Abstract
At four stations in Tokyo Bay, pore water profiles of dissolved organic carbon (DOC), nitrogen (DON), phosphorus (DOP), and inorganic nutrients were determined at 3-month intervals over 6 years. Concentrations of dissolved organic matter (DOM) and nutrients were significantly higher in pore waters than in the overlying waters. Pore water DOC, DON, and DOP concentrations in the upper most sediment layer (0–1 cm) ranged from 246 to 888 μM, from 14.6 to 75.9 μM, and from 0.02 to 9.83 μM, respectively. Concentrations of DOM and nutrients in pore waters occasionally showed clear seasonal trends and were highest in the summer and lowest in the winter. The seasonal trends in the pore water DOM concentrations were coupled with trends in the overlying water temperature and dissolved oxygen concentration. Benthic effluxes of DON and DOP were low compared with those of inorganic nutrients, accounting for only 1.0 and 1.5 % of the total benthic effluxes of nitrogen and phosphorus, respectively; thus benthic DOM fluxes were quantitatively insignificant to the inorganic nutrient fluxes in Tokyo Bay. The DOM fluxes represented about 7, 3, and 10 % of the riverine discharge of DOC, DON, and DOP to Tokyo Bay, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In coastal sediments, part of the particulate organic matter (POM) is directly mineralized to nutrients, while the other part is transformed into dissolved organic matter (DOM) by various biological activities. Microorganisms in the sediment incorporate DOM compounds in pore waters; production and consumption of DOM will occur concurrently during the process of organic matter degradation. Pore water DOM is a heterogeneous mixture of organic components that range in size from large molecules, such as humic substances or proteins, to small molecules, such as short chain organic acids or amino acids (Burdige 2002). Much of the dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) in pore water are of relatively low molecular weight and appear to be refractory (Burdige 2006). In the sediment, pore water DOM concentrations are elevated over overlying water values (Burdige and Gardner 1998). This implies that DOM is released from the sediments into the upper water column. The integrated benthic DOC flux from coastal and continental margin sediment (~180 Tg C year−1; Burdige et al. 1999) is comparable to the estimates of the organic carbon burial rate in all marine sediment (~160 Tg C year−1; Hedge and Keil 1995) and the riverine DOC input (200 Tg C year−1; Meybeck 1982). Furthermore, DON fluxes estimated from estuarine and continental margin sediments (~22 Tg N year−1; Burdige and Zheng 1998) are of the same order of magnitude as global riverine inputs of DON (50 Tg N year−1; Walsh 1991). Thus, benthic DOC and DON fluxes from marine sediments represent an important source of DOC and DON to the oceans, and a significant component of the oceanic carbon and nitrogen cycle.
However, there is little existing knowledge on pore water dissolved organic phosphorus (DOP) in eutrophic estuarine areas. The DOM originates from microbial production through several processes, including excretion, cell lysis, and sloppy feeding (Bronk and Glibert 1993). The DOP may not be refractory, because monophosphate esters predominate, although much of the DOC and DON have been presumed to be refractory in pore waters (Burdige 2006). The DOP is preferentially remineralized relative to DOC and DON in water column (Loh and Bauer 2000). If the DOP is relatively reactive in pore waters as is the case in water column, the dynamics of DOP would be different from those of DOC and DON, and influence the cycle of phosphorus and related elements, such as carbon and nitrogen. DOP is an important component of the P cycle and can be regarded as a quantitatively significant fraction of phosphorus in pore waters (Monbet et al. 2009). Furthermore, there is additional interest in understanding the role of sediments as a source of DON and DOP in the water column, because some of the DOM could be further mineralized to nutrients, and used as nitrogen and phosphorous sources in primary production processes (Burdige and Zheng 1998). Nitrogen and phosphorous released from the sediment has a significant influence on coastal eutrophication (Nixon et al. 1996).
Tokyo Bay is one of the major eutrophic coastal areas in the world (Selman et al. 2008). The sediments in Tokyo Bay are generally hypertrophic, and the overlying water tends to be anoxic during the summer (Kodama et al. 2010). Several studies in Tokyo Bay reported elevated pore water ammonium and phosphate concentrations in the summer, when the temperatures were high and the dissolved oxygen (DO) concentrations were low (Imamura and Matsunashi 1997; Suzumura et al. 2003). Despite the potential importance of DOM in biogeochemical cycles, most studies of pore water in Tokyo Bay have only focused on the inorganic nutrients. Compared with the numerous studies of nutrients, studies of pore water DOM are limited. Further, the contribution of benthic DOM release is not clear as the source of nutrients to the water column. Phosphorus acts as a limiting factor for primary production in Tokyo Bay (Matsumura et al. 2001), and thus the release of DOP and phosphate from sediments may be important. Furthermore, because far less attention has been given to the seasonal variation in pore water DOM, quantitative evaluation of DOM release throughout the year or for longer periods is not yet available.
The purpose of this study is to overview the dynamics of DOM and nutrients occurring in hypertrophic sediments and the overlying waters in Tokyo Bay. Firstly, we carried out time-series observations at four stations in Tokyo Bay over 6 years, and obtained the temporal and spatial distribution of DOC, DON, DOP, and nutrient concentrations in pore water, along with measurements of hydrographic and sediment parameters. Secondly, based on the time-series data, difference in dynamics between DOC, DON, and DOP in the sediment were clarified, and benthic DOM fluxes in terms of carbon, nitrogen, and phosphorus were estimated. Finally, we assessed the importance of the pore water DOM to coastal biogeochemical cycles.
2 Materials and methods
2.1 Study area
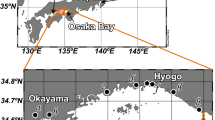
The study area was located in the northwestern area of Tokyo Bay (Fig. 1). In this part of Tokyo Bay, levels of organic matter in the sediment are high, and bottom hypoxia, which is defined as overlying water with DO concentrations ≤2.9 mg l−1, is sustained by thermal and salinity stratification during the summer (Kodama et al. 2010). Sediment cores and overlying water samples were collected at four stations (M1, M2, M3, and M4) (Fig. 1). The water depths at stations M1 and M4 ranged from 10 to 15 m, whereas those at stations M2 and M3 ranged from 22 to 25 m. The organic matter content was generally much higher at stations M2 and M3 than at stations M1 and M4. At all locations, the sediments were silty clay. Bioturbation was virtually absent in these sediments over the study period.
