Abstract
This paper reports the chemistry of fine (PM2.5) and coarse (PM10) aerosols sampled over a period of three years during 2018–2021 at a semi -arid tropical location in the rain shadow region of the peninsular India. The data is classified in to dry (December to May) and wet (June to November) periods. Scavenging effect due to rains have culminated in to less concentrations of both fine and coarse aerosols and their ionic components in the wet period. Significantly high concentrations of the crustal components such as Ca, Na, K and Mg from the local dust resulted in the alkaline pH in both dry and wet periods with Ca and Mg emerging as major neutralizing components. Overall, < 20% samples of both fine and coarse aerosols depicted acidic pH. Concentration of SO4 was comparatively more than NO3 indicating towards more presence of stationary sources (industrial/domestic emissions) than mobile (vehicular emissions) sources. Combustion generated and highly absorbing black carbon aerosols showed high concentration during the dry period. Local activities comprising residential, agricultural, vehicular and industrial emissions were the major sources of aerosols at Solapur however, the contribution from the distant sources were also found to contribute as inferred from the cluster analysis and concentration weighted trajectories (CWT). The observed abundances of the alkaline dust aerosols that could act as cloud condensation nuclei or ice nuclei will have important implications on the studies related to cloud aerosol precipitation interaction over this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the recent past, there has been a significant increase in the anthropogenic emissions of aerosols and their precursor gases in the Asian countries due to the rapid growth in economies that has pushed up enormously the consumption of energy resources (IPCC 2021). Apart from the several anthropogenic activities including vehicular and industrial emissions; biomass burning for agricultural and residential purposes as well as natural phenomena like dust storms, bubble bursting over the oceans, volcanic eruptions, etc. are the major key processes involved in the generation of atmospheric aerosols (Khemani 1989). These prevailing atmospheric aerosols are removed from the atmosphere mainly through dry and wet deposition processes (Yang et al. 2019). Due to the varying microphysical and chemical properties, aerosols behave differently at different levels of the atmosphere (Kumar et al. 2011) The spatial and temporal variation in aerosol properties is also related with the variation in prevailing meteorological conditions and strengths of possible potential sources (Safai et al. 2010). Absorbing type aerosols such as black carbon (BC) warm the surrounding atmosphere whereas scattering type aerosols such as sea salt, sulphate and dust cool the surrounding atmosphere. Therefore, the composition of aerosols is very important for solar radiation balance and thereby the temperature structure in the lower atmosphere which has implications on the cloud micro physical properties and ultimately on the rainfall mechanism (IPCC 2014). It is now well recognized that atmospheric aerosols play an important role in global climate regime. Pan et al. (2015) suggest that over the Indian region, uncertainties in aerosol related impacts are due to their spatial and temporal variations, vertical distribution and chemical heterogeneity which determine the change in the regional climate as well as air quality index. Therefore, sustained observations from different locations are the need of the hour for any assessment and mitigation strategies for aerosol related climatic impacts in India. So far, various studies have tried to address this task but they are mainly limited to urban -industrial and rural-remote locations in India (Safai et al. 2010; Chatterjee et al. 2010; Gawhane et al. 2017; Mukherjee et al. 2018; Buchunde et al. 2022). However, such studies from the arid regions are very few (Reddy et al. 2007; Kumar et al. 2011; Pipal et al. 2011; Soyam et al. 2021) and are essential to understand the aerosol –cloud- rainfall interactions and their plausible impacts on regional precipitation mechanism.
The present study is focused on the measurement of ionic concentrations, sea-salt and non- sea salt (crustal and anthropogenic) fractions of PM2.5 (fine size particles less than or equal to 2.5 μm size) and PM10 (coarse size particles less than or equal to 10 μm size) in different seasons over the semi-arid rain shadow location Solapur in the peninsular India during March 2018 to January 2021.
2 Sampling location and methodology
2.1 Sampling location
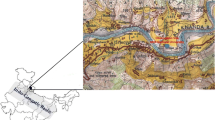
As a part of the major national campaign on cloud aerosol interaction and precipitation enhancement experiment (CAIPEEX) of the Indian Institute of Tropical Meteorology (IITM), ground observational campaign for the determination of chemical characterization of atmospheric aerosols was carried out at the Sinhagad College of Engineering, Solapur, Maharashtra, India (17°43’45.4332” N, 75°.51’24.4476’’ E) during March 2018 to January 2021. Ionic composition of fine (PM2.5) and coarse (PM10) particles has been measured. Solapur falls under a semi-arid, rain shadow zone in peninsular India and the climate is mainly dry with mean annual rainfall of about 700 mm of which about 80% falls in summer monsoon (June to September) and 15% in winter monsoon (October-November). Solapur has a very dry weather with daily maximum and minimum temperature ranging from 30 to 37 °C and 18 – 21 °C respectively. The physiography of the location is a flat, undulated terrain in the Deccan Trap basaltic lava and does not have any prominent hill ranges. The black soil in the district is associated with calcareous kankars. The district has industrial establishments like textile, sugar and cement as major industries. There are about 600 units in the Solapur Textile Cluster of Industries. Solapur has the highest number of sugar factories in Maharashtra state. There are about 30 sugar factories in the Solapur district. Also, there are about 18 cement and concrete manufacturing industries in Solapur. Such large amalgamation of different anthropogenic sources covering industrial to agricultural products apart from the other household activities leads to the mixed type of aerosols over this region and needs to be explored.
2.2 Methodology: sampling and chemical analysis of PM2.5 and PM10
Sampling of PM10 and PM2.5 was carried out using a dust sampler (NETEL India Pvt Ltd). Each sample was collected twice a week for the duration of about 24 h at an average flow rate of 16.7 LPM. Sampling was not continuous due to intermittent rain spells in the wet period. Also, due to the strict restrictions in the COVID pandemic related lockdowns, sampling was not undertaken during April to September 2020. A total of 134 samples of PM2.5 and 110 samples of PM10 were collected using the pre-combusted 47 mm-diameter quartz filters. After the gravimetric analysis, samples were extracted for about an hour with ultrapure water using ultrasonic bath and were analyzed for the major water-soluble ions by using an Ion Chromatograph (Metrohm 850 Professional IC). The detailed description of the sampling and analysis procedures and the detection limits is given elsewhere (Budhavant et al. 2016). Field blanks were taken frequently and were analyzed using procedures similar to those adopted for the samples and necessary correction factors were taken into consideration while computing the ionic concentrations. The error in analytical reproducibility for the major components ranges from 0 to 5%. In addition, we are participating in the international inter-comparison studies, i.e., EANET’s Inter-laboratory comparison projects and WMO’s Laboratory Inter-comparison Studies (LIS). The results from these comparison studies showed good agreement, i.e., the variation is ≤ ± 10%.
