Abstract
Measurements of fine (PM2.5) and inhalable (PM10) particles were collected at the Alaknanda Valley in the heart of Garhwal region during December 2015–December 2016. Collected samples of PMs were analyzed for major chemical species as anions (F−, Cl−, SO42−, NO2− and NO3−) and cations (Na+, NH4+, K+, Ca2+ and Mg2+) by ion chromatography. During the study period, the concentrations of PM2.5 and PM10 were 78.7 ± 25.1 and 111.8 ± 23.4 μg m−3, respectively. The measured chemical species were found a large variability in different seasons due to the impact of emissions and meteorological parameters. The measured water-soluble (WS) chemical species of PM2.5 and PM10 were 43% (33.7 μg m−3) and 57% (64.2 μg m−3), respectively, with the highest concentrations during the winter season followed by summer, monsoon and post-monsoon seasons. A significant positive correlation was observed between NH4+ and other species (Cl−, SO42− and NO3−) in both PM2.5 and PM10, which indicates its presence in the atmosphere as ammonium salts. As a result, NH4+ play a crucial role in neutralization of acidic species in the winter season; however, the contrary feature was observed in the summer season where Ca2+ was responsible for the main neutralizing species, mainly transported from the inland region during the summer. Principal component analysis shows that secondary aerosol, biomass burning, and soil-driven dust were the possible sources of the measured species over the station. Further, air mass back trajectory analyses indicate that the highest mass concentrations of PMs and WS chemical species were in the post-monsoon season when the air masses pass from eastern Pakistan and western part of India over receptor site. In the winter season, the concentrations of PMs and WS species were highest (second) when the air masses transported from a long distance up to Southern Afghanistan covering with Pakistan and western part of India. In overall, the study suggests the long-range transport of pollutants plays a crucial role in the enhancement of PMs over Alaknanda valley region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The atmospheric aerosol is linked to poor air quality, adverse health effects and heat balance of the Earth directly through absorbing/scattering the solar radiation, indirectly by influencing cloud microphysics and possibly by changing the heterogeneous chemistry of reactive greenhouse gases (IPCC 2007). The enhanced pollutant emissions associated with the fast-growing economies of Southeastern Asian countries have led to the progressive increase of aerosol concentrations above the natural background (Ram et al. 2008; 2010; Chatterjee et al. 2010). Aerosol-enriched boundary layer air can be transported to the higher altitudes by valley breeze processes on the Himalayan slopes, which is a matter of concern as glaciers are noted to retreat over the Himalayan sites (Mayewski and Jeschke 1979; Ashish et al. 2006; Gautam et al. 2013). Tropospheric concentrations of atmospheric aerosol particles increased significantly over the last 150 years (Lavanchy et al. 1999), and continue to increase further in developing countries due to urbanization and industrialization. The direct and indirect effects of aerosols depend on size distribution and chemical composition of atmospheric aerosols (Andreae 1995; Jung et al. 2018). Apart from the size of aerosols, assessment of their chemical composition as a function of time is of crucial importance to understand atmospheric processes such as radiative transfer, cloud droplet nucleating ability, acidification, precipitation chemistry and dry deposition (Tiwari et al. 2009).