2.2 Sampling
Sediment cores and overlying water samples were collected at 3-month intervals from stations M1 and M2 from May 2007 to February 2013 (24 times), and from stations M3 and M4 from May 2007 to January 2011 (16 times). Sediments were collected by scuba divers using Plexiglas tubes. The 16 cores collected from May 2007 to February 2008 were segmented at depths of 0–1, 1–2, 2–4, 4–6, and 6–10 cm. Only the 0–1 cm segment was used from the 64 cores collected from May 2008 to February 2013.
Samples were centrifuged for 10 min at 3000 rpm (approximately 1500g) to obtain pore water. The supernatant was filtered through a pre-combusted GF/F filter (GE Healthcare UK Ltd., Little Chalfont, UK). Before analysis for silicic acid, samples were filtered through a cellulose acetate filter with a pore size of 0.45 μm (Toyo Roshi Kaisha Ltd., Tokyo, Japan). Pore water DON, DOP, and nutrients samples are usually frozen without adding HCl. However, precipitates formed in the pore water samples in Tokyo Bay when they were frozen. In the phosphate and silicic acid samples, there were significant differences in measured concentrations between frozen and unfrozen samples. In contrast, there were no significant differences between the fresh samples and the samples preserved with HCl in a refrigerator. Thus, samples were preserved with 6 M HCl (1 % of the sample volume) and stored in the dark at 5 °C until analysis for DOC, DOP, phosphate, and silicic acid. Samples were stored at −20 °C before analysis for DON, ammonium, nitrate, and nitrite.
Scuba divers collected samples of overlying water. The overlying water was filtered as described above. Samples were acidified by adding 6 M HCl (1 % of the sample volume) and were stored in the dark at 5 °C before analysis for DOC. Samples for DON, DOP, and nutrient analyses were stored at −20 °C. Temperature and DO concentrations in the overlying water were monitored by portable sensors (Clorotec ACL1183-PDK, JFE Advantech Co. Ltd., Hyogo, Japan).
2.3 Analysis
Nutrient concentrations were determined by a QuAAtro-Marine 5 channel auto-analyzer (SEAL analytical Ltd., Fareham, UK) following the methods of Kanda (1995), Strickland and Parsons (1972), and Hansen and Koroleff (1999) for ammonium, nitrate and nitrite, and phosphate and silicic acid, respectively. DOC concentrations were determined by a high temperature catalytic oxidation technique, using a TOC-VCSH total carbon analyzer (Shimadzu Inc., Kyoto, Japan). Total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP) were determined by persulfate oxidation (modified after Hansen and Koroleff 1999) with a QuAAtro TN-TP analyzer (SEAL analytical Ltd.). In this analyzer, the sample was mixed with K2S2O8, and the mixture was passed through pressurized Teflon pipe at 110 °C in an oil bath for 40 min. The reaction was started under alkaline conditions for TDN, and acidic conditions for TDP (Hansen and Koroleff 1999). Nitrate and phosphate concentrations in the oxidized samples were then determined by the same colorimetric method as for the nutrient analysis. The DON concentration was calculated as the difference between TDN and dissolved inorganic nitrogen (DIN, the sum of the ammonium, nitrate, and nitrite). DOP concentration was estimated as the difference between TDP and phosphate.
In pore water samples, ammonium concentrations were very high compared with DON concentrations. If the samples were analyzed directly, pore water DON concentrations would be determined as the small difference between the two large values of TDN concentration and DIN concentration. Hence, the DON concentration would reflect a large analytical error. Therefore, before analysis, we removed ammonium from the TDN and DIN samples that were to be used for the calculation of DON. The protocol of ammonia stripping was based on that described by Burdige and Zheng (1998) and Miyajima et al. (2005): We added 0.8 M borate-buffered solution (pH 12.5) to the samples and sprayed them with nitrogen gas at 60 °C to strip off the ammonia. Before these samples were analyzed for TDN and DIN, any water lost during the ammonia removal was replaced with deionized distilled water. The removal efficiency of ammonium was almost 100 %.
2.4 Calculation of benthic DOM and nutrient fluxes
The diffusive fluxes of DOM and nutrients between sediments and the water column were calculated from the interfacial concentration gradients, using Fick’s first law of diffusion (Berner 1980).
where J is the diffusive flux, ϕ is the porosity at the sediment surface (depth = 0–1 cm), D sed is the molecular diffusion coefficient in sediment, and dC/dX is the interfacial concentration gradient across the sediment–water interface. ϕ was calculated from the water content (weight loss on drying at 110 °C) and total density data. dC/dX was assumed to be represented by the difference between the concentration in the overlying water and in the pore water concentration in the first sediment sample below the sediment–water interface, which was divided by the depth of the mid-point of this sediment sample (in our case, 0.5 cm for a 0–1 cm sediment sample). In determining D sed, the diffusion coefficients in seawater for DOM at the in situ temperature were estimated from the Stokes–Einstein equation assuming an average molecular weight for DOM of 3000 Da. This molecular weight represents the intermediate value of previous estimates for pore water DOM. Alperin et al. (1994) reported an average molecular weight of 5000 Da, whereas Burdige and Gardner (1998) suggested that the molecular weight of the majority of pore water DOM in Chesapeake Bay was less than 3000 Da. The diffusion coefficients for nutrients in seawater at the in situ temperature were estimated from Li and Gregory (1974). The resulted molecular diffusion coefficients in sediment were 1.3–2.1 × 10−6 cm2 s−1 for DOM, 9.1–17.7 × 10−6 cm2 s−1 for ammonium, 8.5–16.2 × 10−6 cm2 s−1 for nitrate, 8.0–15.2 × 10−6 cm2 s−1 for nitrite, 5.3–10.1 × 10−6 cm2 s−1 for phosphate, 3.5–6.6 × 10−6 cm2 s−1 for silicic acid, depending on temperature and sediment porosity.