The correctness of the analysis is further assessed following the principle of electrical neutrality by adopting the ionic balance (IB) i.e. balance between the equivalent weights of cations and anions. Since the analysis usually represent the major dissolved inorganic ions in aerosols (SO4, NO3, Cl, NH4, Ca, Mg, Na and K); equivalent amounts of these ions should be nearly equal except when the concentrations of bicarbonate ion (HCO3−) are significant. The ionic balance (IB) in percentage was calculated as below:
This data quality objective method is referred from the WMO manual (WMO GAW TD no. 1251 2004) and only those samples having IB within the threshold limits (20% for total ionic strength of > 100 µeq/L) were considered. Totally 99 samples each for PM2.5 and PM10 that were sampled on the same days during this period have been considered for the present study. It was observed that the inclusion of HCO3− was essential to obtain the ionic balance within prescribed limits. Similar results indicating important role of HCO3 ion in ionic balance have been reported by many studies, especially from the Indian region (Khemani 1989 and references therein, Bhaskar and Rao 2017).The HCO3− ion was computed from the pH value of the sample using the Henderson-Hasselbalch equation as:
where, pCO2 means partial pressure of CO2 in the atmosphere (presently 410 ppm global mean). Such methods using pH for computation of HCO3 are widely reported by several studies (Granat 1972; Kulshrestha et al. 2003; Rao et al. 2016).
The ionic balance for the water soluble extracts of PM2.5 and PM10 for all the four seasons was within the WMO prescribed limits indicating the good quality of analysis. The IB values in summer, monsoon, post-monsoon and winter season for PM2.5 were 16, 16 10 and 4%, respectively and these for PM10 were 18, 19, 10 and 17%, respectively.
3 Results and discussion
3.1 Mass concentration and pH of PM2.5 and PM10
As seen from Fig. 1, the mass concentration of both PM2.5 and PM10 showed similar seasonal variation with maximum concentration in winter (52 ± 22 µg/m3 for PM2.5 and 97 ± 43 µg/m3 for PM10) and minimum in monsoon season (28 ± 18 µg/m3 for PM2.5 and 51 ± 29 µg/m3 for PM10). PM10 showed equal concentration in winter and summer (97 ± 33 µg/m3) seasons whereas the concentration of PM2.5 was comparatively less in summer (38 ± 14 µg/m3). However, post- monsoon season showed moderate concentrations for both PM2.5 (44 ± 24 µg/m3) and PM10 (72 ± 30 µg/m3). Apart from this regular convention of classifying the data in four seasons, we have separated the data in two major periods representative of dry (very few rainy days making up for ≤ 5% of annual rains) and wet (mostly rainy days that make up for ≥ 95% of annual rains) periods. The dry period comprises December to May months (winter and summer seasons) and wet period comprises June to November (monsoon and post-monsoon or south west and north east monsoon). Hereafter, to avoid the repetition of data interpretation and also to get the more broader view of variation in the aerosol composition with respect to precipitation; we will discuss the results for these two major periods i.e. dry and wet periods only. The mean mass concentration of PM2.5 and PM10 was obviously less during the wet period (36 ± 22 µg/m3 for PM2.5 and 60 ± 31 µg/m3 for PM10) than that observed in the dry period (46 ± 20 µg/m3 for PM2.5 and 97 ± 39 g/m3 for PM10). However, the enhancement in mass concentration of PM2.5 was about 28% whereas that in PM10 was about 62% during dry period than that in the wet period. The observed difference in the mean mass concentration of PM2.5 and PM10 in the dry and wet periods is mainly attributed to the prevailing meteorological conditions over the sampling site. The scavenging effect of intermittent rains during the wet period causes significant reduction in both fine and coarse size aerosols, especially more in the coarse aerosols. Also during the dry period, low ventilation coefficients (in winter) restrict the dispersion of particles leading to their accumulation near the surface whereas more insolation gives rise to more turbulence thereby uplifting the particles, especially those in the coarse size at surface and making them airborne for longer duration (in summer). In addition, the local combustion activities related to residential and agricultural practices are increased during the dry period that generates more aerosol particles, both in fine and coarse size. It was observed that the threshold limit (60 µg/m3 for 24 h for PM2.5 and 100 µg/m3 for 24 h for PM10 as per the National Ambient Air Quality Standard set by Central Pollution Control Board in India in the year 2009 (http://cpcb.nic.in/National_Ambient_Air_Quality_Standards.php) was exceeded on about 16% of occasions in the dry and 14% in wet periods for PM2.5 whereas, for PM10, the threshold limit was exceeded for about 44% of occasions in the dry period and about 15% of occasions in the wet period.
3.2 Ionic composition of water soluble extracts of PM2.5 and PM10
As seen from Fig. 2, the significant dominance of dust (Ca and Mg) and sea salt (Na and Cl) over the secondary aerosols (SO4, NO3 and NH4) is clearly depicted in the ionic composition of PM2.5 and PM10 aerosols during both dry and wet periods at Solapur. The overall contribution of the anthropogenically originated secondary aerosols was around 10 to 14% across both dry and wet seasons for PM2.5 and PM10. Similarly, the precursor gases i.e. SO2 for SO4 and NO2 for NO3 also showed concentrations within the prescribed threshold limits under the national ambient air quality monitoring standards by the central pollution control board in India in 2009 (http://cpcb.nic.in/National_Ambient_Air_Quality_Standards.php). The mean SO2 concentration at Solapur was around 21 µg/m3 with zero incidences exceeding the threshold limit of 80 µg/m3. Similarly, the mean NO2 concentration was around 35 µg/m3 with zero incidences exceeding the threshold limit of 80 µg/m3. The decreasing order of percentage wise dominance in the dry season for PM2.5 is HCO3 (28%) > Ca and Na (17% each) > Mg (15%) > Cl (10%) > SO4 (5%) > NO3 and NH4 (3% each) whereas that for PM10 is HCO3 (23%) > Ca (19%) > Na (18%) > Mg (16%) > Cl (8%) > SO4 (7%) > NH4 (4%) > NO3 (3%). Similarly, the decreasing order of percentage wise dominance in the wet season for PM2.5 is HCO3 (28%) > Ca(19%) > Na (18%) > Mg (17%) > Cl (9%) > SO4 (4% ) > NO3 and NH4 (2% each) whereas that for PM10 is HCO3 (26%) > Ca (20%) > Na (18%) > Mg (17%) > Cl (9%) > SO4 (4%) > NH4 (3%) > NO3 (2%). In fact, the crustal sources are more predominant than marine sources as Na at Solapur is found to be primarily from crustal source as observed from the Cl/Na ratio and enrichment of Na from crust with Ca as reference for crustal source (these aspects are discussed in more detail in the Section 3.4). Being a site in the arid zone and having very scanty rainfall due to its location in the drought prone rain shadow region (leeward side of the Western Ghat mountainous ranges in Peninsular India); the aerosol chemistry at Solapur shows a typical feature indicative of enrichment from the highly alkaline ionic components mainly of crustal origin, irrespective of the season of sampling and the size of aerosols. Calcium and magnesium carbonates are reported to be the major components of soil in this region that occur in the form of calcite (CaCO3) and dolomite (CaMg (CO3)2) rocks. The high concentration of HCO3 (derived from pH as explained in Eq. (2) in Section 2.2) in both PM2.5 and PM10 in dry and wet periods corroborate this assumption. Mulani et al. (2019) have reported more calcareous nature of soil with pH values ranging from 6.9 to 8.1 in the Solapur district whereas Das et al. (2005) have reported high pH values for river water of Bhima and its tributaries in this region with values ranging from 7.4 to 9.2. Das and Krishnaswami (2007) have reported significant abundances of Na, Ca, Mg and K in the river sediments from the Deccan Trap with high CaCO3 in the basins of Krishna and Bhima rivers and Nira (tributary of Bhima) that flow in the Solapur region and which are supersaturated with calcite. It can be assumed that these elements released from the river basins could be responsible for the formation of the origin of particulate matter suspended over this region. Bhargava and Bhattacharjee (1982) have reported occurrences of saline and alkaline soils in the Deccan Plateau which contain more amounts of Na, Cl, Mg and SO4. It is reported by several studies that the mineral dust aerosols have the potential to act as cloud condensation nuclei (CCN) and/or ice nuclei (IN) (Koehler et al. 2009; Yamashita et al. 2011) and thereby contribute towards cloud microphysical properties and precipitation mechanism (Twomey, 1977; Rosenfeld et al. 2008). This feature has important implications especially, over the rain shadow area in the present study location in semi-arid region of the peninsular India, where mineral dust aerosols have been observed to be in abundance.
