Abstract
The nutrients essential for primary production are inorganic salts and it is important to fully understand nutrient dynamics in coastal oceans. There are three major sources of nutrients in the Seto Inland Sea: loadings from terrestrial run-off, advection from the open sea, and release from bottom sediments. Among these sources, information concerning release from bottom sediments has been limited due to infrequent observations. In this study, we took monthly samples over a two-year period, measuring nutrient concentrations in pore water from 0 to 12 cm at a sampling station in Harima Nada (depth 43 m), with the aim of clarifying the variation of nutrient concentrations in, and fluxes from, the sediment. Nutrient concentrations in the pore water were characterized by high concentrations of dissolved ammonium, dissolved inorganic phosphorus (DIP) and dissolved silicate (DSi), with minor amounts of nitrate and nitrite. The daily nutrient supply to the water column was 8.7 ± 4.6 mg-N m−2 day−1 for dissolved inorganic nitrogen (DIN), 2.5 ± 3.0 mg-P m−2 day−1 for DIP and 65.1 ± 36.7 mg-Si m−2 day−1 for DSi. DIN and DIP fluxes from sediments were compared to those of terrestrial loadings into Harima Nada, which were estimated to be comparable to or about two times higher than river loadings for DIN and more than ten times higher than terrestrial loading for DIP. Overall, the results of this study provide important basic information on nutrient dynamics from sediments and their management in Harima Nada.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nutrients are a generic term for dissolved inorganic nitrogen (DIN), phosphorus (DIP), and silicate (DSi), which are potential limiting factors in the growth of macro- and microalgae. These three nutrients are present in seawater as nitrate (NO3−), nitrite (NO2−), ammonium (NH4+), phosphate (PO43−), and silicic acid (Si(OH)4). Because the abundance of primary producers (phytoplankton) is linked to the abundance of marine fish resources, areas with higher nutrient concentrations tend to have larger resources of marine fish (Nixon 1982). However, excessive nutrient supply causes eutrophication, leading to massive phytoplankton blooms including ‘red tides’. When these blooms decompose, the result is anoxia in addition to harmful substances that may be released during the occurrence of red tides (Karim et al. 2002). Since the 1960s, eutrophication caused by excessive nutrient loading from land has led to the formation of unsound marine ecosystems in the world’s coastal oceans, so nutrient management is recognized as important for maintaining a sustainable marine coastal environment (Boesch 2019).
The Seto Inland Sea, Japan is a coastal sea that experienced eutrophication in the past, beginning in the 1960s. In the 1970s and 1980s, red tides became more frequent and caused extensive damage to fisheries (Yanagi 2015). In response, in the 1970s, the Special Law for the Conservation of the environment of the Seto Inland Sea was enacted by the Environment Agency (now the Ministry of the Environment of Japan, MOEJ) to regulate chemical oxygen demand (COD), total nitrogen (TN) and total phosphorus (TP) in the Seto Inland Sea. This law has resulted in a 40% reduction in TN and a 60% reduction in TP today compared to the 1970s. The Seto Inland Sea is thus a pioneering example of successful escape from the eutrophication crisis common to many of the world’s semi-enclosed seas (Yanagi 2015). In recent years, however, a decline in fish catch has become a problem in the Seto Inland Sea, declining from 370 t in the 2000s to 160 t in recent years (SetoUchi Net 2023). One reason for this, is considered to be the decrease in nutrients, leading to a decline in primary production, as a result of which the number of consumers in the food chain has also declined (Tanda et al. 2014; Tada 2021; Tada et al. 2024). In response, the law was amended in 2021 to incorporate a system that allows for the supply of nutrients to the sea area as necessary. The specific measures for nutrient management require a conservation plan appropriate to the conditions of each bay or sea, so there is an urgent need to understand the state of the nutrient environment in each area and to determine the policy for nutrient management appropriate to that area.
