental monitoring, and food safety. Nanotechnology and materials science advances continue to improve the efficiency of amperometric biosensors, resulting in useful tools for accurate and consistent detection in a variety of environments. In the research study conducted by Doldan et al., in 2016, an amperometric electrochemical biosensor-based-sandwich assay was constructed for the detection of exosomes present in the gold electrode. In this study, the rabbit antihuman CD9 antibody (a transmembrane protein that functions as a suitable biomarker) was immobilized onto a gold electrode, and in order to capture exosomes, monoclonal antibodies were utilized against the CD9 antibodies. Once the samples were successfully sandwiched, it was followed by signal amplification, and an HRP-conjugated (horseradish peroxidase) α-mouse IgG antibody was applied at last. Eventually, the amperometric diagnostic approach was used, which relied on the efficacy of the enzymatic process and the electrochemical degradation of tetramethylbenzidine (TMB) on the outer coating of the gold electrode (Fig. 5). The developed sensor operates with a 1.5 L analyte volume and can detect as few as 200 exosomes/µL, with a LOD of 2 × 102 particles/µL [142].
Biosensors based on electrochemical (Amperometric) detection of exosomes. The diagrammatic representation is of the sandwich assay with an HRP-enzymatic readout of the CD9 exosomes by the gold electrodes surface conjugated with α-CD9 antibodies. The Biosensor chip will be used for the detection of the signals. Abbreviations: BSA (Bovine Serum Albumin), MUA (11-Mercaptoundecanoic acid), HRP (Horseradish peroxidase). [Adapted with permission Doldan et al. 2016 Copyright 2016 American Chemical Society] [142]
Voltammetric Method
Voltammetric techniques, in which current is measured in relation to electrode potential, are the most commonly used approach in electrochemical biosensors. This preference comes from their technological advantage over other electrochemical processes. These methods excel in quantifying ions and molecules and have fewer restrictions than alternative techniques [143, 144]. The method has also been applied for the exosome detection. An example of exosome detection using the voltammetric method can be seen in the research studies conducted in the year 2017 by Boriachek et al., wherein they demonstrated a square-wave anodic stripping voltammetry (SWASV)-based electrochemical nano-immunosensor. This utilized QDs as the signal amplifier and was used for the recognition of colon and breast tumor-specific exosomes. In this study the LOD was 100 exosomes/µL, with the relative standard-deviation of < 5.5% in the cancer cell lines [145]. In some of the assay studies of voltammetric detection of exosomes, aptamers were used instead of antibodies. Aptamers are small, single-stranded DNA or RNA molecules chosen for their high affinity binding to certain targets. Their adaptability makes them useful in a variety of applications, such as biosensors and targeted drug administration. Aptamers are less expensive and less complicated when compares to antibodies. Aptasensors are biosensors that use an aptamer as a biorecognition, and they have been used to detect exosomes even more than antibody-based biosensors [146, 147].
Electrical Nanobiosensors
Exosomes’ ability to hold electrical energy when they are electrically polarised has led to the conclusion that they can be electrically detected based on earlier research. When voltage is given to exosomes, the interaction between the exosome surface and its surrounding environment creates an interface-effective layer [148]. The favorable electrical characteristics of exosomes, coupled with the advantages of electrical biosensors, such as low cost, low power, real-time analysis, and on-site application due to tiny size, collectively position electrical biosensors as viable diagnostic instruments for exosome detection [149, 150]. Electrical biosensors also have the advantage of being readily incorporated into chips, and since they are real-time assessment systems, they can complete tests immediately [54]. Ibsen et al. have described an Altering Current Electrokinetic (ACE) microarray chip for effectively separating and identifying exosomes using unadulterated human plasma in their investigations on electrical biosensors [151]. ACE microarrays apply alternating current to generate a dielectrophoretic separation force [152], attracting nanoparticles and nanoscale entities. This microchip successfully gathered plasma exosomes from glioblastoma cells containing surface markers and RNAs. The DEP high-field zones selectively captured bio-nanoparticles while subsequently enabling the removal of larger particles and biomolecules during the washing process. The whole procedure, including the exosome segregation and on-chip fluorescence measurement, was accomplished in three quick phases in less than 30 min, indicating great efficiency. Moreover, the microchip facilitated on-chip immunofluorescence identification of protein biomarkers as well as provided viable mRNA for RT-PCR [151]. The ability to combine segregation and identification approaches in a single tool is a significant benefit of electrical bio-based sensors for exosome detection.
Additionally, exosome-encapsulated miRNA detection is a promising technique for non-invasive cancer diagnosis [153]. Traditional approaches, such as RT-PCR, demand a high concentration of RNA isolated from exosomes, which requires significant sample volumes, time-consuming operations, multiple equipment, and chemical kits. To address these challenges, Taller et al. developed a microfluidic chip that combines a surface acoustic wave (SAW) exosome-lysis chip with a second chip for RNA detection. The SAW on the piezoelectric crystal’s surface induces effective turbulent mixing, lysing isolated exosomes without interfering with RNA detection. The RNA detection sensor employs an anion-exchange nanoporous membrane, demonstrating a lysis rate of 38%, a detection limit of 2 pM, and a reduced analysis time of ~ 1.5 h (~ 30 min for lysing and ~ 1 h for detection). his technique is appropriate for mouse model research as well as the analysis of fine needle aspiration (FNA) samples from clinical patients [153].
To conclude, a detailed outline for the exosome detection, isolation, and diagnosis is schematically represented in Fig. 6.