From the SO4 / NO3 ratio, it was observed that SO4 was dominant in both PM2.5 (1.8 and 2.8 in dry and wet periods, respectively) and PM10 (2.8 and 3.3 in dry and wet periods, respectively) indicating towards more impact of stationary sources (industrial/domestic emissions) than those from the mobile (vehicular emissions) on the aerosol acidic components. This assumption is used by many researchers in their interpretation of pollutant sources (Xu et al. 2015; Tripathee et al. 2016; Gawhane et al. 2017; Keresztesi et al. 2019). The normal practice of temporary closure of certain industries in the wet period such as sugar and textile manufacturing factories which are the major sources for many of these aerosols such as SO4, NO3, (sugar and textile factories) Cl (textile factories); could have resulted in their comparatively less concentrations in the wet period.
The degrees of oxidation of SO2 to SO4 and NO2 to NO3 are represented as sulfur oxidation ratio (SOR) and nitrogen oxidation ratio (NOR), respectively. Daily mean concentrations of SO2 and NO2 at Solapur were obtained for the dry and wet periods for the period of Apr 2017 to Mar 2020 from the central pollution control board of India (app.cpcbccr.com/ccr/#/caaqm-dashboard-all/caaqm-landing) and these values have been used to derive SOR and NOR using the corresponding days data for SO4 and NO3 of PM2.5 and PM10 samples at Solapur. The SOR and NOR values have been derived using the following equations (Jiang et al. 2019):
Higher SOR and NOR for PM2.5 than for PM10 in both dry and wet periods clearly indicates towards more oxidation of gaseous SO2 and NO2 in the atmosphere to SO4 and NO3, respectively for fine size aerosols across all the seasons. Further, the SOR and NOR values were observed to be always more during the dry period than during wet period which infers to the possible negative correlation between relative humidity (RH) with SOR and NOR. The mean RH at Solapur during the dry period is about 40% whereas that during the wet period is around 65%. For PM2.5, the ratio SORdry / SORwet was higher (2.7) than the ratio NORdry / NORwet (1.2). However, in case of PM10, the ratio SORdry / SORwet was equal to the ratio NORdry / NORwet (1.3 in both). The mean concentration of ozone was higher in the dry period (53.1 ± 21.2 µg/m3) than in the wet period (40.1 ± 15.9 µg/m3). Higher ozone facilitates more oxidation of SO2 to SO4 and NO2 to NO3 during the dry period (Jiang et al. 2019). The overall effect of reduced RH, higher ambient temperatures and increased ozone concentrations together along with the higher concentrations of the precursor gases in the dry period than in the wet period might have resulted in higher concentrations of SO4 and NO3 in the dry period through the enhanced oxidation rates of their respective precursor gases. This is clearly visible from the ratio values of (SO4)dry / (SO4)wet for PM2.5 (1.13) and PM10 (1.35). Similarly, the ratio values of (NO3)dry / (NO3)wet were 1.40 and 1.62 for PM2.5 and PM10, respectively. Apart from the anthropogenically originated secondary aerosols (SO4 and NO3), the combustion generated absorbing type carbonaceous particles termed as black carbon (BC) aerosols which were monitored at Solapur using the next generation Aethalometer (Aerosol d.o.o., AE-33) also showed significant enhancement during the dry period (3.7 ± 1.9 µg / m3) than in the wet period (1.0 ± 0.15 µg / m3) as reported by Soyam et al. (2021). However, the increased concentrations of the acidic components SO4 and NO3 did not culminate into the lowering of pH values during the dry period for both PM2.5 and PM10 that could be mainly attributed to the significantly high concentrations of alkaline earth elements Ca, Na and Mg. This feature is discussed in more details in the succeeding section.
3.3 pH, original pH and neutralisation factors
One of the basic and primary indicators for aerosol acidity is the pH of water soluble aerosol extracts. The CO2-equilibrated neutral pH value of 5.65 is widely considered to be the threshold for acidification of aerosols and precipitation though with its own limitations (Safai et al. 2004).
The water soluble extracts of both PM2.5 and PM10 samples were initially analyzed for the pH before subjecting them for their analysis for the ionic composition. Figure 3 depicts the mean pH of PM2.5 and PM10 for the above mentioned four seasons as well as for the dry and wet periods. It is clearly seen that the chemical nature of both fine and coarse aerosols was highly alkaline across all the seasons/periods with mean pH of 7.27 ± 0.24, 7.47 ± 0.10, 7.53 ± 0.04 and 7.48 ± 0.14 for PM2.5 during summer, monsoon, post-monsoon and winter season, respectively while the same values for PM10 were respectively 7.31 ± 0.13, 7.43 ± 0.09, 7.52 ± 0.11 and 7.48 ± 0.05. Overall, the mean pH for dry period was 7.38 ± 0.20 for PM2.5 and 7.42 ± 0.11 for PM10 and during the wet period, it was 7.50 ± 0.08 for PM2.5 and 7.47 ± 0.11 for PM10. Dry period depicted comparatively more acidic samples of PM2.5 (16%) and PM10 (18%) than in wet period for PM2.5 (12%) and PM10 (13%). As already mentioned, the reason for this high alkaline nature of pH of both fine and coarse size aerosols is indicative of the acid neutralizing capacity of the alkaline components i.e. Ca, Na, K and Mg.