3 Results
3.1 Environmental parameters
At all stations, the temperatures of the overlying water were between 9.5 and 27.1 °C (Fig. 2a). The high temperatures were observed during August and October/November. The DO concentrations in overlying water ranged from 0.1 to 11.8 mg l−1, and hypoxic or anoxic conditions dominated in August (Fig. 2b). The porosity in the uppermost layer of sediment ranged from 0.74 to 0.94 (Fig. 2c). Minimum values were generally observed at station M4. There was no obvious seasonal variability at all stations.
3.2 Pore water DOM
Pore water DOC concentrations increased logarithmically with depth in many cases, sometimes approaching asymptotic concentrations at depth (Fig. 3). Pore water DOC concentrations in the uppermost layer of sediment were greater than those in the overlying water (t test, P < 0.01, n = 80) by four times on average. The DOC concentrations in pore water in the uppermost layers of the sediment ranged from 218 μM (station M1, February 2008) to 888 μM (station M2, August 2011) (Fig. 4). There were significant seasonal differences in pore water DOC concentrations at stations M1, M2, and M3 (ANOVA, P < 0.05; M1 and M2, n = 6; M3, n = 4). Concentrations at these stations were lowest in January/February (Tukey test, P < 0.05; M1 and M2, n = 6; M3, n = 4), from which time they progressively increased until they reached a maximum in August (Tukey test, P < 0.05; M1 and M2, n = 6; M3, n = 4). In contrast, there was no obvious seasonal variation in concentrations at station M4 (ANOVA, P > 0.05, n = 4). Station M4 was located at the mouth of the river, and thus surface sediments may have been influenced by riverine deposits.
As with DOC, the vertical profile of pore water DON concentrations showed a logarithmical increase with depth (Fig. 3). Pore water DON concentrations in the uppermost layer of sediment were higher than those in the overlying water (t test, P < 0.01, n = 80) by 3 times on average. The concentrations of pore water DON in the uppermost layer ranged from 14.6 μM (station M2, May 2010) to 75.9 μM (station M4, February 2009) (Fig. 4). There was little seasonality in DON concentrations at stations M1, M3, and M4 (ANOVA, P > 0.05; M1, n = 6; M3 and M4, n = 4). On the other hand, there were significant seasonal differences in DON concentrations at station M2 (ANOVA, P < 0.05, n = 6), and DON concentrations were highest in August at station M2 (Tukey test, P < 0.05, n = 6).
Pore water DOP concentrations showed no obvious increasing trend with depth (Fig. 3), and were higher in the uppermost layer of sediment than in the overlying water (t test, P < 0.01, n = 80) by 13 times on average. Pore water DOP in the uppermost layer ranged from a minimum value of 0.02 μM in the winter (station M1, February 2013) to a maximum of 9.83 μM in the summer (station M4, August 2009) (Fig. 4). The pore water DOP concentrations at the four stations were similar, and the concentrations increased sharply in August. There was a significant difference between seasons in pore water DOP concentrations at stations M1, M2, and M4 (ANOVA, P < 0.05; M1 and M2, n = 6; M4, n = 4). Pore water DOM concentrations did not show significant interannual variation (ANOVA, P > 0.05, n = 8–16).
The C/N, C/P, and N/P ratios of the pore water DOM in the uppermost layer of the sediment ranged from 7.0 (station M1, February 2008) to 21.5 (station M3, November 2007), from 48.6 (station M2, May 2007) to 759 (station M4, January 2011), and from 3.8 (station M2, May 2007) to 67.8 (station M1, February 2009), respectively (Fig. 5). The C/N ratios were 12.6 ± 2.4 (mean ± standard deviation, n = 20), 13.7 ± 2.1, 14.0 ± 3.5, and 10.6 ± 2.4, respectively, in May, August, October/November, and January/February. The C/N ratios in all seasons were higher than the Redfield ratio of 6.6 (Redfield et al. 1963). The C/P ratios were 171 ± 78, 183 ± 151, 251 ± 103, 406 ± 191, respectively, in May, August, October/November, and January/February. The N/P ratios were 14.1 ± 7.2, 14.1 ± 13.2, 18.7 ± 8.6, 37.7 ± 14.0, respectively, in May, August, October/November, and January/February. N/P ratios were highest in January/February (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3 and M4, n = 4).
3.3 Pore water nutrients
Except for nitrate and nitrite, the nutrient concentrations in pore water increased with depth (Fig. 3). In pore water, DIN was dominated by ammonium. The pore water concentrations of ammonium in the uppermost layer of sediment ranged from 52.9 μM (station M3, January 2010) to 661.5 μM (station M2, August 2011) (Fig. 6). There was a clear seasonal pattern in pore water ammonium concentrations in the uppermost layer of sediment. At all stations, pore water ammonium concentrations were highest in August (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3 and M4, n = 4). Pore water ammonium concentrations in the uppermost layer of sediment were higher than those in the overlying water (t test, P < 0.01, n = 80) by 32 times on average (Fig. 3). In contrast, concentrations of nitrate and nitrite were very low and showed no seasonal variability (ANOVA, P > 0.05, M1 and M2, n = 6; M3 and M4, n = 4) (Fig. 6). Nitrate and nitrite concentrations were lower in pore water than in the overlying water (t test, P < 0.01, n = 80) (Fig. 3). Pore water phosphate and silicic acid concentrations in the uppermost layer of sediment were greater than those in the overlying water (t test, P < 0.01, n = 80) by 19 and 14 times on average, respectively. The pore water concentrations of phosphate and silicic acid in the uppermost layer of sediment ranged from 0.48 μM (station M4, January 2010) to 154.1 μM (station M2, August 2012), and from 121.8 μM (station M4, February 2008) to 840.8 μM (station M2, August 2007), respectively (Fig. 7). Pore water phosphate and silicic acid concentrations followed a seasonal pattern, with higher concentrations observed at all stations in August (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3 and M4, n = 4). Concentrations of these nutrients were highest at station M2 (ANOVA and Tukey test, P < 0.05, n = 4–6). Pore water nutrients concentrations did not show significant interannual variation (ANOVA, P > 0.05, n = 8–16).