A Schematic representation of exosome as a potential target for detection of cancer biomarker. [Adapted from Singh et al. 2022] [154]
Conclusion
Cancer is one of the leading contributors to fatalities across the globe, as previously highlighted in the current article. Early detection of cancer is very crucial to commence immediate treatment and slow down the progression of the malignancies. In order to increase the survival chances of the cancer patient, impressive developments have been successfully attained in the discipline of preliminary diagnosis and therapy for cancer in the past few decades. In conjunction with this, the development of nanotechnology—specifically, the use of nano-mediated biosensors—has significantly benefited cancer diagnostics, notably in the domain of detection of cancer-derived exosomes. While these advancements are promising, substantial progress is needed to fully develop these technologies and improve their accuracy for early cancer diagnosis. The present study reviewed the most significant latest advancements in nano-mediated biosensors, including target selection, detection mode, and limits, to identify exosomes produced from cancer. Exosomes are overexpressed in a variety of cancer types; the specific targeting surely helps to identify cancer at early stages easily. Some exosomes, such as glypicans, are cleaved and are available in the extracellular fluids, thus enhancing the level of detection with the help of nano-based biosensors. These findings highlight that exosome detection nano-biosensors are beginning to emerge as a viable substitute for traditional methods (such as, ELISA, qPCR, etc.), delivering positive aspects in early cancer assessment and prognosis including shorter detection times, higher sensitivity, and lower expenditures. For instance, the application of gold nanoparticles, quantum dots, and magnetic nanoparticles has exhibited unparalleled sensitivity and specificity with the utilization of SERS, Electrochemical, SPR, Chemiluminescence, and Fluorescence-based biosensors for the detection.
Additionally, recent investigations employed novel nanomaterials and composites to develop reliable and potent biosensors for exosome-based illness diagnostics. Their implementation is projected to advance healthcare diagnosis for multiple diseases. Further exploration into biocompatible, non-invasive, and cost-effective nanomaterials will be important for widespread clinical adoption. Enhancing the specificity and sensitivity of biosensors is essential to ensure accurate detection. Therefore, by integrating artificial intelligence to analyze complex data patterns can further refine the diagnostic process, leading to more precise and earlier detection methods. In summary, nano-mediated biosensors symbolize the convergence of nanotechnology and biomedicine, providing significant breakthroughs in the biology of cancer and opening new techniques for diagnosis and treatment advancements. Future studies on nano-based biosensors have to concentrate on several substantial variables, including cost, reproducibility, simplicity of application, point-of-care applications, stability, and specificity. By addressing these areas, future advancements might enhance earlier diagnosis, better patient care, and higher survival rates.
Data Availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/CAAC.21660
Crosby D, Bhatia S, Brindle KM, et al (2022) Early detection of cancer. Science (1979) 375:. https://doi.org/10.1126/SCIENCE.AAY9040/ASSET/0A3087E0-148F-45C4-B9D9-5558E774A080/ASSETS/IMAGES/LARGE/SCIENCE.AAY9040-F6.JPG
Yang Y, Huang Q, Xiao Z, et al (2022) Nanomaterial-based biosensor developing as a route toward in vitro diagnosis of early ovarian cancer. Mater Today Bio 13:100218. https://doi.org/10.1016/j.mtbio.2022.100218
Wang X, Tian L, Lu J, Ng IOL (2022) Exosomes and cancer - Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022 11:1 11:1–12. https://doi.org/10.1038/s41389-022-00431-5
Xu L, Shoaie N, Jahanpeyma F, et al (2020) Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview. Biosens Bioelectron 161
Sharma A (2018) Role of stem cell derived exosomes in tumor biology. Int J Cancer 142:1086–1092. https://doi.org/10.1002/IJC.31089
Hadavand M, Hasni S (2019) Exosomal biomarkers in oral diseases. Oral Dis 25:10–15. https://doi.org/10.1111/ODI.12878
Console L, Scalise M, Indiveri C (2019) Exosomes in inflammation and role as biomarkers. Clinica Chimica Acta 488:165–171. https://doi.org/10.1016/J.CCA.2018.11.009
Vallabhaneni KC, Hassler MY, Abraham A, et al (2016) Mesenchymal Stem/Stromal Cells under Stress Increase Osteosarcoma Migration and Apoptosis Resistance via Extracellular Vesicle Mediated Communication. PLoS One 11:e0166027. https://doi.org/10.1371/JOURNAL.PONE.0166027
Zuo B, Qi H, Lu Z, et al (2020) Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nature Communications 2020 11:1 11:1–16. https://doi.org/10.1038/s41467-020-15569-2