The pH measurement shows the acidity of aerosols after neutralization by alkaline components particularly Ca and Mg which show more abundance at Solapur; therefore the concentration of these two components should be added to the H+ ion concentration to get the original acidity of aerosols in the absence these two components which is depicted by the original pH or OpH (Galloway et al. 1987). The mean OpH of PM2.5 at Solapur during the dry and wet period was 3.46 and 3.40, respectively whereas the OpH of PM10 during the dry and wet period was 3.64 and 3.63, respectively. This indicates that the original pH of PM2.5 and PM10 was magnitude wise about four orders more acidic than the measured pH in dry and wet period which clearly infers to the vital role played by Ca and Mg in neutralizing the acidification of aerosols over Solapur. The acidic potential of both PM2.5 and PM10 was neutralized by the alkaline ionic components and the measure of this neutralizing potential is attained by the computation of neutralization factors (NF) for each of the alkaline components (Possanzini et al. 1988) as:
where, X can be any one of the alkaline components such as Ca (nss Ca is not considered as Ca has been assumed to be a reference for the crustal source as explained in the Section 3.4), Nss Mg, Nss K and NH4.
.
The NF values for Ca, NH4, Mg and K are depicted in Fig. 4a. It is observed that Ca has been the dominant neutralizer of aerosol acidity at Solapur with NF values of 2.7 ± 1.2 and 3.7 ± 2.9, respectively in dry and wet period for PM2.5; and 2.4 ± 1.0 and 3.9 ± 1.8, respectively in dry and wet period, for PM10. The next strong neutralizer was Mg for both PM2.5 and PM10 in the dry and wet period. Neutralization factors were comparatively much less for NH4 and K indicating their marginal role in arresting the aerosol acidity. As reported from various studies (Khemani 1989 and references therein; Safai et al. 2004), Ca contributes more to the neutralization of aerosol acidity followed by NH4. In fact, over Pune, an urban metropolis in SW India, it has been reported that NH4 showed more NF value than Ca during post monsoon and winter seasons (Safai et al. 2010). However, as observed from the NF values; Ca and nss Mg were found to be contributing more towards neutralisation of aerosol acidity than NH4 and nss K in both fine and coarse particles and in both dry and wet seasons in the present study over Solapur. The term nss depicts non sea salt fraction which is computed using Cl as reference for marine source as shown in Eq. (8) in Section 3.4.
It is reported that Ca (from local dust or soil source) is mainly responsible for containing the spread of acidification of aerosols and thereby of the rain water over the Indian region (Khemani 1989). In order to assess the balance between acidity and alkalinity; the ratio of neutralization potential (NP) to acidic potential (AP) is computed and the more the ratio value, the more is the alkaline contribution to aerosol composition. Normally, AP is the addition of concentrations of two major acidifying components namely, nss SO4 and NO3 whereas, NP is the addition of concentrations of nssCa and NH4 as these two are the premier alkaline components responsible for countering the aerosol acidity. However in the present study, we have taken the liberty to derive NP as addition of Ca and nss Mg because these two have shown much higher NF values than NH4 and nssK. Therefore NP/AP in the present study has been computed as below:
Figure 4b depicts the mean values of NP / AP for PM2.5 and PM10 in dry and wet periods. The mean value of NP / AP for PM2.5 was 4.7 ± 1.9 and 5.6 ± 2.8, respectively during the dry and wet period. Whereas, the NP/AP ratio was estimated to be 4.5 ± 1.9 and 7.0 ± 3.2 for PM10, respectively in dry and wet period. This feature again emphasizes the major contribution of both the alkaline earth components (Ca and Mg) of PM2.5 and PM10 in the neutralization of aerosol acidity at Solapur. Especially, the neutralization potential is more dominant during the wet period for both PM2.5 and PM10 which is also clearly visible from the higher SOR and NOR values during the dry period than during the wet period as well as higher mean pH values during wet period than during the dry period for both PM2.5 and PM10.
3.4 Marine, Crustal and anthropogenic fractions of PM2.5 and PM10
Generally the widely used method for computation of the non-sea salt (Nss) and sea salt (ss) fractions of different ionic components is by considering Na as the reference for marine source and by using the standard sea water ratios for different ionic components (Keene et al. 1986). The Cl/Na ratio is also one of the widely used tests to assess any depletion or enrichment of Cl with respect to its normal molecular form of sea salt (NaCl) from marine sources. The standard sea water ratio of Cl/Na is 1.8 if concentrations are in mg/L (Keene et al. 1986) and any deviation from this ratio is a measure in terms of Cl depletion or enrichment. Okada et al. (1978) have given an elaborate discussion on Cl deficiency and it is also reported by Khemani et al. (1985) and Chatterjee et al. (2010) in their studies. In the present study, it is found that with respect to the standard sea water ratio, Cl was depleted in dry period (mean Cl/Na ~ 0.93) whereas in wet period, there was more Cl depletion (mean Cl/Na ~ 0.77) for PM2.5. Whereas, in case of PM10, the Cl/Na ratio showed more Cl depletion in dry period (mean Cl/Na ~ 0.68) than during wet period (mean Cl/Na ~ 0.80). Normally, in the absence of sources other than sea for Na, the depletion of Cl is related with the probable reaction of sea salt with HNO3 or H2SO4 (Okada et al. 1978). However, in the present study, we have considered the deviation in Cl/Na ratio from the standard sea water ratio of 1.8 due to the high enrichment of Na instead of Cl depletion due to the considerably high concentrations of Na. Also, to check the feasibility of Cl depletion due to the possible reaction of Cl with acidic components; the ratios of Cl + NO3 + SO4 to Na in both dry and wet periods for both PM2.5 and PM10 were computed and it was observed that these ratios were still less (varying between 0.9 and 1.1) than the standard sea water ratio of 1.8. This indicates that the reaction of sea salt with acidic components was not responsible for the significant deviation from standard sea water ratio and it was not depletion of Cl but rather it was due to the enrichment of Na. Even though, the Cl/Na ratio did not indicate both Cl and Na in sea salt form (NaCl), a good correlation coefficient was observed between Na and Cl (r ~ 0.70, p = 0.005) for both PM2.5 and PM10. But at the same time, a good correlation between Na and Ca (r ~ 0.70, p > 0.001) and Na and Mg (r ~ 0.70, p = 0.002) was also observed indicating that a good correlation merely does not indicate towards similar sources. Considering the possible contribution from other sources for Na (especially dust from the local soil and anthropogenic activity in cement making industries); we have computed the non-sea salt fractions for all the measured ionic components of PM2.5 and PM10 by using Cl as the marine reference, which is the second highest component of sea water (Goldberg 1971). Keene et al. (1986) have given detailed deliberations on the use of different components including Na, Cl, Mg and K as reference markers for marine source. For the selection of Cl as a marine source reference, we have used the rules adopted by Church et al. (1982) and Zhang et al. (2012) as follows:
-
(a)
If the ratios of C1/Na and Mg/Na in the sample ≥ corresponding values of the standard sea-water (C1/Na = 1.8, Mg/Na = 0.23) then Na is the sea-salt tracer ion;
-
(b)
If the ratios of Na/Cl and Mg/Cl in the sample ≥ corresponding values of the standard sea-water (Na/Cl = 0.86, Mg/Cl = 0.20) then Cl is the sea-salt tracer ion;
-
(c)
If the ratios of Na/Mg and Cl/Mg in the sample ≥ corresponding values of the standard sea-water (Na/Mg = 4.40, Cl/Mg = 5.13) then Mg is the sea-salt tracer ion.