3.4 DOM and nutrients in the overlying water
Figure 8 shows seasonal variations in DOM concentrations and C/N, C/P, and N/P ratios of DOM in the overlying water at all stations. DOM concentrations and elemental ratios of DOM in the overlying water showed no obvious seasonal variations (ANOVA, P > 0.05, M1 and M2, n = 6; M3 and M4, n = 4). There were no correlations in DOC, DON, and DOP concentrations between overlying water and pore water in the uppermost layer of sediment (Spearman’s rank-order correlation, r s = 0.009, 0.014, −0.007; P > 0.05, n = 80). Figure 9 shows seasonal variations in nutrient concentrations in the overlying water at all stations. Nitrate and nitrite concentrations in the overlying water were lowest in August (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3 and M4, n = 4). The highest concentrations of phosphate and silicic acid in the overlying water occurred in August at stations M1, M2, and M3 (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3, n = 4). There was a significant correlation between phosphate in the overlying water and in pore water in the uppermost layer of the sediment (r s = 0.492, P < 0.01, n = 80). In contrast, there were no significant correlations between overlying water and pore water for ammonium, nitrate, nitrite, and silicic acid concentrations (r s = 0.189, 0.148, 0.122, 0.270; P > 0.01, n = 80).
3.5 DOM and nutrients flux
The DOM and nutrient fluxes between the sediment and overlying water are presented in Fig. 10. DOC fluxes ranged from 11.3 to 104.5 μmol m−2 h−1, with major peaks in August (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3 and M4, n = 4). DON fluxes ranged from 0.26 to 6.24 μmol m−2 h−1. Seasonal trends in DON fluxes were less obvious (ANOVA, P > 0.05; M1 and M2, n = 6; M3 and M4, n = 4). DOP fluxes were low throughout the year. They ranged from −0.05 to 1.08 μmol m−2 h−1, and were highest in August (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3 and M4, n = 4).
Seasonal variations in DOM and nutrient fluxes from the sediment to the water column at stations M1 (circle), M2 (triangle), M3 (square), and M4 (diamond) (positive values imply a source from the sediment, while negative values indicate the sediment as a sink). The dashed lines indicate August, and the fine dashed lines indicate January/February
Ammonium, nitrate and nitrite fluxes ranged from 32.1 to 721.9, from −34.8 to −0.06, and from −4.59 to −0.01 μmol m−2 h−1, respectively. At all stations, nitrate and nitrite fluxes were from the overlying water into the sediments. Phosphate and silicic acid fluxes ranged from −0.78 to 91.8, and from 20.6 to 345.2 μmol m−2 h−1, respectively. Ammonium, phosphate and silicic acid fluxes were highest in August at stations M1, M2, and M3 (ANOVA and Tukey test, P < 0.05; M1 and M2, n = 6; M3, n = 4). Comparing the fluxes of inorganic and organic matter indicates that most of the nitrogen and phosphorus fluxes were in dissolved inorganic forms, and DON and DOP fluxes were only a small fraction of the TDN and TDP fluxes from the sediments, respectively.
4 Discussion
4.1 Pore water DOM
At all stations, DOM concentrations were higher in the pore water than in the overlying water (Fig. 3). The relatively high DOM concentrations in pore water compared with the concentrations in the overlying water indicate DOM release from the sediments to the overlying water. Profiles of pore water DOC and DON for this study are similar to those described in other coastal sediments (Burdige and Zheng 1998; Alperin et al. 1999; Komada et al. 2004; Alkhatib et al. 2013). Generally, DOC and DON concentrations in pore water increased with depth (Fig. 3). Burdige (2002) showed that pore water DOM increased with depth, probably because of increases in relatively refractory DOM produced from the oxidation of POM. In contrast, DOP concentrations in our study appeared to be relatively constant from the sediment surface to depth (Fig. 3). In comparison to DOC and DON, DOP in marine environments contains relatively reactive components including monophosphate esters which are rapidly and easily formed and hydrolyzed (Karl and Björkman 2002). Furthermore, it is considered that in the sediment surface, particulate organic phosphorus is more rapidly hydrolyzed than particulate organic carbon and nitrogen (Burdige 2006). This rapid hydrolysis may thus be responsible for the differing vertical profiles of pore water DOP compared with DOC and DON.
Pore water DOM concentration generally increased steadily with depth during the summer and autumn in our study area (Fig. 3), similar to trends reported for the anoxic sediments (Burdige and Zheng 1998; Komada et al. 2004). Komada et al. (2004) investigated the factors controlling the accumulation of pore water DOC in mixed-redox (suboxic, or oscillating between oxidizing and reducing conditions) sediments and anoxic sediments in the Raritan-New York Bay complex and the Inner New York Bight. They suggested that the accumulation of DOC was suppressed in the mixed-redox sediments relative to persistently the anoxic sediments. This was likely due to the rapid hydrolysis of high molecular weight DOC, and the limited production and enhanced consumption of the less reactive polymeric low molecular weight component of the DOC in the mixed-redox sediments.
In recent years, many studies have examined pore water DOM concentrations and their vertical distributions in the marine environment. Pore water DOC concentrations obtained in this study were within the range reported for other estuarine sediments, such as Chesapeake Bay (Burdige and Homstead 1994; Burdige and Zheng 1998) and the St. Lawrence Estuary (Alkhatib et al. 2013), and continental margins, such as the North Carolina Continental Slope (Alperin et al. 1999), California continental margin (Burdige et al. 1999) and the Mexican margin (Holcombe et al. 2001). In contrast, pore water DOC concentrations were lower in Tokyo Bay than those measured at Cape Lookout Bight (Alperin et al. 1994), probably because the water depth in the latter is much shallower and the area receives larger inputs of organic matter than our study area. DON concentrations in Tokyo Bay pore water were of the same order of magnitude as concentrations reported for other estuarine and continental shelf sites (Burdige and Zheng 1998; Landén and Hall 2000; Alkhatib et al. 2013). Pore water DOP concentrations in this study were similar to those observed in intertidal sediments (Monbet et al. 2009), and mangrove sediments (Lee et al. 2008). However, no data have been reported on pore water DOP concentrations in estuarine and continental shelf sites.