Harima Nada is the name of the part of the Seto Inland Sea surrounded by Hyogo, Okayama, Tokushima, and Kagawa prefectures and is one of the areas affected by nutrient decline. The maximum production rate of phytoplankton in Harima Nada and the adjacent Shido Bay is around 1500 mg-C m−2 day−1, and nitrogen is the limiting factor for primary production there (Tada et al. 1998; Yamaguchi et al. 2020). In Harima Nada, the decrease in nutrient concentrations is also evident, with DIN of about 10 µM in the 1970s being reduced to about 5 µM in the 2000s (Nishikawa et al. 2010). Thus, how marine fish resources have responded to this decline is an important research question that has been investigated in recent years. For example, in Kagawa Prefecture, where cultivation of nori (Porphyra) accounts for one-third of the total value of mariculture production, a large-scale discoloration of nori due to nutrient deficiency occurred in 2002, resulting in a 60% fall in production (Matsuoka et al. 2005; Tada et al. 2010). The production of planktivorous fish such as western sand lance (Ammodytes japonicus) and Japanese anchovy (Engraulis japonicus) has also decreased in recent years due to a decline in food (zooplankton), which probably was caused by increasing water temperature and low nutrient levels (Hashiguchi et al. 2021; Nishikawa et al. 2020; Yoneda et al. 2022).
There are three main sources of nutrients in the Seto Inland Sea: run-off from terrestrial deposits, advection from the open ocean and/or adjacent sea areas, and release from sediments (Tada 2021). Several studies have been conducted to try to understand the balance between these three sources and their variations (Abo et al. 2015; Yanagi and Ishii 2004). Measured values of each source are required but, in particular, seasonal changes in release from bottom sediments are not well understood and there has not been enough information available for accurate estimation of their effects on nutrient environment in the water column (Kamiyama et al. 1997, 1998; Tada 2015). However, nutrient supply from bottom sediments plays an important role in coastal marine ecosystems. For example, in Shido Bay, it was reported that nutrients released from sediments were responsible for 33, 22, and 38% of requirements of DIN, DIP, and DSi, respectively, for primary production of phytoplankton (Srithongouthai et al. 2003a, b). Therefore, in the present study, monthly analyses of nutrients in bottom sediments were conducted at a monitoring station in Harima Nada over a two-year period to clarify the seasonal changes in nutrient concentrations in bottom sediments and to estimate their contribution to nutrient supply.
2 Material and methods
2.1 Sediment samples
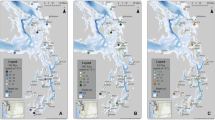
Sampling was conducted monthly at Site H off the eastern coast of Shodo Island, Harima Nada (34° 26.26N, 134° 22.80E, water depth ~ 43 m) from April 2020 to March 2022 (Fig. 1). The observations were conducted using Kagawa University research vessel, Calanus III. Before sediment sampling, the water column profiles of temperature and dissolved oxygen were measured using a conductivity-temperature-depth (CTD) sensor (AAQ-1183; JFE Advantech, Tokyo, Japan). Sediment was collected with a core sampler (G.S. type core sampler ‘Asyura’; Rigo Co., Tokyo, Japan) which can collect three cores at one time. From the three cores obtained, the one in best condition (e.g., flat, least disturbed sediment surface) was selected for analysis. The survey was conducted during a day trip, taking approximately 30 min to return ashore after collecting sediment samples. In the intervals of travel between subsequent sampling localities, core samples were maintained at the ambient seawater temperature corresponding to the sampling localities and in darkness. The following operations were carried out as soon as possible after retuning ashore. Prior to sectioning the core samples, overlying water was collected from the core tube using a siphon for nutrient analysis. The core sample was sectioned at intervals of 0–1, 1–2, 2–4, 4–8, and 8–12 cm below the surface. A part of the cut sample was weighed when wet and after drying at 60 °C for overnight to determine the water content.
Map of sampling site, Site H. The upper map shows the location of the study area among the main islands of the Japanese Archipelago, and the lower map shows Harima Nada in more detail. The orange dotted line in the lower figure indicates the western boundary of the area of Harima Nada (~ 3426 km2) as defined by the Ministry of the Environment. Site H is just east of Shodo Island. The water depth is about 43 m. The black solid circles with lowercase letters (a–j) in the lower figure are the locations of the rivers: Asahi river (a), Yoshii river (b), Chikusa river (c), Ibo river (d), Yumesaki river (e), Ichi river (f), Kako river (g), Akashi river (h), Shin river (i), Kotou river (j)
2.2 Nutrient analysis
The cut sediment sample was centrifuged for 15 min at 3000 r.p.m., and the supernatant liquid (i.e., pore water) was collected. The collected pore water including the overlying water was filtered with a 0.45 μm pore-size membrane filter and kept in a freezer at −20 °C until nutrient analysis with an auto-analyzer (Auto Analyzer III; BL Tec, Japan). Each sample was analyzed for four types of dissolved inorganic nutrient: nitrate and nitrite (NO2 + NO3), ammonium (NH4), phosphate (PO4), and silicic acid (Si(OH)4). Concentrations for each of NO2 + NO3-N and NH4-N were summed and expressed as dissolved inorganic nitrogen (DIN), PO4-P as dissolved inorganic phosphorus (DIP), and Si(OH)4-Si as dissolved silicate (DSi).