In the present study, the Cl/Na and Mg/Na ratios for PM2.5 and PM10 in both the dry and wet periods were less than the standard sea water ratios. Similarly, the Na/Mg and Cl/Mg ratios for PM2.5 and PM10 in both the dry and wet periods were less than the standard sea water ratios. However, the Na/Cl and Mg/Cl ratios for PM2.5 and PM10 in both the dry and wet periods were more than the standard sea water ratios. This clearly infers towards the appropriateness of using Cl as the reference for marine source for the present study. Accordingly, the sea salt fractions (ss) for all the measured ionic components were computed by using Cl as a marine reference by using the formula:
where, ssX is the concentration of ionic species X in the aerosol sample originating from sea salts. [Cl]aerosol is the concentration of Cl in the aerosol sample.
The non- sea salt fraction (NssX) was the total concentration of ionic species X minus sea salt fraction of X (ssX) as given below.
After computation of sea salt fraction, crustal fraction was separated from remaining non sea salt fraction of the aerosol sample. For the crustal source, Ca is used as reference (normally, Al, Fe or Si are used as reference for crustal source however these components of PM2.5 and PM10 were not measured in the present study). Ca correlated well with Na (r ~ 0.70, p > 0.001) and Mg (r ~ 0.85, p > 0.0001). However, HCO3 showed comparatively less correlation with Ca (r = 0.27, p = 0.08) which might be due to possible formation of bicarbonates of aluminum or silicon or iron which could not be ascertained due to the non- measurement of Al, Si and Fe in the present study. Crustal fractions were assessed with Ca as tracer of crust-originated element in some studies for atmospheric deposition (Lammel et al. 2003; Lu et al. 2011). The crustal fraction was estimated using Ca as the crust-originated tracer using the following equation:
where CrX is the crust originated concentration of constituent X in the aerosol sample. [Ca]aerosol is the concentration of Ca in aerosol sample. (X / Ca) crust is derived from the crustal composition data (Horn and Adams 1966). The non-crustal fraction of X (NcrX) was computed in the similar manner as mentioned earlier for Nss X. Further, we have checked the enrichment factors (EF) for Cl and Na with Ca as a reference for crustal source and found that EF values for Na were significantly less (~1.1) as compared to that for Cl (≥ 100) which is clearly indicative of non-enrichment of Na (i.e. Na mainly from crustal source) and substantial enrichment of Cl (i.e. Cl mainly from non-crustal or presumably from the marine source). This again justifies our selection of Cl as reference for marine source instead of Na.
After computations of marine and crustal fractions for each ionic component of PM2.5 and PM10, the remaining fraction is assumed to be from the anthropogenic sources (Safai et al. 2010).
As seen from Fig. 5, significant contribution from anthropogenic source was observed for secondary aerosols i.e. SO4, NO3 and NH4 for both PM2.5 and PM10 during the dry and wet period. For SO4, anthropogenic sources contributed about 70 to 80% whereas for NO3 and NH4, their contribution was from 94 to 99%. Crustal source was more predominant for K with about 85 to 89% contribution whereas marine source contributed about 11 to 17% for both PM2.5 and PM10 during dry and wet period. Surprisingly, anthropogenic (generally combustion related activities) sources showed very marginal contribution for K. For Mg, major contribution was from crustal source (about 55 to 60%) followed by anthropogenic sources (about 30 to 35%) and marine source showed only about 11% contribution for Mg. However, the more interesting thing was the significant contribution of crustal source for Na (40 to 60%) for PM2.5 and PM10 during both the dry and wet periods with marine sources showing about 40 to 45% contribution. This is contrary to the normal feature observed over other parts of the Indian region wherein the marine source contributes almost entirely for Na and therefore making it the first choice for considering it as a reference element for this source. The significant contribution from the crustal source observed for Na indicates the most possible impact of emissions from the cement industries around this region as well as the important composition of the local soil. Higher contribution from the marine sources (around 20%) was observed for SO4 especially in PM2.5 during both dry and wet periods; however, in PM10 it varied from 13% in dry period to 19% in wet period. Several studies have reported marine emissions (through sea spray consisting dimethyl sulphide emissions) as an important source for atmospheric SO4 (Khemani et al. 1985; Safai et al. 2010).
3.5 Cluster and CWT analysis
At any location, existence of aerosols depends upon the local as well as distant sources. The possible long range transport of PM2.5 and PM10 from the distant sources was assessed by performing the cluster analysis during dry and wet periods utilizing data obtained from the Hybrid Single Particle Lagrangian Integrated Trajectory (HYSPLIT) back trajectories. Five day back trajectories ending at the observation site at a height of 100 m above the ground level (AGL) were extracted with hourly resolution by using the HYSPLIT model (Draxler and Rolph 2003). In this analysis, the optimum number of clusters is chosen on the basis of Total Spatial Variance i.e. TSV (Tang et al. 2015; Petit et al. 2017). The maximum percentage change in the TSV was used to identify the optimum cluster number which was retained for the final representation of cluster trajectories. For the present study, maximum change was observed below three clusters hence three cluster solution was opted for the cluster analysis. The details of this analysis are given elsewhere (Petit et al. 2017; Mukherjee et al. 2020). Figure 6a shows the origin and pathways traveled for different clusters for PM2.5 and PM10 during the dry period and Fig. 6b shows the statistical details for PM2.5 and PM10 concentrations during the dry period. Similarly, Fig. 7a, b show the same for PM2.5 and PM10 during the wet period.
Three clusters were estimated to dominate during the dry period. Cluster 1 originated from north of Solapur starting in the Vidarbha region and travelled over Wardha and other districts before reaching Solapur and contributed towards about 34% and 33.5% of the back trajectories and accounted for 49 µg/m3 and 96 µg/m3 mean mass concentration for PM2.5 and PM10, respectively. Cluster 2 originated from the south east of Solapur starting from the coastal regions near Visakhapatnam and passed over Andhra Pradesh before reaching Solapur and contributed to about 33% and 34% of the back trajectories and accounted for 55 µg/m3 and 100 µg/m3 mean mass concentration for PM2.5 and PM1o, respectively. Cluster 3 originated from north west of Solapur starting from the Arabian Sea and passed over the metro cities of Mumbai and Pune before reaching Solapur and it contributed to about 33% of the back trajectories and accounted for 36 µg/m3 and 94 µg/m3 mean mass concentration for PM2.5 and PM10, respectively. Two out of three clusters showed air mass trajectories from continental regions (which contributed about 67% of the observed air mass trajectories for both PM2.5 and PM10) wherein the anthropogenic sources are located majorly whereas, one cluster showed air mass trajectories originating from marine region (Arabian Sea) but this too passed over the continental part covering metro cities and contributed about 33% of the observed air mass trajectories. The contribution of coarse (PM10) particles could be attributed to the incursion of dust from continental regions and sea salt from marine region (both generally in coarse size). Different types of anthropogenic activities leading to the formation of secondary aerosols that are generally formed from their precursor gases through the gas to particle conversion mechanism could contribute to the fine (PM2.5) particle formations.