Among studies of pore water DOM, studies of seasonal variation in pore water DOM are limited, with the present study providing the most comprehensive data set. There was a seasonal trend in pore water DOC and DON concentrations in the uppermost layer of the sediments, such that concentrations were highest in the summer and lowest in the winter in our study area (Fig. 4). This seasonal trend is similar to that observed for DOC concentrations in Cape Lookout Bight and Chesapeake Bay (Alperin et al. 1994; Burdige and Zheng 1998). However, pore water DON concentrations in the uppermost layer of the sediments showed no obvious seasonal variability at stations M1, M3, and M4. Pore water DON concentrations were irregularly high in the winter at all stations. Pore water DON concentrations were high in February 2009, with samples taken just days after the flood events. High DON concentrations presumably resulted from particle supply by floodwater. There was a seasonal pattern in pore water DON concentrations at station M3 if the value observed in February 2009 was excluded (ANOVA, P < 0.05, n = 3–4). In contrast, there was no clear seasonal pattern at station M4, even with the exclusion of the February 2009 value. This is suggested to be due to the location of the station at the mouth of the river, where surface sediments may have been influenced by riverine deposits.
Spearman’s rank-order correlation analysis on pore water DOM concentrations and the potentially influential environmental parameters are summarized in Table 1. At our study area, the temperature of the overlying water was significantly correlated with pore water DOC (r s = 0.467, P < 0.01, n = 80) and DOP (r s = 0.429, P < 0.01, n = 80), but not DON (r s = 0.155, P > 0.01, n = 80) (Table 1). Previous studies reported that temperature of the overlying water was generally similar to the temperature of the pore water (Burdige 2006). Alperin et al. (1994) suggested that high temperatures promote elevated hydrolysis and activities of fermentative microorganisms, and thus stimulate the hydrolysis of POM, resulting in higher concentrations of pore water DOM in the summer.
Some studies have reported that when reactive organic matter was gradually added to experimental sediments cores, pore water DON concentrations generally increased (Enoksson 1993; Sloth et al. 1995). The major POM portion supplied to the sediments in the inner part in Tokyo Bay was produced autochthonously in the water column (Ogawa et al. 1994; Kubo 2015). The relationship between pore water DOC, DON and DOP, and the organic matter content of carbon, nitrogen and phosphorus in the uppermost layer of sediments was, however, not significant (r s = −0.101, −0.159, 0.087; P > 0.05; n = 80) (Table 1). Furthermore, there was no significant correlation between pore water TDN and TDP, and the organic matter content of nitrogen and phosphorus in the uppermost layer of sediments (r s = 0.150, 0.135; P > 0.05; n = 80), probably because the sampling methods could not detect the freshly deposited POM, which accumulates in the very thin layer of the sediments surface.
Skoog and Arias-Esquivel (2009) suggested that after the onset of anoxia in incubated overlying water, concomitant increases in DOC and iron fluxes resulted from the dissolution of previously co-precipitated iron oxyhydroxides and organic carbon. They reported that increases in the DOM flux under anoxic conditions could be the result of the release of DOM adsorbed by mineral oxyhydroxides. Metal oxides that accumulate near the sediment–water interface may act as DOM filters (Skoog et al. 1996; Skoog and Arise-Esquivel 2009). The reductive dissolution of DOM adsorbed to mineral phases may increase pore water DOM concentrations in the sediment surface. Chin et al. (1998) showed that a strong correlation was observed between Fe(II) and DOM in pore water, which suggests that both constituents accumulate in pore water. They hypothesized that the reduction of iron oxyhydroxides coated with organic matter releases both DOM and Fe(II) in freshwater wetlands. When anoxia in overlying water occurs, pore water DOM concentration would increase, because of the reductive dissolution of DOM adsorbed to mineral phases. This would appear to be linked to an inverse correlation between pore water DOM concentrations and DO concentrations of overlying water. In our study, DO concentrations of the overlying water showed negative correlations significantly, with pore water DOC concentrations (r s = −0.473, P < 0.01, n = 80), with DON concentrations (r s = −0.281, P < 0.05, n = 80), and with DOP concentrations (r s = −0.521, P < 0.01, n = 80) (Table 1). During the summer, DOM may desorb from mineral oxyhydroxides, and increase in concentration in pore water, because seasonal anoxia in overlying water generally occurs during the summer in our study area.
As discussed above, both temperature and DO concentrations in overlying water have significant correlations with pore water DOM concentrations. Further statistical analysis such as multiple regression analysis, principal component analysis, and ANOVA, was attempted to identify the major factors that control pore water DOM concentrations. However, none of these statistical analyses were able to evaluate the effects of temperature and dissolved oxygen quantitatively, because temperature and DO were strongly correlated.
4.2 Pore water nutrients
Pore water ammonium and phosphate concentrations obtained in this study were in the same range as those previously reported by Imamura and Matsunashi (1997) and Suzumura et al. (2003) in their studies of Tokyo Bay. Pore water ammonium, phosphate, and silicic acid concentrations were similar to the values reported in coastal sediment from semi-enclosed areas, such as the Gulf of Finland (Conley et al. 1997) and Aburatsubo Bay (Takayanagi and Yamada 1999), and continental shelf areas, such as the Mexican margin (Holcombe et al. 2001) and Massachusetts Bay (Hopkinson et al. 2001). However, the nutrient concentrations were much higher than those reported in intertidal sediments of the Satilla River Estuary (Jahnke et al. 2003), the Seto Inland Sea (Magni and Montani 2006),and the Marano and Grado Lagoons (De Vittor et al. 2012), and in estuarine sediments in the Gulf of the St. Lawrence (Thibodeau et al. 2010) and the Pearl River Estuary (Zhang et al. 2013). The low concentrations in these areas may reflect the fact that the sediment surfaces and overlying water in these areas were oxygenated and received lower inputs of organic matter than our study area.