The rapid growth of industries, vehicles, population, and anthropogenic activities resulted in higher concentrations of fine mode particles in the megacities of most of the rapidly developing countries in Asia (Menon et al. 2002; Akimoto, 2003; Tiwari et al. 2009; Bisht et al. 2015a; Kishore et al. 2018). Numerous studies have been directed to understand the climatic effects of atmospheric aerosols (Hobbs et al. 1997; Penner et al. 2001 and references therein). Many studies have indicated that particulates in the accumulation mode (d < 2.5 µm) are the most critical to human health, air quality degradation, etc. (Pope et al. 1995; Dholakia et al. 2014; Bisht et al. 2015a). Till date, no studies were made for fine particulates (PM2.5: particles less than 2.5 µm) and inhalable particle (PM10: ≤ 10 µm) over the Alaknanda basin, located in the state of Uttarakhand. The present study is the first attempt to take the observations of particulate matters (PMs) and its water-soluble chemical constituents over Alaknanda basin to understand their loading over high altitude region. Very limited studies were conducted over the Himalayas (Ram et al. 2008; 2010; Chatterjee et al. 2010; Srivastava et al. 2015; Kumar and Attri 2016); however, several studies were made over Ganga Basin (Tare et al. 2006; Srivastava et al. 2014; Bisht et al. 2015a) and other parts of India (Safai et al. 2007; Rastogi and Sarin 2006; Pipal et al. 2011; Bisht et al. 2015b). Generally, the chemical composition of these aerosols highly depends on the source regions and variability in meteorological conditions (Gabriel et al. 2002). In addition, it was observed that the contribution of carbonaceous aerosol in total suspended particulates was much higher in the Indian regions (Safai et al. 2007; Ram et al. 2008; Beegum et al. 2009; Tiwari et al. 2013; Bisht et al. 2015a; Sharma et al. 2017). Due to the above-mentioned importance and lack of physical and chemical data of atmospheric aerosols over Himalayan environment, a year-long measurements of PMs (PM2.5 and PM10) and its chemical constituents were made over Srinagar, Garhwal, Uttarakhand along with meteorological parameters (MPs). The obtained data of PMs and MPs were separated on different time scales such as monthly, seasonally and yearly. The impact of meteorological parameters on PMs and its chemical constituents were also studied to quantify the accumulation or dispersion of the measured secondary aerosols in the study region because the basin region has a tendency to accumulate the pollutants by the circulation of air masses. Further, we have tried to study the transportation of measured atmospheric aerosols over the study region using air mass back trajectory analyses.
Experimental Site and Meteorological Conditions
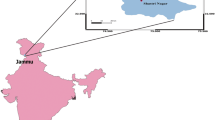
The experimental site is located in the heart of Srinagar city in the premises of Hemwati Nandan Bahuguna Garhwal University (HNBGU) “Chauras campus”, Garhwal (30.22°N 78.78°E, 560 amsl), Uttarakhand (total of 53,483 km2). The whole state is a hilly area and has drastic climate variability, which is totally different from the plain region. The university is 132 km away from Haridwar (nearest inland location) and is on Haridwar–Badrinath Highway in the central Himalayan region. The Srinagar is located on the bank of Alaknanda River which is approximately 560 m above sea level (Fig. 1). The Alaknanda valley is the widest in the Garhwal hills in Central Himalaya. The site has no major industrial activities in and around the city; however, the possibility of transported pollutants can be expected over the study region.
The sampling of PM2.5 (particle size < 2.5 µm) and PM10 aerosols was carried out during the period from December 2015 to December 2016. The average annual rainfall of the state is 1550 mm and temperatures range from 0 to 43 °C (FSI 2009). The forest area of the state is 34,691 km2, which constitutes 64.79% of its geographical area (FSI 2009). Meteorological parameters (MPs) such as wind speed, wind direction, relative humidity, and temperature were simultaneously measured by automatic weather station along with PMs measurement. Figure 2a,b, shows monthly variations in MPs such as temperature, relative humidity, wind speed and wind direction. The month of January was the coldest winter month with an average temperature of 12.5 °C, which rises as the summer approaches and attains maximum during the summer (30.5 °C in June) season. The temperature was almost consistent in the summer and monsoon seasons and commences decreasing thereafter (Fig. 2a). The annual mean temperature was found to be about 22.0 °C during the study period. The relative humidity (RH) was varied from 38% (April) to 83% (August) during the study period. The prevailing winds were mostly from east–west direction. The wind speed was varying from 5.8 km h−1 (December) to 16.56 km h−1 (June).
Instrumentations and Methodology
The sampling of PMs was carried out on the rooftop of the building (~ 10 m above the ground) once in a week using APM-550 (for PM2.5) and APM-460 (for PM10) samplers (M/s Envirotech Pvt. Ltd., India) with a flow rate of one cubic meter per hour. The quartz filter papers were used for PM sampling. Before and after sampling, the filters were kept in a desiccator (24 h) to remove the moisture content of the filter papers. The desiccated filter papers were weighted using electronic microbalance (Model GR202, A&D Company Ltd Japan) with 0.01 mg resolution. The particle concentrations were determined gravimetrically by the difference in their weights before and after the sampling (Bisht et al. 2015b).