Three clusters were obtained for the wet period for PM2.5, PM10. Cluster 1 originated from north-west of Solapur starting from the Arabian Sea and passed over the metro cities of Mumbai and Pune before reaching Solapur and contributed to about 17% and 16% of the back trajectories and accounted for 25 µg/m3 and 61 µg/m3 mean mass concentration for PM2.5 and PM10, respectively. Cluster 2 originated from south west of Solapur starting from the Arabian Sea and passed over parts of the Konkan region in coastal Maharashtra before reaching Solapur and contributed to about 49% and 50% of the back trajectories and accounted for 30 µg/m3 and 52 µg/m3 mean mass concentration for PM2.5 and PM10, respectively. Cluster 3 originated from north east of Solapur starting from the Chhattisgarh region and then travelled across parts of the Telangana region before reaching the sampling site and contributed to about 35% and 34% of the back trajectories and accounted for 46 µg/m3 and 70 µg/m3 mean mass concentration for PM2.5 and PM10, respectively.
The Concentration weighted trajectory (CWT) analysis (Ashbaugh et al. 1985; Stohl et al. 1996) was also undertaken for both dry and wet periods to mark the potential sources for PM2.5 and PM10 utilizing the earlier mentioned 5 day HYSPLIT back trajectories dataset. In CWT analysis, observed mass concentrations of PM2.5, PM10 are distributed along with the trajectories depending on the number and the corresponding residence time of trajectories in a particular grid. The CWT analysis was performed using Zefir (Petit et al. 2017) tool. Figure 6c describes the results of the CWT analysis for the dry period and Fig. 7c describes the same for the wet period. The results of the CWT analysis are also complementary to the cluster analysis and infer towards possible impact of distant sources, apart from that of surrounding local sources at Solapur especially more during the dry period. In addition to the distant sources, emissions from the local sources such as thermal power plant and cement, textile and sugar industries share a major fraction in the observed abundance of fine and coarse particles and their ionic components. During the dry period, the notable effect of transported pollutant particles especially from the continental regions of north-east (Chhattisgarh region where major coal mines and power plants are located and parts of Telangana), south-east (Visakhapatnam where shipping industry and steel plant, etc. are located.) and north-west (Mumbai-Pune where different type of industries are situated) was observed on the pH of PM2.5 and PM10. As seen in Fig. 3, mean pH was 7.38 in dry period and 7.50 in wet period for PM2.5 whereas for PM10, it was 7.42 in dry and 7.47 in wet period, respectively. Similarly, mean NP / AP ratio (Fig. 4b) for PM2.5 and PM10 was 4.7 and 4.5 respectively during the dry period and it was 5.6 and 7.0, respectively for PM2.5 and PM10 during the wet period indicating more acidic fraction in both fine and coarse aerosols during the dry period. The possible effect of long range transport of dust (from Gulf Coast) and sea salt particles (from Arabian Sea) along with some contribution from the Mumbai-Pune industrial belt was visible during the wet period. The impact of local tourist and residential/agricultural burning activities might be comparatively less during the wet period as compared to the dry period.
4 Conclusions
A three yearlong study was conducted on the chemical composition of fine (PM2.5) and coarse (PM10) aerosols at a semi-arid rain shadow location, Solapur in the peninsular India. The major highlights of the study are as follows:
-
1.
High mass concentrations of both fine and coarse aerosols in the dry period with dominance of crustal components (Ca, Mg, k and Na) from local dust in both dry and wet periods leading to the alkaline (pH ≥ 7) nature of aerosols.
-
2.
Sodium originated from both the crustal and marine sources in almost equal amounts thereby making Cl as a reference for marine source in the computation of sea salt fractions of other measured components.
-
3.
SO4 showed comparatively more concentration than NO3 inferring to more impact of emissions from stationary sources such as domestic and industrial activities than mobile ones such as vehicular exhaust.
-
4.
The original pH of fine and coarse aerosols (in absence of Ca and Mg) was about four orders more acidic than the measured pH in dry and wet period which indicates to the important role of Ca and Mg in neutralizing the aerosol acidity.
-
5.
The cluster and CWT analysis suggested possible important role of transported aerosols from distant source regions in both dry and wet periods with about 67% contribution from inland continental sources in dry period and near equal contribution from inland as well as marine sources in wet period.
-
6.
Considering the rich abundance of mineral dust aerosols observed over Solapur and their potential role as CCN/ and/or IN in the cloud formation; the results obtained from this study will be useful for the on-going and future observational as well as modeling initiatives towards the understanding of the cloud aerosol interaction and thereby for the precipitation enhancement experiments in India.
Data Availability
Data used in the present study can be obtained by making a request through http://www.tropmet.res.in/~caipeex/ registration form.php or contacting thara@tropmet.res.in.
References
Ashbaugh, L.L., Malm, W.C., Sadeh, W.Z.: A residence time probability analysis of sulfur concentrations at grand Canyon National Park. Atmos. Environ. 19, 1263–1270 (1985). https://doi.org/10.1016/0004-6981(85)90256-2
Bhargava, G.P., Bhattacharjee, J.C.: Morphology, genesis and classification of salt affected soils. Rev. Soil Res. India, Part II. 508–528 (1982)
Bhaskar, V.V., Rao, P.S.P.: Annual and decadal variation in chemical composition of rain water at all the ten GAW stations in India. J. Atmos. Chem. 74, 23–53 (2017). https://doi.org/10.1007/s10874-016-9339-3
Buchunde, P.S., Safai, P.D., Mukherjee, S., Raju, M.P., Meena, G.S., Sonbawne, S.M., Dani, K.K., Pandithurai, G.: Seasonal abundances of primary and secondary carbonaceous aerosols at a high-altitude station in the western Ghat Mountains, India. Air Qual. Atmos. Heal. 15, 209–220 (2022). https://doi.org/10.1007/s11869-021-01097-5