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40:D1241–D1244. https://doi.org/10.1093/NAR/GKR828
Munich S, Sobo-Vujanovic A, Buchser WJ, et al (2012) Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 1:1074–1083. https://doi.org/10.4161/ONCI.20897
Melo SA, Luecke LB, Kahlert C, et al (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015 523:7559 523:177–182. https://doi.org/10.1038/nature14581
Sun Z, Shi K, Yang S, et al (2018) Effect of exosomal miRNA on cancer biology and clinical applications. Molecular Cancer 2018 17:1 17:1–19. https://doi.org/10.1186/S12943-018-0897-7
Jakobsen KR, Paulsen BS, Bæk R, et al (2015) Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 4:1–10. https://doi.org/10.3402/JEV.V4.26659
Zorrilla SR, Pérez-Sayans M, Fais S, et al (2019) A Pilot Clinical Study on the Prognostic Relevance of Plasmatic Exosomes Levels in Oral Squamous Cell Carcinoma Patients. Cancers 2019, Vol 11, Page 429 11:429. https://doi.org/10.3390/CANCERS11030429
Dai S, Wan T, Wang B, et al (2005) More Efficient Induction of HLA-A*0201-Restricted and Carcinoembryonic Antigen (CEA)–Specific CTL Response by Immunization with Exosomes Prepared from Heat-Stressed CEA-Positive Tumor Cells. Clinical Cancer Research 11:7554–7563. https://doi.org/10.1158/1078-0432.CCR-05-0810
Chen Z, Liang Q, Zeng H, et al (2020) Exosomal CA125 as A Promising Biomarker for Ovarian Cancer Diagnosis. J Cancer 11:6445. https://doi.org/10.7150/JCA.48531
Kowal EJK, Ter-Ovanesyan D, Regev A, Church GM (2017) Extracellular Vesicle Isolation and Analysis by Western Blotting. Methods Mol Biol 1660:143–152. https://doi.org/10.1007/978-1-4939-7253-1_12/COVER
Brown BA, Zeng X, Todd AR, et al (2020) Charge Detection Mass Spectrometry Measurements of Exosomes and other Extracellular Particles Enriched from Bovine Milk. Anal Chem 92:3285–3292. https://doi.org/10.1021/ACS.ANALCHEM.9B05173/ASSET/IMAGES/LARGE/AC9B05173_0004.JPEG
Lee J, Kim H, Heo Y, et al (2019) Enhanced paper-based ELISA for simultaneous EVs/exosome isolation and detection using streptavidin agarose-based immobilization. Analyst 145:157–164. https://doi.org/10.1039/C9AN01140D
Soo CY, Song Y, Zheng Y, et al (2012) Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 136:192–197. https://doi.org/10.1111/J.1365-2567.2012.03569.X
Jing S, Shiping S (2017) A review of the quantitative detection and diagnostic and therapeutic applications of exosomes. Journal of Radiation Research and Radiation Processing 35:. https://doi.org/10.11889/J.1000-3436.2017.RRJ.35.030101
van der Pol E, Coumans FAW, Grootemaat AE, et al (2014) Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. Journal of Thrombosis and Haemostasis 12:1182–1192. https://doi.org/10.1111/JTH.12602
Baptista FR, Belhout SA, Giordani S, Quinn SJ (2015) Recent developments in carbon nanomaterial sensors. Chem Soc Rev 44:4433–4453. https://doi.org/10.1039/C4CS00379A
Tripathi AD, Katiyar S, Chaurasia AK (2023) Nanomaterials for Biosensing Applications. In: Mishra A (ed) Recent Advances in Biosensor Technology. BENTHAM SCIENCE PUBLISHERS, pp 1–29
Sharma S, Masud MK, Kaneti YV, et al (2021) Extracellular Vesicle Nanoarchitectonics for Novel Drug Delivery Applications. Small 17
Atay S, Godwin AK (2014) Tumor-derived exosomes. Commun Integr Biol 7:. https://doi.org/10.4161/CIB.28231
Erdbrügger U, Lannigan J (2016) Analytical challenges of extracellular vesicle detection: A comparison of different techniques. Cytometry Part A 89:123–134. https://doi.org/10.1002/CYTO.A.22795
Gusachenko ON, Zenkova MA, Vlassov V V. (2013) Nucleic acids in exosomes: Disease markers and intercellular communication molecules. Biochemistry (Moscow) 78:1–7. https://doi.org/10.1134/S000629791301001X/METRICS
Taylor DD, Gercel-Taylor C (2008) RETRACTED: MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110:13–21. https://doi.org/10.1016/J.YGYNO.2008.04.033
Tripathi AD, Katiyar S, Mishra A (2023) Glypican1: a potential cancer biomarker for nanotargeted therapy. Drug Discov Today 103660
Witwer KW, Buzás EI, Bemis LT, et al (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2:. https://doi.org/10.3402/JEV.V2I0.20360
Lobb R, Möller A (2017) Size Exclusion Chromatography: A Simple and Reliable Method for Exosome Purification. Methods Mol Biol 1660:105–110. https://doi.org/10.1007/978-1-4939-7253-1_9/COVER
Boriachek K, Masud MK, Palma C, et al (2019) Avoiding pre-isolation step in exosome analysis: Direct isolation and sensitive detection of exosomes using gold-loaded nanoporous ferric oxide nanozymes. Anal Chem 91:3827–3834. https://doi.org/10.1021/acs.analchem.8b03619
Sharma P, Ludwig S, Muller L, et al (2018) Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles 7:. https://doi.org/10.1080/20013078.2018.1435138
Kabe Y, Suematsu M, Sakamoto S, et al (2018) Development of a Highly Sensitive Device for Counting the Number of Disease-Specific Exosomes in Human Sera. Clin Chem 64:1463–1473. https://doi.org/10.1373/CLINCHEM.2018.291963