One-fourth of sample filters were extracted with ultrapure water (50 ml) via ultrasonication for 60 min, thereafter the liquid samples were filtered through a prewashed Whatman filter No. 41 into pre-cleaned polypropylene bottles. All filtered samples were preserved at 4 °C in a refrigerator. The major anions (F−, CI−, SO42−, NO2− and NO3−) and cations (Na+, NH4+, K+, Ca2+ and Mg2+) were quantitatively determined by ion chromatography (D1ONEX-2000, USA) for measurement of anions and cations using analytical column (Ion Pac-AS15 with micro-membrane suppressor ASRS ultra II 2 mm, 38 mM potassium hydroxide and the IonPac-CS17 column with micro-membrane suppressor CSRS ultra II 2 mm, 6 mM methyl sulfonic acid as eluents and ultrapure water as regenerator) were used, respectively. The cations and anions standards were procured from Dionex for calibration (Tiwari et al. 2009). For quality control, field blanks were also collected during the study period and it was analyzed as samples. In addition, the samples have rechecked the concentrations after three injections in IC during analysis. Collected field blank filters were treated in the same procedures as samples filters during storage, sampling and chemical analysis. The concentrations of chemical constituents were corrected using field blank filters concentrations. Most of the field blank values were found within the detection limits (Bisht et al. 2015b). Chemical analysis procedures were strictly quality controlled to avoid any possible contamination of the samples.
Results and Discussion
Mass Concentrations of PM2.5 and PM10
Figure 3 shows monthly mean mass concentrations of PM2.5 and PM10 along with their respective Indian National Ambient Air Quality Standards (NAAQS). The PM10 concentration was varied between 79.6 µg m−3 (August) and 154.1 µg m−3 (December) with an annual mean of 111.8 (± 23.4) µg m−3 which is about two times higher than its annual standard set by NAAQS (60 µg m−3 for PM10). The higher PM10 levels dust during the summer (pre-monsoon) months may be due to windblown from the adjoining regions together with the thermodynamic conditions in the planetary boundary layer. Li et al. (2017) have found that atmospheric aerosols interact strongly with meteorological parameters over planetary boundary layer region. During the study period, the annual mean PM2.5 concentration was 78.7 (± 25.1) µg m−3 which is about more than two times higher than the annual standard set by NAAQS (40 µg m−3 for PM2.5) and varied between 39.4 µg m−3 (August) and 125.3 µg m−3 (December). It is due to the frequent thermal inversions, and stable atmospheric boundary layer causes stagnation of particulates in the lower atmosphere in valley region (Tripathi et al. 2006; Srivastava et al. 2012; Bisht et al. 2015a). In a recent study, Sen et al. (2017) suggested that the deteriorating air quality over the Indo-Himalayan Range is due to the pollutants undergo long-range transport from their nearby source regions over the Indo-Gangetic Basin (IGB). Tripathi et al. (2006) reported ~ 75% contribution of fine particles to the total aerosol mass at Kanpur over the IGB and significant association with the prevailing foggy conditions in the winter season. However, coarse mode particles (d > 2.5 µm) are generally produced by natural processes such as sea salt aerosols, produced by direct dispersal of ocean water and crustal aerosols, originated from the solid surface. At this point, it is worthy to note that although PMs is a better indicator of total suspended particulate (TSP), it may not necessarily represent a true picture of more hazardous fine particulate. This situation is particularly important in the Indian context, where a significant proportion of PM10 may be due to locally generated windblown dust in the coarse fractions (i.e., PM10) and which was not as harmful as PM2.5 (Sharma and Maloo 2005). In another study, Sharma et al. (2014) observed a mean concentration of PM2.5 about 42 ± 8 µg m−3 over the northwestern Himalayan region, which is relatively lower as compared to the present study and could be due to the consideration of limited period of data during 12–22 March 2013. The coarse fraction (PM10–2.5) was estimated by the differentiating from PM10 and PM2.5 concentrations and its variation are shown in Fig. 3. The monthly mean variation in PM10–2.5 concentrations provides a clear picture of the fraction of coarser aerosol particles over the observation site, which shows significantly higher fractions during the summer months and lower during the winter months. To understand the contributions of PM2.5 in PM10, the ratio of PM2.5/PM10 is estimated and found to be ~ 0.69 ± 0.1 (mean), varying from 0.49 (August) to 0.82 (December). The ratio is much smaller during the monsoon and summer months, indicating relatively less fine particle fraction in PM10 suggesting the dominance of coarser particles due to mineral dust transported from the adjacent desert regions (Srivastava et al. 2012). However, the ratio is much higher during the winter months, indicating relatively large fine particle fractions in PM10. These high fine mode fractions are attributed to low-level inversion and favorable meteorological conditions for accumulation of pollutants in the lower atmosphere in valley region. In addition to this, it is due to anthropogenic emissions such as biomass burning in open fields, domestic fuel in rural settings, emissions from thermal power plants, brick kilns, fossil fuel burning, and vehicular exhausts (Bisht et al. 2015a; Kishore et al. 2018). The ratio of PM2.5/PM10 is found to be comparable at the other Himalayan sites (Sen et al. 2017).