2.3 TOC and TN analysis
Surface sediment samples (0–1 cm) from January and February 2022 were analyzed for analyzing total organic carbon (TOC) and total nitrogen (TN) in the sediments. After the sediment sample was freeze-dried, 1 N HCl was added to an approximately 500 mg sediment sample, and the sample was left for 24 h at room temperature to remove non-organic carbon, carbonate (Hedges and Stern 1984). Then, the remaining HCl was removed with de-ionized water until pH ~ 7, and the sample was freeze-dried again. Approximately 15 mg of the freeze-dried sample was wrapped in a tin capsule for analysis with a CHN coder (JM10; J-Science Lab, Kyoto, Japan). Weight loss due to loss of carbonate was obtained as the difference between the weights of pre-HCl and HCl treatment. Each sediment sample was analyzed three times.
2.4 Data analysis
Nutrient fluxes (J) in DIN, DIP, and DSi were estimated by nutrient concentration gradients between the surface sediment (0–1 cm) pore water and the overlying water using the following equation, Fick’s first law (Yamamoto et al. 1998).
where \(\phi\) = the porosity of the surface sediment (0–1 cm);
\({D}_{s}\) = a diffusion coefficient;
\(dC/dz\) = the nutrient concentration gradient between the pore water and the overlying water.
Difference in depth (\(dz\)) was determined as the difference (0.5 cm) between the overlying water as 0 cm and the mean depth of the surface sediments of 0–1 cm.
The porosity of the surface sediment (0–1 cm) (\(\phi\)) was obtained using the following equation.
where \(\rho\) = the density of the dried sediment;
\(\omega\) = water content of the sediment.
In this study, the value of \(\rho\) = 2.5 g m−3 obtained in Osaka Bay (which is part of the Seto Inland Sea) was used (Yasuhara and Yamazaki 2005).
A diffusion coefficient (\({D}_{s}\)) was calculated using the following equation.
where \({D}_{0}^{0}\) = the diffusion coefficient at 0 °C for each nutrient;
\(\alpha\) = constant for anions or cations;
\(t\) = bottom water temperature.
The constant (\(\alpha\)) used was 0.040 for anions and 0.048 for cations (Lerman 1979). The bottom water temperature was that obtained from CTD data. Values for the diffusion coefficient at 0 °C were taken from previous studies (Li and Gregory 1974; Rebreanu et al. 2008; Yamamoto et al. 1998), as follows: NH4, 0.98 × 10−5 cm2 s−1; NO3, 0.987 × 10−5 cm2 s−1; PO4, 6.1 × 10−6 cm2 s−1; and Si(OH)4, 0.47 × 10−5 cm2 s−1. Note that because nitrate and nitrite cannot be separated in this experiment, the diffusion coefficient value for nitrate was used to calculate the flux of NO2 + NO3.
2.5 Statistical analyses
Linear correlations between two data sets were assessed using Pearson's product moment correlation coefficient (r) with Microsoft Excel (Microsoft). The statistical significances of the linear correlations were determined by using the p-value. That is, if the p-value was less than 0.05, the correlation was considered significant.
3 Results
3.1 Bottom temperature, dissolved oxygen, porosity, and TOC/TN contents
The bottom water temperature varied with the seasons between a minimum of 8.7 °C in winter and maximum 26.0 °C in summer (16.9 °C ± 5.2 °C, mean annual temperature ± SD, n = 24; Fig. 2). Winter seasonal low temperatures occurred between February and March, at around 10 °C; seasonal high temperatures were observed to occur between September and October, at values around 25 °C.