Lim J, Choi M, Lee H, et al (2019) Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnology 17:. https://doi.org/10.1186/s12951-018-0433-3
Mahapatra S, Chandra P (2020) Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. Biosens Bioelectron 165:112361. https://doi.org/10.1016/J.BIOS.2020.112361
Shetti NP, Malode SJ, Roy S, et al (2020) Electroanalytical techniques for investigating biofilms: Applications in biosensing and biomolecular interfacing. Nanomaterials in Diagnostic Tools and Devices 293–329. https://doi.org/10.1016/B978-0-12-817923-9.00011-0
Shi J, McLamore ES, Marshall Porterfield D (2013) Nanomaterial based self-referencing microbiosensors for cell and tissue physiology research. Biosens Bioelectron 40:127–134. https://doi.org/10.1016/J.BIOS.2012.06.059
Mahmoudpour M, Ezzati Nazhad Dolatabadi J, Torbati M, Homayouni-Rad A (2019) Nanomaterials based surface plasmon resonance signal enhancement for detection of environmental pollutions. Biosens Bioelectron 127:72–84. https://doi.org/10.1016/J.BIOS.2018.12.023
Topkaya SN, Azimzadeh M, Ozsoz M (2016) Electrochemical Biosensors for Cancer Biomarkers Detection: Recent Advances and Challenges. Electroanalysis 28:1402–1419. https://doi.org/10.1002/ELAN.201501174
Ligler FS, Gooding JJ (2019) Lighting Up Biosensors: Now and the Decade to Come. Anal Chem 91:8732–8738. https://doi.org/10.1021/ACS.ANALCHEM.9B00793/ASSET/IMAGES/LARGE/AC-2019-00793G_0006.JPEG
Clark LC, Lyons C (1962) ELECTRODE SYSTEMS FOR CONTINUOUS MONITORING IN CARDIOVASCULAR SURGERY. Ann N Y Acad Sci 102:29–45. https://doi.org/10.1111/J.1749-6632.1962.TB13623.X
Morales MA, Halpern JM (2018) Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug Chem 29:3231–3239. https://doi.org/10.1021/ACS.BIOCONJCHEM.8B00592/ASSET/IMAGES/LARGE/BC-2018-005923_0006.JPEG
Naresh V, Lee N (2021) A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, Vol 21, Page 1109 21:1109. https://doi.org/10.3390/S21041109
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975 256:5517 256:495–497. https://doi.org/10.1038/256495a0
Watson JD, Crick FHC (1953) Genetical implications of the structure of deoxyribonucleic acid. Nature 171:964–967. https://doi.org/10.1038/171964B0
SHIMOMURA O, JOHNSON FH, SAIGA Y (1962) Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J Cell Comp Physiol 59:223–239. https://doi.org/10.1002/JCP.1030590302
Su S, Sun Q, Gu X, et al (2019) Two-dimensional nanomaterials for biosensing applications. TrAC Trends in Analytical Chemistry 119:115610. https://doi.org/10.1016/J.TRAC.2019.07.021
Shabaninejad Z, Yousefi F, Movahedpour A, et al (2019) Electrochemical-based biosensors for microRNA detection: Nanotechnology comes into view. Anal Biochem 581:113349. https://doi.org/10.1016/J.AB.2019.113349
Campuzano S, Yáñez-Sedeño P, Pingarrón JM (2018) Nanoparticles for nucleic-acid-based biosensing: opportunities, challenges, and prospects. Analytical and Bioanalytical Chemistry 2018 411:9 411:1791–1806. https://doi.org/10.1007/S00216-018-1273-6
Xu L, Shoaie N, Jahanpeyma F, et al (2020) Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview. Biosens Bioelectron 161
Chia BS, Low YP, Wang Q, et al (2017) Advances in exosome quantification techniques. TrAC Trends in Analytical Chemistry 86:93–106. https://doi.org/10.1016/J.TRAC.2016.10.012
Im H, Yang KS, Lee H, Castro CM (2018) Nanotechnology Platforms for Cancer Exosome Analyses. Diagnostic and Therapeutic Applications of Exosomes in Cancer 119–128. https://doi.org/10.1016/B978-0-12-812774-2.00007-9
Yoo SM, Lee SY (2016) Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends Biotechnol 34:7–25. https://doi.org/10.1016/j.tibtech.2015.09.012
Lan L, Yao Y, Ping J, Ying Y (2017) Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens Bioelectron 91:504–514. https://doi.org/10.1016/J.BIOS.2017.01.007
Xuan X, Yoon HS, Park JY (2018) A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens Bioelectron 109:75–82. https://doi.org/10.1016/J.BIOS.2018.02.054
Jalalvand AR, Haseli A, Farzadfar F, Goicoechea HC (2019) Fabrication of a novel biosensor for biosensing of bisphenol A and detection of its damage to DNA. Talanta 201:350–357. https://doi.org/10.1016/J.TALANTA.2019.04.037
Shao B, Ma X, Zhao S, et al (2018) Nanogapped Au(core) @ Au-Ag(shell) structures coupled with Fe3O4 magnetic nanoparticles for the detection of Ochratoxin A. Anal Chim Acta 1033:165–172. https://doi.org/10.1016/J.ACA.2018.05.058
Liu Y, Dong P, Jiang Q, et al (2019) Assembly-enhanced fluorescence from metal nanoclusters and quantum dots for highly sensitive biosensing. Sens Actuators B Chem 279:334–341. https://doi.org/10.1016/J.SNB.2018.10.016
Chen Y-T, Lee Y-C, Lai Y-H, et al (2020) Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors (Basel) 10:209. https://doi.org/10.3390/bios10120209
Yang Y, Li C, Shi H, et al (2019) A pH-responsive bioassay for paper-based diagnosis of exosomes via mussel-inspired surface chemistry. Talanta 192:325–330. https://doi.org/10.1016/J.TALANTA.2018.09.067