Water-Soluble Ions in PM2.5 and PM10
The monthly mean variability of chemical species in PM2.5 and PM10 was separated during the study period and shown in Fig. 4a–b. In PM2.5, all the species have peaked during the month of December, 2015 and started increasing from September to November 2016, which indicated the influence of anthropogenic sources because of biomass burning from western part of India (Kaskaoutis et al. 2014). On the other hand, enhancement in the mass concentration of PM10 was observed during summer and monsoon months. Large seasonal variability, with large magnitude, is observed in most of these species in PM2.5 and PM10, which is shown in Fig. 5a, b, respectively. In PM2.5, all the species (except NO2) were found to be lower during the summer. However, the concentrations of CI−, SO42−, NO3−, Na+, NH4+, Ca2+ and Mg2+ were found to be highest during the post-monsoon period. However, it was opposite in case of PM10, the concentrations of CI−, SO 24 , Na+ and Ca2+ were found to be highest in the summer period.
The annual mean, standard deviation (± δ), maximum and minimum values of water-soluble ionic species (F−, CI−, SO42−, NO2−, NO3−, Na+, NH4+, K+, Mg2+ and Ca2+) in PM2.5 and PM10 are given in Table 1. Among all the chemical species in PM10, the mean concentration of chloride was highest (12.99 µg m−3) followed by SO42− (mean 11.5 µg m−3) which is the second highest concentration varied from 3.1 to 17.8 µg m−3. However, SO42− concentrations (8.0 ± 4.8) µg m−3 in PM2.5 varied between 0.6 and 17.7 µg m−3. In PM10, the concentration of fluoride (F−) ion was 0.4 µg m−3; however, in PM2.5, it was low concentration (0.03 µg m−3). The ionic abundance, showed in the order of SO42− > Cl− > Na+ > Ca2+ > NH4+ > NO3− > K+ > Mg2+ in PM2.5 and CI− > SO42− > NO3− > Na+ > Ca2+ > K+ > NH4+ > Mg2+ in PM10.
The percentage contributions of each chemical species in both PMs sample are shown in Fig. 6. The measured total water-soluble ionic fraction contributed approximately 43% of the total PM2.5, of which anions and cations account for about 32 and 11%, respectively (Fig. 6a). On the other hand, it was about 57% of the total PM10 with 30 and 27% for anions and cations, respectively (Fig. 6b). The major unanalyzed portion in PM10 (43%) and PM2.5 (56%) samples was due to carbonaceous aerosols such as black carbon and organic carbons, and other insoluble hard metals. However, in PM10, the analyzed ionic species consists of secondary inorganic aerosols (SO42− and NO3−) which contribute approximately 18% of the PM10 mass (19.12 µg m−3) followed by salt aerosols (Na+ and CI− 20% (23.31 µg m−3), and mineral dust (K+, Mg2+ and Ca2+), around 13% (14.97 µg m−3). However, in PM2.5, the secondary inorganic aerosols (SO42− and NO3−) were approximately 14.9% (12 µg m−3), 16% salt aerosols, (12.97 µg m−3) and 9% mineral dust (7 µg m−3). However, the difference in PM10 could be due to soil derived. Parmar et al. (2001) found an equal distribution of sodium in both fine and coarse modes in their study at Agra during the monsoon period because of marine sources. The source for fine mode sodium is largely unknown. Hong and Chak (1997) reported that the combustion of fossil fuel and biomass may be an important source for significant emission of fine mode sodium into the atmosphere during a dry period. The percentage contributions of NO3− were found to be approximately equal in both PM2.5 and PM10 samples. Finlayson-Pitts and Pitts (2000) reported that NO3− can be found in the atmosphere both in the fine and coarse modes with highly variable amounts, wherein the fine particles are generally from urban origin while the larger particles are from marine origin. As the experimental site is far away from the oceanic regions, the possible source of NO3− in coarse mode is to be soil derived (Parmar et al. 2001; Tare et al. 2006), The less variability was seen of ammonium ion in both PM10 and PM2.5 samples in the present study; however, much of the water-soluble ammonium ion (~ 77%) was obtained from fine mode aerosols over Kanpur (Tare et al. 2006).