Temperature and dissolved oxygen (DO) in the overlying water, sediment porosity, and pore water nutrient concentrations at different sediment depths during the sampling period (April 2020 to March 2022). The ordinate axis for each nutrient concentration is logarithmic. DIN is the sum of NO2 + NO3 and NH4+; DIP is PO4, and DSi is Si(OH)4. Each length (e.g., 0‒1 cm) is the interval of the sediment section
The trend of dissolved oxygen (DO) showed a typical inverse relationship with temperature (Fig. 2): relatively high (~ 8–10 mg L−1) in winter and ~ 3 mg L−1 in summer. The minimum value of DO occurred in July and August, 1–3 months earlier than the period of maximum bottom water temperature. The mean annual value of DO was 6.6 ± 2.0 mg L−1 (mean ± SD, n = 24).
Porosity varied from 0.77 to 0.94, but the mean value at each sediment depth was ~ 0.85 with no large variations (Fig. 2). For example, the mean value and the standard deviation of the porosity were 0.89 ± 0.03 (n = 24) for 0–1 cm, 0.87 ± 0.02 (n = 24) for 2–4 cm, and 0.84 ± 0.02 (n = 24) for 8–12 cm.
TOC contents were 18.9 ± 0.8 mg-C g−1 (n = 3) for January 2022 and 19.4 ± 0.1 mg-C g−1 (n = 3) for February 2022. The mean TOC for both months combined was 19.1 mg-C g−1. TN contents was 2.4 ± 0.1 mg-N g−1 and 2.4 ± 0.05 mg-N g−1 for January 2022 and February 2022, respectively.
3.2 Nutrient concentrations in pore water
The vertical distribution of pore water nutrients shows an increasing trend with depth from the surface to deeper layers, except for NO2 + NO3 (Figs. 2 and 3, Table 1). For these nutrients, the overlying water concentrations were 1–2 orders lower compared with their pore water concentrations (Fig. 2, Table 1). Seasonal variations of DIN, DIP, and DSi in both pore water and overlying water show trends of increasing in summer to fall and decreasing in winter to spring (Figs. 2 and 3). Correlation tests between bottom water temperature versus nutrients in overlying water or pore water revealed significant relationships (r: 0.39–0.68, p < 0.05), excepting some of the results (i.e., 4–8 cm of DIN and DIP and 8–12 cm of DIN) (Table 2).
Vertical distributions in pore water nutrient concentrations at each season in sediments. Each value is expressed as a mean value for each period. Plots on the depth axis are located at the mean value of the widths cut (e.g., 8–12 cm is 10 cm). DIN, DIP and DSi are as defined in the legend of Fig. 2
No general trends were apparent in NO2 + NO3 concentrations (Figs. 2 and 3) but in the overlying water values sometimes exceeded concentrations in the pore water, a phenomenon not observed for other nutrients. The mean of NO2 + NO3 concentration in the pore water was highest for the surface layer of 0–1 cm and lower than ~ 2 µM for the other sediment depths (Table 1). The concentration of NH4 in the overlying water was low, much higher in the pore water and increasing markedly with sediment depth (Table 1). When comparing NO2 + NO3 and NH4 concentrations in the pore water, the combined NO2 + NO3 concentration never exceed that of NH4, except for April 2020 (Figs. 2 and 3, Table 1). Consequently, the contribution of NH4 to DIN in the pore water is very high, comprising 98%.
DIP concentration in the overlying water ranges from 0.3 to 1.9 µM and is much higher in the pore water, increasing with sediment depth (Figs. 2 and 3, Table 1). DSi was present in the highest concentration compared with other nutrients in both the overlying and pore water. DSi in the overlying water was never much more than 60 µM, while the pore water mean never fell below the order of hundred at any depth and increased with depth, as with DIN and DIP (Figs. 2 and 3, Table 1).
3.3 Variation in nutrient flux
When comparing the mean values of DIN, DIP, DSi fluxes from the pore water to the overlying water, they ranked in the order DSi (65.1 ± 36.7 mg-Si m−2 day−1) > DIN (8.7 ± 4.6 mg-N m−2 day−1) > DIP (2.5 ± 3.0 mg-P m−2 day−1) (Table 1). As a molar ratio their mean values are approximately 7:1:28.
All flux values tended to be lower in winter, higher in summer to fall (Fig. 4). Bottom temperature showed a significant correlation with each flux (r > 0.6, p < 0.01) (Table 2). DIN flux was ~ 4 mg-N m−2 day−1 in winter, and ~ 10–15 mg-N m−2 day−1 in summer (Fig. 4). DIP flux was < 2 mg-P m−2 day−1 in winter but in fall reached > 10 mg-P m−2 day−1 (Fig. 4). DSi flux was < 40 mg-Si m−2 day−1 in winter, > 100 mg-Si m−2 day−1 in summer, with a maximum value of 155 mg-Si m−2 day−1 (Fig. 4).