Budhavant, K.B., Rao, P.S.P., Safai, P.D., Leck, C., Rodhe, H.: Black carbon in cloud-water and rain water during monsoon season at a high altitude station in India. Atmos. Environ 129, 256–264 (2016). https://doi.org/10.1016/J.ATMOSENV.2016.01.028
Chatterjee, A., Adak, A., Singh, A.K., Srivastava, M.K., Ghosh, S.K., Tiwari, S., Devara, P.C.S., Raha, S.: Aerosol Chemistry over a high Altitude Station at northeastern Himalayas, India. PLoS One 5, e11122 (2010). https://doi.org/10.1371/journal.pone.0011122
Church, T.M., Galloway, J.N., Jickells, T.D., Knap, A.H.: The chemistry of western Atlantic precipitation at the mid-atlantic coast and Bermuda. J. Geophys. Res. 87, 11013–11018 (1982). https://doi.org/10.1029/JC087iC13p11013
Das, A., Krishnaswami, S.: Elemental geochemistry of river sediments from the Deccan Traps, India: Implications to sources of elements and their mobility during basalt–water interaction. Chem. Geol. 242, 232–254 (2007). https://doi.org/10.1016/j.chemgeo.2007.03.023
Das, A., Krishnaswami, S., Sarin, M.M., Pande, K.: Chemical weathering in the Krishna Basin and Western Ghats of the Deccan Traps, India: Rates of basalt weathering and their controls. Geochim. Cosmochim. Acta 69, 2067–2084 (2005). https://doi.org/10.1016/J.GCA.2004.10.014
Draxler, R.R., Rolph, G.D.: HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Silver Spring, MD Model. HYSPLIT.php. (2003). http://ready.arl.noaa.gov/
Galloway, J.N., Dianwu, Z., Jiling, X., Likens, G.E.: Acid rain: China, United States, and a remote area. Science 80(236), 1559–1562 (1987). https://doi.org/10.1126/science.236.4808.1559
Gawhane, R.D., Rao, P.S.P., Budhavant, K.B., Waghmare, V., Meshram, D.C., Safai, P.D.: Seasonal variation of chemical composition and source apportionment of PM2.5 in Pune, India. Environ. Sci. Pollut Res 24, 21065–21072 (2017). https://doi.org/10.1007/s11356-017-9761-3
Goldberg, E.D., Broecker, W.S., Gross, M.G., Turekian, K.K.: Radioactivity in the Marine Environment. National Academies Press, Washington, D.C (1971)
Granat, L.: On the relation between pH and the chemical composition in atmospheric precipitation. Tellus A Dyn. Meteorol. Oceanogr. 24, 550 (1972). https://doi.org/10.3402/tellusa.v24i6.10682
Horn, M.K., Adams, J.A.S.: Computer-derived geochemical balances and element abundances. Geochim. Cosmochim. Acta. 30, 279–297 (1966). https://doi.org/10.1016/0016-7037(66)90003-2
IPCC.: Climate Change : The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.(2021). https://doi.org/10.1017/9781009157896
IPCC.: Climate Change 2014: Working Group III Contribution to the IPCC Fifth Assessment Report. Cambridge: Cambridge University Press (2014). https://doi.org/10.1017/CBO9781107415416
Jiang, F., Liu, F., Lin, Q., Fu, Y., Yang, Y., Peng, L., Lian, X., Zhang, G., Bi, X., Wang, X., Sheng, G.: Characteristics and formation mechanisms of sulfate and nitrate in size-segregated Atmospheric particles from Urban Guangzhou, China. Aerosol Air Qual. Res 19, 1284–1293 (2019). https://doi.org/10.4209/aaqr.2018.07.0251
Keene, W.C., Pszenny, A.A.P., Galloway, J.N., Hawley, M.E.: Sea-salt corrections and interpretation of constituent ratios in marine precipitation. J. Geophys. Res. 91, 6647 (1986). https://doi.org/10.1029/JD091iD06p06647
Keresztesi, Ã., Birsan, M.V., Nita, I.A., Bodor, Z., Szép, R.: Assessing the neutralisation, wet deposition and source contributions of the precipitation chemistry over Europe during 2000–2017. Environ. Sci. Eur. 31, 1–15 (2019). https://doi.org/10.1186/s12302-019-0234-9
Khemani, L.T.: Physical and chemical characteristics of atmospheric aerosol in air pollution control 2. In: Cheremisinoff, P.N. (eds.) Encyclopaedia of Environmental Control Techniques, pp. 401–452. Gulf Publishing U.S.A. (1989)
Khemani, L.T., Momin, G.A., Naik, M., Prakasa Rao, P.S., Kumar, R., Murty, R.: Impact of alkaline particulates on pH of rain water in India. Water Air Soil Pollut. 25, (1985). https://doi.org/10.1007/BF00283789
Koehler, K.A., Kreidenweis, S.M., DeMott, P.J., Petters, M.D., Prenni, A.J., Carrico, C.M.: Hygroscopicity and cloud droplet activation of mineral dust aerosol. Geophys. Res. Lett. 36, 1–5 (2009). https://doi.org/10.1029/2009GL037348
Kulshrestha, U.C., Kulshrestha, M.J., Sekar, R., Sastry, G.S.R., Vairamani, M.: Chemical characteristics of rainwater at an urban site of south-central India. Atmos. Environ. 37, 3019–3026 (2003). https://doi.org/10.1016/S1352-2310(03)00266-8
Kumar, K.R., Narasimhulu, K., Balakrishnaiah, G., Reddy, B.S.K., Gopal, K.R., Reddy, R.R., Satheesh, S.K., Moorthy, K.K., Babu, S.S.: Characterization of aerosol black carbon over a tropical semi-arid region of Anantapur, India. Atmos. Res. 100, 12–27 (2011). https://doi.org/10.1016/j.atmosres.2010.12.009
Lammel, G., Brüggemann, E., Gnauk, T., Müller, K., Neusüss, C., Röhrl, A.: A new method to study aerosol source contributions along the tracks of air parcels and its application to the near-ground level aerosol chemical composition in central Europe. J. Aerosol Sci. 34, 1–25 (2003). https://doi.org/10.1016/S0021-8502(02)00134-9
Lu, X., Li, L.Y., Li, N., Yang, G., Luo, D., Chen, J.: Chemical characteristics of spring rainwater of Xi’an city. NW China. Atmos. Environ. 45, 5058–5063 (2011). https://doi.org/10.1016/j.atmosenv.2011.06.026
Mukherjee, S., Singla, V., Meena, G.S., Aslam, M.Y., Safai, P.D., Buchunde, P., Vasudevan, A.K., Jena, C.K., Ghude, S.D., Dani, K., Pandithurai, G.: Sub micron aerosol variability and its ageing process at a high altitude site in India: Impact of meteorological conditions. Environ. Pollut. 265, 115019 (2020). https://doi.org/10.1016/j.envpol.2020.115019
Mukherjee, S., Singla, V., Pandithurai, G., Safai, P.D., Meena, G.S., Dani, K.K., Kumar, A.: Seasonal variability in chemical composition and source apportionment of sub-micron aerosol over a high altitude site in western ghats, India. Atmos. Environ. 180, 79–92 (2018). https://doi.org/10.1016/j.atmosenv.2018.02.048
Mulani, R.D., Gaikwad, G.A., Bhosale, B.D., Upadhay, V.B.: Study of Micronutrient Status in Soil of Solapur District. Int. J. Agric. Environ. Biotechnol. 12, (2019). https://doi.org/10.30954/0974-1712.06.2019.8.