Pore water ammonium and phosphate concentrations were highest in summer, perhaps owing to an increased temperature in the overlying water and a greater supply of organic matter to the sediment (Enoksson 1993; Banta et al. 1995; Cowan and Boynton 1996; Landén and Hall 1998; Hopkinson et al. 2001). Furthermore, anoxic conditions in the overlying water may have a similar influence on concentrations of phosphate and DOM in pore water. The increase in pore water phosphate concentrations under anaerobic conditions could be the result of redox-dependent release of phosphate adsorbed by iron oxyhydroxides and carbonates (Viktorsson et al. 2013; Lehtoranta et al. 2015), liberation from manganese dioxide (Baldwin et al. 2001) and anaerobic degradation of organic phosphorus adsorbed to sediment, such as inositol hexaphosphate (Suzumura and Kamatani 1995). The seasonal variations in silicic acid concentrations were consistent with the seasonal variability in the temperature of the overlying water (r s = 0.504, P < 0.01, n = 80) (Table 1). Temperature was the major factor controlling the dissolution process, and temperature increases in the summer strengthened the rate of silica dissolution from diatoms, resulting in elevated silicic acid concentrations in the sediments (Kamatani 1982; Yamada and D’Elia 1984).
4.3 Significance of DOM fluxes
Benthic DOC, DON, DOP, ammonium, phosphate, and silicic acid were released from the sediments to the water column all year around (Fig. 10). The annual average fluxes (± standard deviation) were 0.30 ± 0.15 mol m−2 year−1 for DOC, 0.02 ± 0.01 mol m−2 year−1 for DON, 0.002 ± 0.002 mol m−2 year−1 for DOP, 1.88 ± 1.30 mol m−2 year−1 for ammonium, −0.10 ± 0.06 mol m−2 year−1 for nitrate, −0.01 ± 0.01 mol m−2 year−1 for nitrite, 0.15 ± 0.18 mol m−2 year−1 for phosphate, and 1.18 ± 0.69 mol m−2 year−1 for silicic acid. The DON and DOP fluxes contributed 1.0 and 1.5 % of the TDN and TDP fluxes, respectively. Data from this study indicate that most of the nitrogen and phosphorus fluxes were in dissolved inorganic forms, and DOM fluxes represented a small fraction of the sediments in Tokyo Bay. Burdige and Zheng (1998) showed that benthic DON fluxes represented 3 % of TDN fluxes in Chesapeake Bay, and Hopkinson (1987) reported a value of approximately 10 % in Georgia Bight, USA. The contribution of DOM fluxes in Tokyo Bay appeared to be lower than the values reported for other coastal and estuarine sediment. Furthermore, benthic DON flux in Tokyo Bay was smaller than that in other coastal areas, such as Chesapeake Bay (0.07 ± 0.02 mol m−2 year−1) (Burdige and Zheng 1998). Considering that the benthic flux of ammonium released from sediments in Tokyo Bay was similar to those in the Chesapeake Bay (1.96 ± 0.17 mol m−2 year−1) (Burdige and Zheng 1998), most DOM in Tokyo Bay likely be mineralized in the sedimentary layer, and that may result in smaller contribution of the benthic DOM flux.
The benthic fluxes of DOM and nutrients also varied seasonally. The fluxes of DOC, DOP, ammonium, phosphate, and silicic acid were highest in the summer. Most of the seasonal variation appeared to reflect the variation in pore water concentration.
We extrapolated the average fluxes of DOM and nutrient calculated in this study for the total sediment surface area of Tokyo Bay (922 km2), and estimated the annual release from the sediments to the water column. Since our study area is located at the innermost part of the bay where biological activity is relatively high, the estimated annual release should be considered as the maximum estimate. Calculation results for benthic DOM and nutrients fluxes for Tokyo Bay are shown in Table 2. Benthic fluxes of DOC, DON, and DOP were estimated as 3.3 ± 0.6 Gg C year−1, 0.22 ± 0.07 Gg N year−1, and 0.059 ± 0.005 Gg P year−1, respectively. Kubo et al. (2015) estimated that the riverine discharge of DOC into Tokyo Bay was 50 Gg C year−1, whereas riverine discharges of DON and DOP were estimated at 6.6 Gg N year−1 and 0.57 Gg P year−1, respectively, by Suzumura and Ogawa (2001) and Matsumura and Ishimaru (2004). A comparison of the estimates of benthic DOM fluxes from this study with estimates of riverine discharges of DOM shows that the benthic fluxes were equivalent to approximately 7, 3, and 10 % of the riverine discharges of DOC, DON, and DOP to Tokyo Bay, respectively. The DOM fluxes from sediment in Tokyo Bay were small in comparison with the riverine input of DOM, but were not negligible.
5 Conclusions
We measured the concentrations of pore water DOM (DOC, DON, and DOP) and nutrients over 6 years at four stations in the inner part of Tokyo Bay. Pore water DOM and nutrient concentrations were highest in the summer and lowest in the winter. In our study area, temporal variations in pore water DOM concentrations were significantly correlated with the temperature (DOC and DOP) and DO concentrations (DOC, DON, and DOP) in the overlying water. Rapid decomposition of organic matter under high temperature might be one of the main reasons for the seasonal cycles of pore water DOM concentrations. The reductive dissolution of DOM, adsorbed to mineral phases caused by anoxia in overlying water, might also increase the pore water DOM concentrations of the sediment surface. The DON and DOP fluxes comprised 1.0 and 1.5 % of the TDN and TDP fluxes, respectively, showing that the benthic DOM fluxes were insignificant to the benthic nutrients fluxes. Furthermore DOC, DON, and DOP fluxes were equivalent to about 7, 3, and 10 % of the riverine discharge of DOC, DON, and DOP to Tokyo Bay, respectively. Benthic DOM release was smaller than the river discharge of DOM in Tokyo Bay, but was not negligible.