Nutrient fluxes from sediment to water column within the sampling period (April 2020 to March 2022). DIN, DIP and DSi are as defined in the legend of Fig. 2. Each nutrient flux value was calculated from the difference in nutrient concentrations between the overlying water and the surface sediment (0‒1 cm) using Fick’s first law
4 Discussion
4.1 Pore water nutrients and their fluxes to the overlying water
Nutrient concentrations and their fluxes were higher in seasons when bottom water temperature was high, with significant relationships between temperature and nutrient concentrations and their fluxes (Table 2). Therefore, water temperature is considered to be a key factor in controlling the nutrient behaviors in sediments. Such a trend of high pore water nutrients with high water temperature has been observed in other Japanese coastal areas, too (Muta et al. 2020; Yasui et al. 2016), and water-temperature variability is recognized as an important factor in promoting organic matter degradation (Arndt et al. 2013). Such responsivity of organic matter degradation rate to temperature is related to bacterial processes, which are temperature dependent, affecting bacterial activation and/or shifts in dominant bacterial species and, consequently, varying the activity of organic matter degradation in the sediment (Robador et al. 2009; Tsuboi et al. 2014).
Anoxic conditions of bottom water, in addition to bottom water temperature, have an important influence on nutrient fluxes, especially for phosphorus. Indeed, DIP in sedimentary pore water elutes to the water column in the presence of anaerobic bottom water (Patrick and Khalid 1974; Watanabe and Tsunogai 1984; Tanaka 1994). Correspondingly, the effects of such high DIP flux and DIP concentration in overlying water under bottom-water anoxic conditions have been confirmed for various marine environments including the Seto Inland Sea (Srithongouthai et al. 2002; Viktorsson et al. 2013; Muta et al. 2020). In line with previous reports, we confirmed that DIP flux at our site was high under conditions of lower bottom-water DO levels. On the other hand, form measurements of activity or potential bioavailability of phosphorus in sediments, it was found that more than half the total phosphorus in most sediments (including representative natural sampling points around Harima Nada) contain organic phosphorus (Nakakuni et al. 2023; Tada et al. 2023). In contrast, exchangeable or loosely sorbed phosphorus and Fe-bound phosphorus account for approximately 10% each (Nakakuni et al. 2023; Tada et al. 2023). If Site H has a similar phosphorus composition, the degradation of organic material might also have a significant influence on the availability of inorganic phosphorus. Indeed, the availability and releasability of phosphorus in sediments is not only determined by changes in redox conditions, but is also related to the phosphorus-forms present in those sediments (Cavalcante et al. 2018; Gächter and Müller 2003; Tu et al. 2019). At Site H, a decrease in bottom water DO, facilitating Fe-bound phosphorus removal, and an increase in bottom water temperature, accelerating microbial activity, occur almost simultaneously. This coincidence makes it difficult to determine which of the two factors is more influential.
The distribution of pore water nutrients shows higher concentrations with substrate depth for NH4, DIP, and DSi, whereas NO2 + NO3 concentration was sometimes higher in the overlying water rather than that in the sediment (Fig. 2). NH4 is generated by the degradation of organic nitrogen, being oxidized to nitrate and nitrite when dissolved oxygen is supplied to the sediment (Berner 1980). From the sediment surface to deeper in the sediment, the dominance of NH4 (> 90% of DIN) in the pore water rather than NO2 + NO3 indicates that the sediment lacks dissolved oxygen beneath a few millimeters from the surface, which is supported by vertical profiles of dissolved oxygen measured in sediments at millimeter intervals in Hiroshima Bay, also in the Seto Inland Sea (Sayama et al. 2002).