Air Mass Back trajectory Analysis for Source Apportionment
The air mass back trajectory analysis is an important tool to identify the source region of any pollutants that are associated with different emission sources on the way over the receptor site (Sen et al. 2017). In the present study, analysis of daily 5-day air mass back trajectories was carried out over the sampling site at Srinagar using National Oceanic and Atmospheric Administration (NOAA) Air Resources Laboratory (ARL) Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model (Stein et al. 2015). The back trajectories were calculated on sampling day starting from Srinagar at 12:30 pm local time and at the altitude of 1000 m above ground level during the study period and were separated seasonally (Fig. 7). It was observed that the most of the trajectories were transported from the western side during the winter, summer and post-monsoon seasons except the monsoon season. It is clearly evident that the air masses reaching to the receptor side station during the winter season are from the Gulf regions, and have a relatively longer pathway. In the post-monsoon season, the highest mass concentrations of PMs and WS chemical species were observed when the air masses pass from eastern Pakistan and western part of India over receptor site. In the winter season, the concentrations of PMs and WS species were observed second highest when the air masses transported from a long distance up to Southern Afghanistan covering with Pakistan and western part of India. On the other hand, during the monsoon season, the air masses are mostly confined to be localized. In over all, the study suggests that the long-range transport of pollutants plays a crucial role in the enhancement of PMs over Alaknanda Valley region.
Factor Analysis and Correlation Matrix
The Principal component analysis (PCA) was applied to identify the sources of the ionic species in the measured PMs using factor extraction with an Eigenvalue larger than one after varimax rotation and was estimated by SPSS software (version 6.0) (Table 2). In its support, the Pearson correlation coefficient among measured chemical constituents in PMs was made (Table 3). The PCA yielded a total of three significant factors explaining about 75% of the total data variance (Table 2).
Factor 1 explains about 53.4 and 66.4% of the data variance which shows a strong positive loading for NO3−, SO42−, Ca2+, and Mg2+ for PM10 and PM2.5. High loadings of NO3−, SO42−, Ca2+ and Mg2+ in this factor clearly indicate the major sources of these ions from the combustion of fossil fuel and erosion of soil dust. The correlation coefficient between SO42− and NO3− was highest in both (0.53), indicates the similarity in the sources as fossil fuel combustion (Table 2b) and may be associated with long-range transports of sulfate and nitrate from the polluted site. Apart from this, the SO42− may be produced from fertilizer applications, thermal power plant, and oil refineries. Correlation coefficients between Ca2+ and SO42− (0.84 and 0.87), Mg2+ and Cl− (0.40 and 0.72), SO42− and Cl− (0.82 and 0.70) indicate that some parts of these species are originated from crustal soil sources. Mg2+ and Ca2+, derived from natural sources such as soil, play a crucial role in neutralization of the acidic species in the atmosphere (Sharma and Maloo 2005; Bisht et al. 2015b). While NH4+ from anthropogenic sources also plays an important role in neutralization, generally ammonium present in the atmosphere as a secondary inorganic aerosol which is produced from fertilizers used by farmers during agricultural activities and/or urine excretion (Kaya and Tuncel 1997). High loading of nitrate in factor 1 is mainly from nitrogenous fertilizers, cattle wastes, and soil particulates. Association of F− in this factor indicates that the source is from soil dust. A strong correlation (> 0.90) of Na+ and Cl− in both PM10 and PM2.5 indicates that most parts of Na+ and Cl− are derived from marine sources during the monsoon period, which transported from a long distance along with the moisture over receptor site. Earlier studies on rainwater chemistry during the monsoon over Himalayan and plain sites in northern India indicated the marine source region (Tiwari et al. 2012; Bisht et al. 2015c). However, an elevated Cl−/Na+ ratio in the present study, compared to seawater ratio indicates that a part of chloride from other emission sources. Kulshrestha et al. (1996) reported saline soil contribution towards Cl− along with sea salt aerosols during the monsoon period.
Factor 2 for PM10 and PM2.5 explains about 13 and 11% of the variance with a strong positive loading of F−, NH4+, Mg2+ and K+. These high loadings of these species are due to biomass burning activities (Tiwari et al. 2009; Cheng et al. 2013). Wood burning, which is commonly used (main fuel for cooking the food) by the inhabitants of the high altitude area, is a major source of potassium. In addition to this, the people use coal for heating as well as cooking purposes, which produces an enormous amount of fluoride and potassium into the atmosphere. In Factor 3, the NO2− loadings are much higher with positive significant (about 11% of the total variance), indicate that the source may be anthropogenic along with biomass burning (Gadi et al., 2011). Being part of oxides of nitrogen, it may be transported from long distance from the source region.