Okumoto S, Jones A, Frommer WB (2012) Quantitative Imaging with Fluorescent Biosensors. https://doi.org/101146/annurev-arplant-042110-103745 63:663–706. https://doi.org/10.1146/ANNUREV-ARPLANT-042110-103745
Hirata E, Kiyokawa E (2016) Future Perspective of Single-Molecule FRET Biosensors and Intravital FRET Microscopy. Biophysj 111:1103–1111. https://doi.org/10.1016/j.bpj.2016.01.037
Chen X, Lan J, Liu Y, et al (2018) A paper-supported aptasensor based on upconversion luminescence resonance energy transfer for the accessible determination of exosomes. Biosens Bioelectron 102:582–588. https://doi.org/10.1016/J.BIOS.2017.12.012
Zhang P, He M, Zeng Y (2016) Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip 16:3033–3042. https://doi.org/10.1039/C6LC00279J
Liu C, Xu X, Li B, et al (2018) Single-Exosome-Counting Immunoassays for Cancer Diagnostics. Nano Lett 18:4226–4232. https://doi.org/10.1021/ACS.NANOLETT.8B01184/ASSET/IMAGES/LARGE/NL-2018-01184Z_0005.JPEG
He D, Ho SL, Chan HN, et al (2019) Molecular-Recognition-Based DNA Nanodevices for Enhancing the Direct Visualization and Quantification of Single Vesicles of Tumor Exosomes in Plasma Microsamples. Anal Chem 91:2768–2775. https://doi.org/10.1021/ACS.ANALCHEM.8B04509/ASSET/IMAGES/LARGE/AC-2018-04509X_0005.JPEG
Jin D, Yang F, Zhang Y, et al (2018) ExoAPP: Exosome-Oriented, Aptamer Nanoprobe-Enabled Surface Proteins Profiling and Detection. Anal Chem 90:14402–14411. https://doi.org/10.1021/ACS.ANALCHEM.8B03959/ASSET/IMAGES/LARGE/AC-2018-03959M_0005.JPEG
Liu C, Zhao J, Tian F, et al (2019) I-DNA- A nd Aptamer-Mediated Sorting and Analysis of Extracellular Vesicles. J Am Chem Soc 141:3817–3821. https://doi.org/10.1021/JACS.9B00007/ASSET/IMAGES/LARGE/JA-2019-00007Q_0005.JPEG
Lewis JM, Vyas AD, Qiu Y, et al (2018) Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 12:3311–3320. https://doi.org/10.1021/ACSNANO.7B08199/ASSET/IMAGES/LARGE/NN-2017-081998_0006.JPEG
Ibsen SD, Wright J, Lewis JM, et al (2017) Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano 11:6641–6651. https://doi.org/10.1021/ACSNANO.7B00549/ASSET/IMAGES/LARGE/NN-2017-005499_0011.JPEG
Kang YT, Kim YJ, Bu J, et al (2017) High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale 9:13495–13505. https://doi.org/10.1039/C7NR04557C
Zhang H, Wang Z, Zhang Q, et al (2019) Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens Bioelectron 124–125:184–190. https://doi.org/10.1016/J.BIOS.2018.10.016
Wan S, Zhang L, Wang S, et al (2017) Molecular Recognition-Based DNA Nanoassemblies on the Surfaces of Nanosized Exosomes. J Am Chem Soc 139:5289–5292. https://doi.org/10.1021/JACS.7B00319/ASSET/IMAGES/LARGE/JA-2017-00319D_0004.JPEG
Ye Y, Gao J, Zhuang H, et al (2017) Electrochemical gene sensor based on a glassy carbon electrode modified with hemin-functionalized reduced graphene oxide and gold nanoparticle-immobilized probe DNA. Microchimica Acta 184:245–252. https://doi.org/10.1007/S00604-016-1999-9/TABLES/1
Xia Y, Wang L, Li J, et al (2018) A Ratiometric Fluorescent Bioprobe Based on Carbon Dots and Acridone Derivate for Signal Amplification Detection Exosomal microRNA. Anal Chem 90:8969–8976. https://doi.org/10.1021/ACS.ANALCHEM.8B01143/ASSET/IMAGES/LARGE/AC-2018-01143R_0006.JPEG
Cheung LS, Sahloul S, Orozaliev A, Song YA (2018) Rapid Detection and Trapping of Extracellular Vesicles by Electrokinetic Concentration for Liquid Biopsy on Chip. Micromachines 2018, Vol 9, Page 306 9:306. https://doi.org/10.3390/MI9060306
Tian Q, He C, Liu G, et al (2018) Nanoparticle Counting by Microscopic Digital Detection: Selective Quantitative Analysis of Exosomes via Surface-Anchored Nucleic Acid Amplification. Anal Chem 90:6556–6562. https://doi.org/10.1021/ACS.ANALCHEM.8B00189/ASSET/IMAGES/LARGE/AC-2018-00189V_0003.JPEG
Tian Y, Ma L, Gong M, et al (2018) Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry. ACS Nano 12:671–680. https://doi.org/10.1021/ACSNANO.7B07782/ASSET/IMAGES/LARGE/NN-2017-07782V_0006.JPEG
Huang L, Wang DB, Singh N, et al (2018) A dual-signal amplification platform for sensitive fluorescence biosensing of leukemia-derived exosomes. Nanoscale 10:20289–20295. https://doi.org/10.1039/C8NR07720G
He F, Wang J, Yin BC, Ye BC (2018) Quantification of Exosome Based on a Copper-Mediated Signal Amplification Strategy. Anal Chem 90:8072–8079. https://doi.org/10.1021/ACS.ANALCHEM.8B01187/ASSET/IMAGES/LARGE/AC-2018-01187Y_0005.JPEG
Lee JH, Kim JA, Jeong S, Rhee WJ (2016) Simultaneous and multiplexed detection of exosome microRNAs using molecular beacons. Biosens Bioelectron 86:202–210. https://doi.org/10.1016/J.BIOS.2016.06.058
Zhai LY, Li MX, Pan WL, et al (2018) In Situ Detection of Plasma Exosomal MicroRNA-1246 for Breast Cancer Diagnostics by a Au Nanoflare Probe. ACS Appl Mater Interfaces 10:39478–39486. https://doi.org/10.1021/ACSAMI.8B12725/ASSET/IMAGES/LARGE/AM-2018-12725Q_0006.JPEG