The nutrient fluxes observed in the present study are roughly similar to those observed in previous studies including around the Harima Nada area of the Seto Inland Sea. For example, a DIN flux of 0–15.5 mg-N m−2 day−1 was reported in Tsuda Bay (Tada et al. 2018) and 8–20 mg-N m−2 day−1 in Shido Bay (Srithongouthai et al. 2003b). However, in contrast to these values, the fluxes in Tokyo Bay (10–240 mg-N m−2 day−1 in NH4; Yasui et al. 2016) and Osaka Bay (high DIN fluxes reaching 100 mg-N m−2 day−1; Tada et al. 2018) are considerably higher. One reason why the fluxes differ in different coastal areas may be related to the amount of substrate contained (e.g., particulate nitrogen in sediments). Several previous studies have found high nutrient fluxes in sediments containing high levels of TN (Muta et al. 2020; Tada et al. 2018). The sediments in Tsuda Bay and Shido Bay contain ~ 2 mg-N g−1 (Yamaguchi et al. 2014), similar to the amounts reported in the present study, whereas Osaka Bay and Tokyo Bay have values as high as ~ 3 mg-N g−1 (Kubo and Kanda 2017; Tada et al. 2018).
4.2 Contribution of pore water as a source of nutrients in Harima Nada
Comparing nitrogen supply from benthic flux and terrestrial inputs, nitrogen input to Harima Nada from terrestrial sources was estimated at approximately 34 t day−1, (Ministry of the Environment 2015) which divided by the sea area of Harima Nada (3426 km2) is 9.9 mg-N m−2 day−1 (Fig. 5A). This value is comparable to that obtained as the mean benthic flux in the present study (8.7 ± 4.6 mg-N m−2 day−1; Fig. 5A, Table 1). Terrestrial phosphorus inputs to Harima Nada were reported to be 1.6 t-P day−1 (Ministry of the Environment 2015) and the value per unit area of the sea area of Harima Nada is 0.47 mg-P m−2 day−1. The mean DIP benthic flux estimated in the present study was 2.5 ± 3.0 mg-P m−2 day−1, which is 5.3 times higher than the estimated value from land areas (Fig. 5A, Table 1).
Comparison of nutrient inputs from terrestrial areas and nutrient supply from pore water to the water column in Harima Nada, and DIN flux and DIP flux between rivers flowing into Harima-Nada and the pore water. The terrestrial loadings in A were obtained by dividing terrestrial nitrogen loading into Harima Nada (34 t day−1, as estimated by the Ministry of the Environment 2015) by the area of the Harima Nada (3426 km2). The nutrient fluxes in A and B are expressed as the mean value (n = 24 for A, n = 6 for spring [March–May], summer [June–August], fall [September–November], and winter [December–February] in B) and its standard deviation (error bars) during the observation periods. River fluxes were calculated using a river nutrient data set incorporated into Leng et al. (2023). The locations of the rivers are shown in Fig. 1. Nitrogen pore water flux is the sum of the fluxes of NO2 + NO3 and NH4
Additionally, we compared seasonal DIN flux and DIP flux from ten rivers flowing into Harima-Nada with corresponding seasonal benthic fluxes (Fig. 5B). The river data include both class A rivers (Asahi, Yoshii, Ibo, and Kako rivers) and class B rivers (Chikusa, Yumesaki, Ichi, Akashi, Shin, and Kotou rivers; Leng et al. 2023). DIN flux in these rivers did not show large fluctuations between spring (March–May) and fall (September–November), ranging from 4.7 to 5.4 mg-N m−2 day−1. However, the flux decreased slightly to 3.0 mg-N m−2 day−1 in winter (December–February). DIN flux from the sediments varied between 6.9 and 8.6 mg-N m−2 day−1 in the spring to summer (June–August), a high of 13 mg-N m−2 day−1 in the fall, decreasing to 6.4 mg-N m−2 day−1 in the winter. The ratio of DIN flux from the rivers to DIN flux from the sediments was 1.5–1.6 in spring and summer and 2.1–2.4 in fall and winter.
DIP flux from the rivers remained low at ~ 0.4 mg-P m−2 day−1 throughout the year, while DIP flux from the sediments rose markedly during summer and fall (Fig. 5B), varying between 3.5 and 4.7 mg-P m−2 day−1. Consequently, the ratio of DIP flux from the rivers to DIP flux from the sediments was lower in the spring and winter, at ~ 2–4, but rose to ~ 8–12 in summer and fall.