Conclusions
Mass concentrations of particulate matters (PM2.5 and PM10) and their chemical characteristics along with the probable sources were studied at Alaknanda basin (in Himalayan region) in Northern India. The concentrations of PM2.5 and PM10 were 78.7 (± 25.1) and 111.8 (± 23.4) μgm−3, respectively. The ratio (PM2.5/PM10) value was found to be 0.69 (± 0.1), ranging from 0.61 (June) to 0.82 (December), indicating the dominance of fine mode anthropogenic particles. A large seasonal variability in the measured chemical species suggested the impact of different emission sources and meteorological parameters. The total measured water-soluble ionic species in PM2.5 and PM10 were ~ 43 and 57%, respectively, which consist of secondary inorganic aerosols ~ 15% in PM2.5 and 18% in PM10, salt aerosols ~ 16% in PM2.5 and 20% in PM10, and mineral dust ~ 9% in PM10 and 13% in PM10. A significant correlation (p < 0.01) between NH4+ and Cl−, SO42−, NO3− in PM2.5 and PM10, respectively, indicates the presence in their salt forms. Neutralization of acidic species in aerosols was found that NH4+ play a crucial role in neutralization of acidic components in the winter season; however, the contrary feature was observed in the summer season where Ca2+ was responsible for the neutralization of acidic components in the PM samples which were transported from the inland region during the summer. The principal component analysis showed that secondary aerosol, biomass burning, and soil-driven dust are the possible sources in measured species. Cluster analysis indicates that the continental region is affecting the Himalayan region. In overall, the study suggests that the long-range transport of pollutants plays a crucial role in the enhancement of PMs over Alaknanda Valley region.
References
Akimoto H (2003) Global air quality and pollution. Science 302:1716–1719
Andreae MO (1995) Climatic effects of changing atmospheric aerosol levels. In: Henderson-Sellers A (ed) world survey of climatology: future climates of the world, vol 16. Elsevier, Amsterdam, pp 341–392
Ashish A, Joshi V, Sharma A, Anthwal S (2006) Retreat of Himalayan glaciers indicator of climate change. Nat Sci 4:53–60
Beegum SN, Moorthy KK, Babu SS, Satheesh SK, Vinoj V, Badarinath KVS, Safai PD, Devara PCS, Singh S, Vinod Dumka UC, Pant P (2009) Spatial distribution of aerosol black carbon over India during pre-monsoon season. Atmos Environ 43(5):1071–1078
Bisht DS, Dumka UC, Kaskaoutis DG, Pipal AS, Srivastava AK, Soni VK, Attri SD, Sateesh M, Tiwari S (2015a) Carbonaceous aerosols and pollutants over Delhi urban environment: temporal evolution, source apportionment and radiative forcing. Sci Total Environ 521–522:431–445
Bisht DS, Srivastava AK, Pipal AS, Srivastava MK, Pandey AK, Tiwari S, Pandithurai G (2015b) Aerosol characteristics at a rural station in southern peninsular India during CAIPEEX-IGOC: physical and chemical properties. Environ Sci Pollut Res 22:5293–5304
Bisht DS, Tiwari S, Srivastava AK, Singh JV, Singh BP, Srivastava MK (2015c) High concentration of acidic species in rainwater at Varanasi in the Indo-Gangetic plains, India. Nat Hazards 75:2985–3003
Chatterjee A, Adak A, Singh AK, Srivastava MK, Ghosh SK, Tiwari S et al (2010) Aerosol chemistry over a high altitude station at northeastern Himalayas, India. PLoS One 5(6):e11122
Cheng Y, Engling G, He KB, Duan FK, Ma YL, Du ZY, Liu JM, Zheng M, Weber RJ (2013) Biomass burning contribution to Beijing aerosol. Atmos Chem Phys 13:7765–7781
Dholakia HH, Bhadra D, Garg A (2014) Short term association between ambient air pollution and mortality and modification by temperature in five Indian cities. Atmos Environ 99:168–174
Finlayson Pitts BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere. Elsevier, New York
Forest Survey of India (FSI) (2009) Chapter 7: forest and tree resources in states and union territories. India State of Forest Report, Dehradun