Okada, K., Ishizaka, Y., Masuzawa, T., Isono, K.: Chlorine deficiency in coastal aerosols. J. Meteorol. Soc. Japan Ser. II 56, 501–507 (1978). https://doi.org/10.2151/jmsj1965.56.5_501
Pan, X., Chin, M., Gautam, R., Bian, H., Kim, D., Colarco, P.R., Diehl, T.L., Takemura, T., Pozzoli, L., Tsigaridis, K., Bauer, S., Bellouin, N.: A multi-model evaluation of aerosols over South Asia: Common problems and possible causes. Atmos. Chem. Phys. 15, 5903–5928 (2015). https://doi.org/10.5194/acp-15-5903-2015
Petit, J.-E., Favez, O., Albinet, A., Canonaco, F.: A user-friendly tool for comprehensive evaluation of the geographical origins of atmospheric pollution: Wind and trajectory analyses. Environ. Model. Softw. 88, 183–187 (2017). https://doi.org/10.1016/j.envsoft.2016.11.022
Pipal, A.S., Kulshrestha, A., Taneja, A.: Characterization and morphological analysis of airborne PM2.5 and PM10 in Agra located in north central India. Atmos. Environ. 45, 3621–3630 (2011). https://doi.org/10.1016/j.atmosenv.2011.03.062
Possanzini, M., Buttini, P., Di Palo, V.: Characterization of a rural area in terms of dry and wet deposition. Sci. Total Environ. 74, 111–120 (1988). https://doi.org/10.1016/0048-9697(88)90132-5
Rao, P.S.P., Tiwari, S., Matwale, J.L., Pervez, S., Tunved, P., Safai, P.D., Srivastava, A.K., Bisht, D.S., Singh, S., Hopke, P.K.: Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos. Environ. 146, 90–99 (2016). https://doi.org/10.1016/j.atmosenv.2016.06.069
Reddy, R.R., Gopal, K.R., Narasimhulu, K., Reddy, L.S.S., Kumar, K.R.: Aerosol size distribution variation in Anantapur (14.62°N, 77.65°E) Semi Arid Zone and its impact on Aerosol Effective Radius. Aerosol Air Qual. Res. 7, 550–562 (2007). https://doi.org/10.4209/aaqr.2007.01.0004
Rosenfeld, D., Lohmann, U., Raga, G.B., O’Dowd, C.D., Kulmala, M., Fuzzi, S., Reissell, A., Andreae, M.O.: Flood or drought: How do aerosols affect precipitation? Science. 321, 1309–1313 (2008). https://doi.org/10.1126/science.1160606
Safai, P.D., Budhavant, K.B., Rao, P.S.P., Ali, K., Sinha, A.: Source characterization for aerosol constituents and changing roles of calcium and ammonium aerosols in the neutralization of aerosol acidity at a semi-urban site in SW India. Atmos. Res. 98, 78–88 (2010). https://doi.org/10.1016/j.atmosres.2010.05.011
Safai, P.D., Rao, P.S., Momin, G.A., Ali, K., Chate, D.M., Praveen, P.S.: Some observations on the characteristics of aerosols at traffic junctions in Pune city, Indian. J. Radio Sp. Phys. 33, 260–266 (2004)
Soyam, P.S., Safai, P.D., Mukherjee, S., Todekar, K., Bankar, S., Gurnule, D., Malap, N., Prabhakaran, T.: Black carbon aerosols over a semi-arid rain shadow location in Peninsular India: Temporal variability and sources. J. Earth Syst. Sci. 130, 95 (2021). https://doi.org/10.1007/s12040-021-01610-5
Stohl, A.: Trajectory statistics-A new method to establish source-receptor relationships of air pollutants and its application to the transport of particulate sulfate in Europe. Atmos. Environ. 30, 579–587 (1996). https://doi.org/10.1016/1352-2310(95)00314-2
Tang, Y., Pang, H., Zhang, W., Li, Y., Wu, S., Hou, S.: Effects of changes in moisture source and the upstream rainout on stable isotopes in precipitation -a case study in Nanjing, eastern China. Hydrol. Earth Syst. Sci. 19, 4293–4306 (2015). https://doi.org/10.5194/hess-19-4293-2015
Tripathee, L., Kang, S., Rupakheti, D., Zhang, Q., Huang, J., Sillanpää, M.: Water-soluble ionic composition of aerosols at urban location in the foothills of Himalaya, Pokhara Valley, Nepal. Atmos. (Basel) 7, (2016). https://doi.org/10.3390/atmos7080102
Twomey, S.: The influence of Pollution on the Shortwave Albedo of Clouds. J. Atmos. Sci. 34(7), 1149–1152 (1977). https://doi.org/10.1175/1520-0469(1977)034%3C1149:tiopot%3E2.0.co;2
WMO GAW TD no. 1251.: Manual for the GAW precipitation chemistry programme- guidelines, data quality objectives and standard operating procedures. In: Allan M.A. (ed.) Prepared by GAW precipitation chemistry science advisory group (2004)
Xu, Z., Wu, Y., Liu, W.J., Liang, C.S., Ji, J., Zhao, T., Zhang, X.: Chemical composition of rainwater and the acid neutralizing effect at Beijing and Chizhou city. China Atmos. Res. 164–165, 278–285 (2015). https://doi.org/10.1016/j.atmosres.2015.05.009
Yamashita, K., Murakami, M., Hashimoto, A., Tajiri, T.: CCN ability of asian mineral dust particles and their effects on cloud droplet formation. J. Meteorol. Soc. Japan 89, 581–587 (2011). https://doi.org/10.2151/jmsj.2011-512
Yang, L., Pandithurai, G., Chate, D.M., Rao, P.S.P., Waghmare, V., Iyer, U.: Evidence of precedent wind role on controlling PM1 wet scavenging of aerosols during monsoon rain events. Atmos. Environ. 201, 265–277 (2019). https://doi.org/10.1016/j.atmosenv.2018.12.041
Zhang, N., He, Y., Cao, J., Ho, K., Shen, Z.: Long-term trends in chemical composition of precipitation at Lijiang, southeast Tibetan Plateau, southwestern China. Atmos. Res. 106, 50–60 (2012). https://doi.org/10.1016/j.atmosres.2011.11.006
Acknowledgements
The CAIPEEX project in IITM is funded by the Ministry of Earth Sciences (MoES), Government of India. We are thankful to the Director, IITM and Principal of N. B. Navale Sinhgad College of Engineering for their support, guidance and encouragement during the course of this study. Thanks are also due to Dr. A.K. Srivastava and Dr. D.S. Bisht from IITM, New Delhi for their valuable support in the logistic arrangements for sampling of aerosols at Solapur.
Author information
Authors and Affiliations
Contributions
P.S. Soyam: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing - original draft. P. D. Safai: Conceptualization, Investigation, Validation, Writing – review and editing. S. Mukherjee: Review- draft, data analysis. S. Kondle: Data Curation. S. Bankar: Data Curation. K. Todekar: Data Curation. N. Malap: Data curation. D. Surendran: Formal Analysis and logistic support. A. Gaikwad: Formal Analysis. S. Lohogaonkar: Formal Analysis. Thara V. Prabha: Conceptualization, Writing - review and editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soyam, P.S., Safai, P.D., Mukherjee, S. et al. Significant abundances of alkaline components in the fine and coarse aerosols over a tropical rain shadow location in peninsular India. J Atmos Chem 80, 191–209 (2023). https://doi.org/10.1007/s10874-023-09447-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-023-09447-6