References
Alkhatib M, del Giorgio PA, Gelinas Y, Lehmann MF (2013) Benthic fluxes of dissolved organic nitrogen in the lower St. Lawrence estuary and implications for selective organic matter degradation. Biogeosciences 10:7609–7622
Alperin MJ, Albert DB, Martens CS (1994) Seasonal variations in production and consumption rates of dissolved organic carbon in an organic-rich coastal sediment. Geochim Cosmochim Acta 58:4909–4930
Alperin MJ, Martens CS, Albert DB, Suayah IB, Benninger LK, Blair NE, Jahnke RA (1999) Benthic fluxes and porewater concentration profiles of dissolved organic carbon in sediments from the North Carolina continental slope. Geochim Cosmochim Acta 63:427–448
Baldwin DS, Beattie JK, Coleman LM, Jones DR (2001) Hydrolysis of an organophosphate ester by manganese dioxide. Environ Sci Technol 35:713–716
Banta GT, Giblin AE, Hobbie JE, Tucker J (1995) Benthic respiration and nitrogen release in Buzzards Bay, Massachusetts. J Mar Res 53:107–135
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton University Press, Princeton, p 241
Bronk DA, Glibert PM (1993) Contrasting patterns of dissolved organic nitrogen release by two size fractions of estuarine plankton during a period of rapid NH4 + consumption and NO2 − production. Mar Ecol Prog Ser 96:291–299
Burdige DJ (2002) Sediment pore waters. In: Hansell DA, Carlson CD (eds) Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, pp 612–664
Burdige DJ (2006) Geochemistry of marine sediments. Princeton University Press, Princeton, p 609
Burdige DJ, Gardner KG (1998) Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Mar Chem 62:45–64
Burdige DJ, Homstead J (1994) Fluxes of dissolved organic carbon from Chesapeake Bay sediments. Geochim Cosmochim Acta 58:3407–3424
Burdige DJ, Zheng S (1998) The biogeochemical cycling of dissolved organic nitrogen in estuarine sediments. Limnol Oceanogr 43:1796–1813
Burdige DJ, Berelson WM, Coale KH, McManus J, Johnson KS (1999) Fluxes of dissolved organic carbon from California continental margin sediments. Geochim Cosmochim Acta 63:1507–1515
Chin YP, Traina SJ, Swank CR (1998) Abundance and properties of dissolved organic matter in pore waters of a freshwater wetland. Limnol Oceanogr 43:1287–1296
Conley DJ, Stockenberg A, Carman R, Johnstone RW, Rahm L, Wulff F (1997) Sediment-water nutrient fluxes in the Gulf of Finland, Baltic Sea. Estuar Coast Shelf Sci 45:591–598
Cowan JLW, Boynton WR (1996) Sediment-water oxygen and nutrient exchanges along the longitudinal axis of Chesapeake Bay: seasonal patterns, controlling factors and ecological significance. Estuaries 19:562–580
De Vittor C, Faganeli J, Emili A, Covelli S, Predozani S, Acquavita A (2012) Benthic fluxes of oxygen, carbon and nutrients in the Marano and Grado Lagoon (northern Adriatic Sea, Italy). Estuar Coast Shelf Sci 113:57–70
Enoksson V (1993) Nutrient recycling by coastal sediment: effects of added algal material. Mar Ecol Prog Ser 92:245–254
Hansen HP, Koroleff F (1999) Determination of nutrients. In: Grasshoff K, Kremling K, Erhardt M (eds) Methods of seawater analysis. Wiley, New York, pp 159–228
Hedge JI, Keil RG (1995) Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar Chem 49:81–115
Holcombe BL, Keil RG, Devol AH (2001) Determination of pore-water dissolved organic carbon fluxes from Mexican margin sediments. Limnol Oceanogr 46:298–308
Hopkinson CS Jr (1987) Nutrient regeneration in shallow-water sediments of the estuarine plume region of the nearshore Georgia Bight, USA. Mar Biol 94:127–142
Hopkinson CS Jr, Giblin AE, Tucker J (2001) Benthic metabolism and nutrient regeneration on the continental shelf of Eastern Massachusetts, USA. Mar Ecol Prog Ser 224:1–19
Imamura M, Matsunashi S (1997) Nitrogen and phosphorus flux between water and sediments around a Bay head. Proc Coast Eng 44:1081–1085 (in Japanese)
Jahnke RA, Alexander CR, Kostka JE (2003) Advective pore water input of nutrients to the Satilla River Estuary, Georgia, USA. Estuar Coast Shelf Sci 56:641–653
Kamatani A (1982) Dissolution rates of silica from diatoms decomposing at various temperatures. Mar Biol 68:91–96
Kanda J (1995) Determination of ammonium in seawater based on the indophenol reaction with o-phenyl phenol (OPP). Water Res 29:2746–2750
Karl DM, Björkman KM (2002) Dynamics of DOP. In: Hansell DA, Carlson CD (eds) Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, pp 612–664
Kodama K, Oyama M, Kume G, Serizawa S, Shiraishi H, ShibataY Shimizu M, Horiguchi T (2010) Impaired megabenthic community structure caused by summer hypoxia in a eutrophic coastal bay. Ecotoxicology 19:479–492
Komada T, Reimers CE, Luther GW III, Burdige DJ (2004) Factor affecting dissolved organic matter dynamics in mixed-redox to anoxic coastal sediments. Geochim Cosmochim Acta 68:4099–4111
Kubo A (2015) Carbon cycling in Tokyo Bay. Dissertation, Tokyo University of Marine Science and Technology
Kubo A, Yamamoto-Kawai M, Kanda J (2015) Seasonal variations in concentration and lability of dissolved organic carbon in Tokyo Bay. Biogeosciences 12:269–279
Landén A, Hall POJ (1998) Seasonal variation of dissolved and adsorbed amino acids and ammonium in a near-shore marine sediment. Mar Ecol Prog Ser 170:67–84
Landén A, Hall POJ (2000) Benthic fluxes and pore water distributions of dissolved free amino acids in the open Skagerrak. Mar Chem 71:53–68
Lee RY, Porubsky WP, Feller IC, McKee KL, Joye SB (2008) Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (Twin Cays, Belize). Biogeochem 87:181–198