Gabriel R, Mayol-Bracero OL, Andreae MO (2002) Chemical characterization of submicron aerosol particles collected over the Indian Ocean. J Geophys Res 107(D19):8005. https://doi.org/10.1029/2000jd000034
Gadi R, Kulshrestha UC, Sarkar AK, Garg SC, Parashar DC (2011) Emissions of SO2 and NOx from biofuels in India. Tellus B: Chem Phys Meteorol 55(3):787–795. https://doi.org/10.3402/tellusb.v55i3.16370
Gautam R, Hsu NC, Lau WKM, Yasunari TJ (2013) Satellite observations of desert dust-induced Himalayan snow darkening. Geophys Res Lett 40(5):988–993
Hobbs PV, Reid JS, Kotchenruther RA, Ferek RJ, Weiss R (1997) Direct radiative forcing by smoke from biomass burning. Science 275:1776–1778
Hong Z, Chak KC (1997) Size distribution of inorganic aerosols at a coastal site. J Aerosol Sci 28(101):213–214
IPCC (2007) Impacts, Adaptation and Vulnerability. Working group ii contribution to the intergovernmental panel on climate change. Fourth Assessment Report (Cambridge Univ Press), Cambridge
Jung CH, Lee JY, Um J, Lee SS, KIm YP (2018) Chemical composition based aerosol optical properties according to size distribution and mixture types during smog and Asian dust events in Seoul Korea. Asia Pac J Atmos Sci 54(1):19–32
Kaskaoutis DG, Kumar S, Sharma D, Singh RP, Kharol SK, Sharma M, Singh AK, Singh S, Singh A, Singh D (2014) Effects of crop residue burning on aerosol properties, plume characteristics and long, range transport over northern India. J Geophys Res 119:5424–5444
Kaya G, Tuncel G (1997) Trace element and major ion composition of wet and dry deposition in Ankara, Turkey. Atmos Environ 31:3985–3998
Kishore N, Srivastava AK, Nandan H, Pandey CP, Agrawal S, Singh N, Soni VK, Bisht DS, Tiwari S, Srivastava MK (2018) Long-term (2005–2012) measurements of near-surface air pollutants at an urban location in Indo-Gangetic Basin. J Earth Sys Sci (Accepted)
Kulshrestha UC, Sarkar AK, Srivastava SS, Parashar DC (1996) Investigation into atmospheric deposition through precipitation studies at New Delhi (India). Atmos Environ 30:4149–4154
Kumar A, Attri AK (2016) Biomass combustion a dominant source of carbonaceous aerosols in the ambient environment of western himalayas. Aerosol Air Qual Res 16:519–529
Lavanchy MG, Schotterer U, Schwikowski M, Gaggeler HW, Baltensperger U (1999) Historical record of carbonaceous particle concentrations from a European high-alpine glacier. J Geophys Res. 104:21227–21236
Li Z, Guo J, Ding A, Liao H, Liu J, Sun Y, Wang T, Xue H, Zhang H, Zhu B (2017) Air Pollution and Control Aerosol and boundary-layer interactions and impact on air quality. Natl Sci Rev 4:810–833
Mayewski PA, Jeschke PA (1979) Himalayan and Trans-Himalayan glacier fluctuations since AD 1812. Arct Alp Res 11:267–287
Menon S, Hansen J, Nazarenko L, Luo Y (2002) Climate effects of black carbon aerosols in China and India. Science 297:2250–2253
Parmar RS, Satsangi GS, Kumari M, Lakhani A, Srivastava SS, Prakash S (2001) Study of size distribution of atmospheric aerosol at Agra. Atmos Environ 35:693–702
Penner JE, Hegg D, Leaitch R (2001) Unravelling the role of aerosols in climate change. Environ Sci Technol 34:332A–340A
Pipal AS, Kulshrestha A, Taneja A (2011) Characterization and morphological analysis of airborne PM2.5 and PM10 in Agra located in north central India. Atmos Environ 45:3621–3630
Pope CA, Thun M, Namboodira J, Dockery DW, Evans JS, Speizer FW, Heath CW Jr (1995) Particulate air pollution as a predictor of mortality in a prospective study of US adults. Am J Respir Crit Care Med 151:669–674
Ram K, Sarin MM, Hegde P (2008) Atmospheric abundances of primary and secondary carbonaceous species at two high-altitude sites in India: sources and temporal variability. Atmos Environ 42:6785–6796
Ram K, Sarin MM, Hegde P (2010) Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya. Atmos Chem Phys 10(23):11791–11803