Lyu Y, Cui D, Huang J, et al (2019) Near-Infrared Afterglow Semiconducting Nano-Polycomplexes for the Multiplex Differentiation of Cancer Exosomes. Angewandte Chemie International Edition 58:4983–4987. https://doi.org/10.1002/ANIE.201900092
Jørgensen M, Bæk R, Pedersen S, et al (2013) Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles 2:. https://doi.org/10.3402/JEV.V2I0.20920
He D, Wang H, Ho SL, et al (2019) Total internal reflection-based single-vesicle in situ quantitative and stoichiometric analysis of tumor-derived exosomal microRNAs for diagnosis and treatment monitoring. Theranostics 9:4494. https://doi.org/10.7150/THNO.33683
Song Y, Wei W, Qu X (2011) Colorimetric Biosensing Using Smart Materials. Advanced Materials 23:4215–4236. https://doi.org/10.1002/ADMA.201101853
Cheng N, Du D, Wang X, et al (2019) Recent Advances in Biosensors for Detecting Cancer-Derived Exosomes. Trends Biotechnol 37:1236–1254. https://doi.org/10.1016/j.tibtech.2019.04.008
Wang YM, Liu JW, Adkins GB, et al (2017) Enhancement of the Intrinsic Peroxidase-Like Activity of Graphitic Carbon Nitride Nanosheets by ssDNAs and Its Application for Detection of Exosomes. Anal Chem 89:12327–12333. https://doi.org/10.1021/ACS.ANALCHEM.7B03335/ASSET/IMAGES/LARGE/AC-2017-03335Y_0005.JPEG
Shao B, Xiao Z (2020) Recent achievements in exosomal biomarkers detection by nanomaterials-based optical biosensors - A review. Anal Chim Acta 1114:74–84
Casals E, Pfaller T, Duschl A, et al (2010) Time evolution of the nanoparticle protein corona. ACS Nano 4:3623–3632. https://doi.org/10.1021/NN901372T/SUPPL_FILE/NN901372T_SI_001.PDF
Wang X, Hu Y, Wei H (2016) Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front 3:41–60. https://doi.org/10.1039/C5QI00240K
Jiang Y, Shi M, Liu Y, et al (2017) Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angewandte Chemie International Edition 56:11916–11920. https://doi.org/10.1002/ANIE.201703807
Chen J, Xu Y, Lu Y, Xing W (2018) Isolation and Visible Detection of Tumor-Derived Exosomes from Plasma. Anal Chem 90:14207–14215. https://doi.org/10.1021/ACS.ANALCHEM.8B03031/ASSET/IMAGES/LARGE/AC-2018-03031T_0006.JPEG
Xia Y, Liu M, Wang L, et al (2017) A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens Bioelectron 92:8–15. https://doi.org/10.1016/J.BIOS.2017.01.063
Chen Z, Cheng SB, Cao P, et al (2018) Detection of exosomes by ZnO nanowires coated three-dimensional scaffold chip device. Biosens Bioelectron 122:211–216. https://doi.org/10.1016/J.BIOS.2018.09.033
Jiang Y, Shi M, Liu Y, et al (2017) Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angewandte Chemie International Edition 56:11916–11920. https://doi.org/10.1002/ANIE.201703807
Liu W, Li J, Wu Y, et al (2018) Target-induced proximity ligation triggers recombinase polymerase amplification and transcription-mediated amplification to detect tumor-derived exosomes in nasopharyngeal carcinoma with high sensitivity. Biosens Bioelectron 102:204–210. https://doi.org/10.1016/J.BIOS.2017.11.033
Yang Y, Li C, Shi H, et al (2019) A pH-responsive bioassay for paper-based diagnosis of exosomes via mussel-inspired surface chemistry. Talanta 192:325–330. https://doi.org/10.1016/J.TALANTA.2018.09.067
Chen J, Xu Y, Lu Y, Xing W (2018) Isolation and Visible Detection of Tumor-Derived Exosomes from Plasma. Anal Chem 90:14207–14215. https://doi.org/10.1021/ACS.ANALCHEM.8B03031/ASSET/IMAGES/LARGE/AC-2018-03031T_0006.JPEG
Masson JF (2017) Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens 2:16–30. https://doi.org/10.1021/ACSSENSORS.6B00763/ASSET/IMAGES/LARGE/SE-2016-00763X_0004.JPEG
Nguyen HH, Park J, Kang S, Kim M (2015) Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, Vol 15, Pages 10481–10510 15:10481–10510. https://doi.org/10.3390/S150510481
Brolo AG (2012) Plasmonics for future biosensors. Nature Photonics 2012 6:11 6:709–713. https://doi.org/10.1038/nphoton.2012.266
Rojalin T, Phong B, Koster H, Carney RP (2019) Nanoplasmonic approaches for sensitive detection and molecular characterization of extracellular vesicles. Front Chem 7:448979. https://doi.org/10.3389/FCHEM.2019.00279/BIBTEX
Zhang L, Gu C, Wen J, et al Recent advances in nanomaterial-based biosensors for the detection of exosomes. https://doi.org/10.1007/s00216-020-03000-0/Published
Zeng S, Baillargeat D, Ho HP, Yong KT (2014) Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem Soc Rev 43:3426–3452. https://doi.org/10.1039/C3CS60479A
Stockman MI (2015) Nanoplasmonic sensing and detection: Enhanced optical fields in nanoplasmonic systems provide efficient sensing and detection. Science (1979) 348:287–288. https://doi.org/10.1126/SCIENCE.AAA6805/ASSET/7E985E40-7F0A-4673-BB7D-EF2F287118A2/ASSETS/GRAPHIC/348_287_F1.JPEG
Sina AAI, Vaidyanathan R, Wuethrich A, et al (2019) Label-free detection of exosomes using a surface plasmon resonance biosensor. Anal Bioanal Chem 411:1311–1318. https://doi.org/10.1007/S00216-019-01608-5/FIGURES/4