Lehtoranta J, Ekholm P, Wahlström S, Tallberg P, Unsitalo R (2015) Labile organic carbon regulates phosphorus release from eroded soil transported into anaerobic coastal systems. Ambio 44:263–273
Li YH, Gregory S (1974) Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38:703–714
Loh AN, Bauer JE (2000) Distribution, partitioning and fluxes of dissolved and particulate organic C, N and P in the eastern North Pacific and Southern Oceans. Deep-Sea Res Pt. I 47:2287–2316
Magni P, Montani S (2006) Seasonal patterns of pore-water nutrients, benthic chlorophyll a and sedimentary AVS in a macrobenthos-rich tidal flat. Hydrobiologia 571:297–311
Matsumura T, Ishimaru T (2004) Freshwater discharge and inflow loads of nitrogen and phosphorus to Tokyo Bay (from April 1997 to March 1999). Oceanogr Jpn 13:25–36 (in Japanese with English abstract)
Matsumura T, Horimoto N, Xu Y, Ishimaru T (2001) Recent trends of nutrients in Tokyo Bay (1989–1998). La mer 39:19–32 (in Japanese with English abstract)
Meybeck M (1982) Carbon, nitrogen, and phosphorus transport by world rivers. Am J Sci 282:401–450
Miyajima T, Tanaka Y, Koike I (2005) Determining 15 N enrichment of dissolved organic nitrogen in environmental waters by gas chromatography/negative-ion chemical ionization mass spectrometry. Limnol Oceanogr: Methods 3:164–173
Monbet P, McKelvie ID, Worsfold PJ (2009) Dissolved organic phosphorus speciation in the waters of the Tamar estuary (SW England). Geochim Cosmochim Acta 73:1027–1038
Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Boicourt WC, Boynton WR, Church TM, Ditoro DM, Elmgren R, Garber JH, Giblin AE, Jahnke RA, Owens NJP, Pilson MEQ, Seitzinger SP (1996) The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochem 35:141–180
Ogawa H, Aoki N, Kon I, Ogura N (1994) Stable carbon isotope ratio of suspended particulate and sedimentary organic matter during the summer blooming in Tokyo Bay. Geochemistry 28:21–36 (in Japanese with English abstract)
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. In: Hill MN (ed) The sea. Interscience, New York, pp 26–77
Selman M, Greenhalgh S, Diaz R, Sugg Z (2008) Eutrophication and hypoxia in coastal areas: a global assessment of the state of knowledge. WRI Policy Note: Water Qual 1:1–6
Skoog AC, Arias-Esquivel VA (2009) The effect of induced anoxia and reoxygenation on benthic fluxes of organic carbon, phosphate, iron, and manganese. Sci Total Environ 407:6085–6092
Skoog A, Hall POJ, Hulth S, Paxéus N, Rutgers van der Loeff M, Westerlund S (1996) Early diagenetic production and sediment-water exchange of fluorescent dissolved organic matter in the coastal environment. Geochim Cosmochim Acta 60:3619–3629
Sloth NP, Blackburn H, Hansen LS, Risgaard-Petersen N, Lomstein BA (1995) Nitrogen cycling in sediments with different organic loading. Mar Ecol Prog Ser 116:163–170
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa, p 311
Suzumura M, Kamatani A (1995) Mineralization of inositol hexaphosphate in aerobic and anaerobic marine sediments: implications for the phosphorus cycle. Geochim Cosmochim Acta 59:1021–1026
Suzumura M, Ogawa H (2001) Distribution of organic carbon, nitrogen, and phosphorus in surface seawater of Tokyo Bay in summer. Bull Coast Oceanogr 38:119–129 (in Japanese with English abstract)
Suzumura M, Kokubun H, Itoh M (2003) Phosphorus cycling at the sediment-water interface in a eutrophic environment of Tokyo Bay, Japan. Oceanogr Jpn 12:501–516 (in Japanese with English abstract)
Takayanagi K, Yamada H (1999) Effects of benthic flux on short term variations of nutrients in Aburatsubo Bay. J Oceanogr 55:463–469
Thibodeau B, Lehmann MF, Kowarzyk J, Mucci A, Gélinas Y, Gilbert D, Maranger R, Alkhatib M (2010) Benthic nutrient fluxes along the Laurentian Channel: impacts on the N budget of the St. Lawrence marine system. Estuar Coast Shelf Sci 90:195–205
Viktorsson L, Ekeroth N, Nilsson M, Kononets M, Hall POJ (2013) Phosphorus recycling in sediments of the central Baltic Sea. Biogeosciences 10:3901–3916
Walsh JJ (1991) Importance of continental margins in the marine biogeochemical cycling of carbon and nitrogen. Nature 350:53–55
Yamada SS, D’Elia CF (1984) Silicic acid regeneration from estuarine sediment cores. Mar Ecol Prog Ser 18:113–118
Zhang L, Wang L, Yin K, Lü Y, Zhang D, Yang Y, Huang X (2013) Pore water nutrient characteristics and the fluxes across the sediment in the Pearl River estuary and adjacent waters, China. Estuar Coast Shelf Sci 133:182–192
Acknowledgments
Special thanks to IDEA Consultants, Inc., Tokyo-Kyuei Co., Sanyo Techno Marine, Inc. for their cooperation in sediment sampling and collecting pore water. This study was conducted as part of follow-up assessment survey by the technical committee for the extension project of Tokyo International Airport, organized by the Ministry of the Land, Infrastructure, Transport, and Tourism. We thank the scientists and administrators who participated in the assessment for their support for this study. We also thank Drs. Michiyo Yamamoto-Kawai, Fuminori Hashihama and Atsushi Kubo for fruitful discussions. This study was also supported by a Grant-in-Aid for Scientific Research (C) (24510009) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by the Sumitomo Foundation Grant (113214). The authors are grateful to the anonymous reviewers, who provided valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yasui, S., Kanda, J., Usui, T. et al. Seasonal variations of dissolved organic matter and nutrients in sediment pore water in the inner part of Tokyo Bay. J Oceanogr 72, 851–866 (2016). https://doi.org/10.1007/s10872-016-0382-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-016-0382-0