Rastogi N, Sarin MM (2006) Chemistry of aerosols over a semi-arid region: evidence for acid neutralization by mineral dust. Geophy Res Lett 33:L23815
Safai PD, Kevat S, Praveen PS, Rao PSP, Momin GA, Ali K, Devara PCS (2007) Seasonal variation of black carbon aerosols over a tropical urban city of Pune, India. Atmos Environ 41:2699–2709
Sen A et al (2017) Variations in particulate matter over Indo-Gangetic Plains and Indo-Himalayan Range during four field campaigns in winter monsoon and summer monsoon: role of pollution pathways. Atmos Environ 154:200–224
Sharma M, Maloo S (2005) Assessment of ambient air PM10 and PM2.5 and characteristics of PM10 in the city of Kanpur, India. Atmos Environ 39:6015–6026
Sharma SK et al (2014) Measurements of particulate (PM2.5), BC and trace gases over the northwestern Himalayan Region of India. MAPAN J Metrol Soc Ind 29:243–253. https://doi.org/10.1007/s12647-014-0104-2
Sharma D, Srivastava AK, Ram K, Singh A, Singh D (2017) Temporal variability in aerosol characteristics and its radiative properties over Patiala, northwestern part of India: impact of agricultural biomass burning emissions. Environ Pollution 231:1030–1041
Srivastava AK, Sachchidanand Singh, Tiwari S, Kanawade VP, Bisht DS (2012) Variation between near-surface and columnar aerosol characteristics during the winter and summer at Delhi in the Indo-Gangetic Basin. J Atmos Solar Terrest Phys 77:57–66
Srivastava AK, Bisht DS, Ram K, Tiwari S, Srivastava MK (2014) Characterization of carbonaceous aerosols over Delhi in Ganga basin: seasonal variability and possible sources. Environ Sci Pollut Res 21:8610–8619
Srivastava AK, Ram K, Singh S, Kumar S, Tiwari S (2015) Aerosol optical properties and radiative effects over Manora Peak in the Himalayan foothills: seasonal variability and role of transported aerosols. Sci Total Environ 502:287–295
Stein AF, Draxler RR, Rolph GD, Stunder BJB, Cohen MD, Ngan F (2015) NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull Amer Meteor Soc 96:2059–2077. https://doi.org/10.1175/BAMS-D-14-00110.1
Tare V, Tripathi SN, Chinnam N, Srivastava AK, Dey S, Manar M, Kanawade VP, Agarwal A, Kishore S, Lal RB, Shar M (2006) Measurements of atmospheric parameters during indian space research organization geosphere biosphere program land campaign II at a typical location in the Ganga Basin: 2. Chemical properties. J Geophys Res 111(D23210):1–14
Tiwari S, Srivastava AK, Bisht DS, Bano T, Singh S, Behura S, Srivastava MK, Chate DM, Padmanabhamurty B (2009) Black carbon and chemical characteristics of PM10 and PM2.5 at an urban site of North India. J Atmos Chem 62:193–209
Tiwari S, Chate DM, Bisht DS, Srivastava MK, Padmanabhamurty B (2012) Rainwater chemistry in the North Western Himalayan Region, India. Atmos Res 104–105:128–138
Tiwari S, Pervez S, Perrino C, Srivastava AK, Bisht DS, Chate DM (2013) Chemical characterization of atmospheric Particulate Matter in Delhi, India, Part II: source apportionment studies using PMF 3.0. Sustainable. Environ Res 23(5):295–306
Tripathi SN et al (2006) Measurements of atmospheric parameters during Indian space research organization geosphere biosphere programme land campaign II at a typical location in the Ganga basin: 1. Physical and optical properties. J Geophys Res 111(D23209):1–14
Acknowledgements
Author ASG is thankful to the SERB-DST project for financial support (No. SB/EMEQ-043/2014 dated March 08, 2016). Authors are thankful to the Director, IITM to provide the facility for analysis of aerosol samples at IITM Delhi branch.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gautam, A.S., Negi, R.S., Singh, S. et al. Chemical Characteristics of Atmospheric Aerosol at Alaknanda Valley (Srinagar) in the Central Himalaya Region, India. Int J Environ Res 12, 681–691 (2018). https://doi.org/10.1007/s41742-018-0125-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-018-0125-8