Sina AAI, Vaidyanathan R, Dey S, et al (2016) Real time and label free profiling of clinically relevant exosomes. Scientific Reports 2016 6:1 6:1–9. https://doi.org/10.1038/srep30460
Zhu L, Wang K, Cui J, et al (2014) Label-free quantitative detection of tumor-derived exosomes through surface plasmon resonance imaging. Anal Chem 86:8857–8864. https://doi.org/10.1021/AC5023056/ASSET/IMAGES/LARGE/AC-2014-023056_0005.JPEG
Thakur A, Qiu G, NG SP, et al (2017) Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens Bioelectron 94:400–407. https://doi.org/10.1016/J.BIOS.2017.03.036
Liu C, Zeng X, An Z, et al (2018) Sensitive Detection of Exosomal Proteins via a Compact Surface Plasmon Resonance Biosensor for Cancer Diagnosis. ACS Sens 3:1471–1479. https://doi.org/10.1021/ACSSENSORS.8B00230/ASSET/IMAGES/LARGE/SE-2018-00230B_0002.JPEG
Park J, Im H, Hong S, et al (2018) Analyses of Intravesicular Exosomal Proteins Using a Nano-Plasmonic System. ACS Photonics 5:487–494. https://doi.org/10.1021/ACSPHOTONICS.7B00992/ASSET/IMAGES/LARGE/PH-2017-00992Y_0004.JPEG
Im H, Shao H, Park Y Il, et al (2014) Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature Biotechnology 2014 32:5 32:490–495. https://doi.org/10.1038/nbt.2886
Cialla-May D, Zheng XS, Weber K, Popp J (2017) Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: from cells to clinics. Chem Soc Rev 46:3945–3961. https://doi.org/10.1039/C7CS00172J
Zong C, Xu M, Xu LJ, et al (2018) Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem Rev 118:4946–4980. https://doi.org/10.1021/ACS.CHEMREV.7B00668/ASSET/IMAGES/LARGE/CR-2017-00668B_0028.JPEG
Cialla D, März A, Böhme R, et al (2012) Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal Bioanal Chem 403:27–54. https://doi.org/10.1007/S00216-011-5631-X/FIGURES/2
Wang Y, Yan B, Chen L (2013) SERS Tags: Novel optical nanoprobes for bioanalysis. Chem Rev 113:1391–1428. https://doi.org/10.1021/CR300120G/ASSET/IMAGES/MEDIUM/CR-2012-00120G_0004.GIF
Ma D, Huang C, Zheng J, et al (2018) Quantitative detection of exosomal microRNA extracted from human blood based on surface-enhanced Raman scattering. Biosens Bioelectron 101:167–173. https://doi.org/10.1016/J.BIOS.2017.08.062
Pang Y, Wang C, Lu LC, et al (2019) Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens Bioelectron 130:204–213. https://doi.org/10.1016/J.BIOS.2019.01.039
Zong S, Wang Z, Chen H, Cui Y (2013) Ultrasensitive Telomerase Activity Detection by Telomeric Elongation Controlled Surface Enhanced Raman Scattering. Small 9:4215–4220. https://doi.org/10.1002/SMLL.201301372
Zong S, Wang L, Chen C, et al (2016) Facile detection of tumor-derived exosomes using magnetic nanobeads and SERS nanoprobes. Analytical Methods 8:5001–5008. https://doi.org/10.1039/C6AY00406G
Weng Z, Zong S, Wang Y, et al (2018) Screening and multiple detection of cancer exosomes using an SERS-based method. Nanoscale 10:9053–9062. https://doi.org/10.1039/C7NR09162A
Tian YF, Ning CF, He F, et al (2018) Highly sensitive detection of exosomes by SERS using gold nanostar@Raman reporter@nanoshell structures modified with a bivalent cholesterol-labeled DNA anchor. Analyst 143:4915–4922. https://doi.org/10.1039/C8AN01041B
Li T Da, Zhang R, Chen H, et al (2018) An ultrasensitive polydopamine bi-functionalized SERS immunoassay for exosome-based diagnosis and classification of pancreatic cancer. Chem Sci 9:5372–5382. https://doi.org/10.1039/C8SC01611A
Pang Y, Wang C, Lu LC, et al (2019) Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens Bioelectron 130:204–213. https://doi.org/10.1016/J.BIOS.2019.01.039
Lee W, Nanou A, Rikkert L, et al (2018) Label-Free Prostate Cancer Detection by Characterization of Extracellular Vesicles Using Raman Spectroscopy. Anal Chem 90:11290–11296. https://doi.org/10.1021/ACS.ANALCHEM.8B01831/ASSET/IMAGES/LARGE/AC-2018-01831S_0006.JPEG
Kwizera EA, O’Connor R, Vinduska V, et al (2018) Molecular Detection and Analysis of Exosomes Using Surface-Enhanced Raman Scattering Gold Nanorods and a Miniaturized Device. Theranostics 8:2722. https://doi.org/10.7150/THNO.21358
Wang DM, Lin KL, Huang CZ (2019) Carbon dots-involved chemiluminescence: Recent advances and developments. Luminescence 34:4–22. https://doi.org/10.1002/BIO.3570
Roda A, Mirasoli M, Michelini E, et al (2016) Progress in chemical luminescence-based biosensors: A critical review. Biosens Bioelectron 76:164–179. https://doi.org/10.1016/J.BIOS.2015.06.017
Tiwari A, Dhoble SJ (2018) Recent advances and developments on integrating nanotechnology with chemiluminescence assays. Talanta 180:1–11. https://doi.org/10.1016/J.TALANTA.2017.12.031
Wang Y, Liu Z, Wang X, et al (2019) Rapid and Quantitative Analysis of Exosomes by a Chemiluminescence Immunoassay Using Superparamagnetic Iron Oxide Particles. J Biomed Nanotechnol 16:1792–1800. https://doi.org/10.1166/JBN.2019.2809
Jiang Q, Liu Y, Wang L, et al (2019) Rapid Enrichment and Detection of Extracellular Vesicles Enabled by CuS-Enclosed Microgels. Anal Chem 91:15951–15958. https://doi.org/10.1021/ACS.ANALCHEM.9B04485/ASSET/IMAGES/LARGE/AC9B04485_0004.JPEG
Chen Y, Zhou S, Li L, Zhu J jie (2017) Nanomaterials-based sensitive electrochemiluminescence biosensing. Nano Today 12:98–115. https://doi.org/10.1016/J.NANTOD.2016.12.013
Shoaie N, Daneshpour M, Azimzadeh M, et al (2019) Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: a review on recent advances. Microchimica Acta 2019 186:7 186:1–29. https://doi.org/10.1007/S00604-019-3588-1
Xu H, Liao C, Zuo P, et al (2018) Magnetic-Based Microfluidic Device for On-Chip Isolation and Detection of Tumor-Derived Exosomes. Anal Chem 90:13451–13458. https://doi.org/10.1021/ACS.ANALCHEM.8B03272/ASSET/IMAGES/LARGE/AC-2018-03272B_0006.JPEG
Hochendoner P, Zhao Z, He M (2018) Diagnostic Potential of Tumor Exosomes. Diagnostic and Therapeutic Applications of Exosomes in Cancer 161–173. https://doi.org/10.1016/B978-0-12-812774-2.00009-2
Kimmel DW, Leblanc G, Meschievitz ME, Cliffel DE (2012) Electrochemical sensors and biosensors. Anal Chem 84:685–707. https://doi.org/10.1021/AC202878Q/ASSET/IMAGES/LARGE/AC-2011-02878Q_0003.JPEG
Doldán X, Fagúndez P, Cayota A, et al (2016) Electrochemical Sandwich Immunosensor for Determination of Exosomes Based on Surface Marker-Mediated Signal Amplification. Anal Chem 88:10466–10473. https://doi.org/10.1021/ACS.ANALCHEM.6B02421/ASSET/IMAGES/LARGE/AC-2016-02421C_0007.JPEG
Topkaya SN, Azimzadeh M, Ozsoz M (2016) Electrochemical Biosensors for Cancer Biomarkers Detection: Recent Advances and Challenges. Electroanalysis 28:1402–1419. https://doi.org/10.1002/ELAN.201501174
Scholz F (2015) Voltammetric techniques of analysis: the essentials. ChemTexts 1:1–24. https://doi.org/10.1007/S40828-015-0016-Y/TABLES/1
Boriachek K, Islam MN, Gopalan V, et al (2017) Quantum dot-based sensitive detection of disease specific exosome in serum. Analyst 142:2211–2219. https://doi.org/10.1039/C7AN00672A
Alkhamis O, Canoura J, Yu H, et al (2019) Innovative engineering and sensing strategies for aptamer-based small-molecule detection. TrAC Trends in Analytical Chemistry 121:115699. https://doi.org/10.1016/J.TRAC.2019.115699
Sedighian H, Halabian R, Amani J, et al (2018) Manufacturing of a novel double-function ssDNA aptamer for sensitive diagnosis and efficient neutralization of SEA. Anal Biochem 548:69–77. https://doi.org/10.1016/J.AB.2018.02.017
Al Ahmad M (2018) Electrical Detection, Identification, and Quantification of Exosomes. IEEE Access 6:22817–22826. https://doi.org/10.1109/ACCESS.2018.2828038
Li H, Liu X, Li L, et al (2016) CMOS Electrochemical Instrumentation for Biosensor Microsystems: A Review. Sensors 2017, Vol 17, Page 74 17:74. https://doi.org/10.3390/S17010074
Li H, Liu X, Li L, et al (2016) CMOS Electrochemical Instrumentation for Biosensor Microsystems: A Review. Sensors 2017, Vol 17, Page 74 17:74. https://doi.org/10.3390/S17010074
Ibsen SD, Wright J, Lewis JM, et al (2017) Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano 11:6641–6651. https://doi.org/10.1021/ACSNANO.7B00549/ASSET/IMAGES/LARGE/NN-2017-005499_0011.JPEG
Eliza SA, Dutta AK (2010) Ultra-high sensitivity gas sensors based on GaN HEMT structures. ICECE 2010–6th International Conference on Electrical and Computer Engineering 431–433. https://doi.org/10.1109/ICELCE.2010.5700721
Taller D, Richards K, Slouka Z, et al (2015) On-chip surface acoustic wave lysis and ion-exchange nanomembrane detection of exosomal RNA for pancreatic cancer study and diagnosis. Lab Chip 15:1656–1666. https://doi.org/10.1039/C5LC00036J
Singh S, Numan A, Cinti S (2022) Electrochemical nano biosensors for the detection of extracellular vesicles exosomes: From the benchtop to everywhere? Biosens Bioelectron 216:114635. https://doi.org/10.1016/j.bios.2022.114635
Acknowledgements
IIT (BHU) Varanasi made available the laboratory and functional assistance, for which the authors are grateful. We acknowledge DBT India for providing fellowship to Abhay Dev Tripathi. We acknowledge ICMR DHR for providing financial support to author Vivek K. Chaturvedi.
Funding
Abhay Dev Tripathi received a fellowship from the Department of Biotechnology, India, throughout the course of this study (DBT award no. DBT/JRF/BET-19/I/2019/AL/140), which was funded under the DBT JRF Ph.D. program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. ADT and YL conducted the writing of the original manuscript, the figures, the data gathering, and the literature search. SK did the data collection and writing. Data analysis and editing were done by VKC and PS, whereas concept idea, supervision, data analysis, editing, and revision were performed by AM. The final paper was reviewed and approved by all contributors.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tripathi, A.D., Labh, Y., Katiyar, S. et al. Advancements in Nano-Mediated Biosensors: Targeting Cancer Exosome Detection. J Clust Sci 35, 2195–2212 (2024). https://doi.org/10.1007/s10876-024-02676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-024-02676-z