These results demonstrate clearly that benthic fluxes in Harima Nada include a high proportion of DIN and DIP sources from within Harima Nada itself. The importance of benthic fluxes as nutrient sources has been proposed in previous studies. For example, Yanagi and Ishii (2004) estimated the origins of TN and TP in the Seto Inland Sea using a unit response function method, reporting that the open ocean origin was ~ 60% (which is in close agreement with other studies [e.g., Fujiwara et al. 1997; Hayami et al. 2004; Takeoka 2006; Yanagi and Ishii 2004), ~ 10% from rivers, and ~ 30% originating from bottom sediments. In Harima Nada, Abo et al. (2015) estimated DIN sources in winter after dividing the area into northern and southern parts. They reported that 48% of the DIN source is derived from the open ocean, and 26% each from rivers and from bottom sediments in the northern Harima Nada; and in southern Harima Nada, open ocean, riverine, and pore water discharges were estimated at 27, 4, 69%, respectively. All studies, including the present study, concluded that nutrient supply from the bottom sediment plays a major role in nutrient supply to the water column in the Seto Inland Sea.
For comparison, in Tokyo Bay, terrestrial inputs were estimated at 76,000 t year−1 for DIN and 4100 t year−1 for DIP, and benthic flux was 22,300 ± 4900 t year−1 for DIN and 4000 ± 400 t year−1 for DIP (Yasui et al. 2016). Also, in Ariake Bay, Kyushu, the DIN from sediment was estimated to be equivalent to 48% of the DIN input from rivers, and benthic DIP was estimated to be equivalent to 117% of that from rivers (Koriyama et al. 2013). Therefore, the importance of benthic nutrient fluxes as a nutrient source is not restricted to the Seto Inland Sea but is recognized in other sea areas, too. Note that our estimation was calculated assuming an equal supply of river-derived nutrients to Harima Nada. However, in practice, the balance between river-derived versus bottom-sediment-derived nutrient supplies would be altered in estuary areas due to the strong influence of the proximate riverine input.
Note that the figures arrived at in the present study clarify that, in comparison with terrestrial inputs and river fluxes, benthic fluxes contribute more as nutrient sources to Harima Nada in terms of DIP flux than of DIN (Fig. 5). This feature might be related to a difference in degradation rates between nitrogen and phosphorous components. Montani et al. (1991) investigated nitrogen and phosphorus contents and cycling in phytoplankton, zooplankton, suspended particles, sinking particles, and surface sediments at sampling points in the Osaka Bay a part of the Seto Inland Sea, over three years. They found that the proportions of nitrogen and phosphorus reaching the surface sediments were 8 and 16%, respectively, of the supply of these components to the bay. In Harima Nada, the ratio of particulate nitrogen to particulate phosphorus in surface seawater is ~ 12, while the ratio of TN to TP in sediments is 7.6–9.0, which is obviously lower than that of the sea water (Asahi et al. 2014; Nakakuni et al. 2022), implying relatively high phosphorus in sediments. These results suggest that degradation fields (e.g., water column, surface sediment, or deeper sediment) are different for nitrogenous and phosphorous organic materials, and phosphorus is rather refractory in comparison with nitrogen. Additionally, release and absorption of phosphorous in the bottom sediments depending on DO concentrations also might be related to the high proportion of phosphorous in the sediment (Patrick and Khalid 1974; Watanabe and Tsunogai 1984; Tanaka 1994). Therefore, sediment probably becomes a high phosphorus supplier. Indeed, the DIN:DIP flux ratio estimated in this study was a mean of 7:1, which is markedly higher phosphorus than the Redfield ratio.
There was a large difference between nitrogen input to Harima Nada from terrestrial sources estimated by the Ministry of the Environment and the DIN flux from the river (Fig. 5). This is because the terrestrial sources are calculated as the amount of TN including industrial sources (Ministry of the Environment 2015), while nitrogen of river origin is estimated as DIN (Leng et al. 2023). Comparison of seasonal DIN or DIP fluxes from sediments with those from the rivers revealed that the relationship between them is not constant (Fig. 5B). The higher ratios of DIN flux from sediments relative to its flux from the rivers in the summer and fall was conceivable, because benthic fluxes were higher in these seasons. However, the ratio in winter was maintained at a high value of ~ 2, the same level as in the fall. This indicates that although DIN fluxes from the sediments are lower in winter, they are still an important source of DIN in winter relative to the rivers. Surprisingly, the sediment contribution of phosphorus in spring and winter was only 2–4 times higher than that of the rivers, whereas in summer and fall the ratio of DIP flux from sediments relative to its flux from the rivers was > 7, resulting in the contrastingly large ratio observed between seasons. The large variation in DIP flux from the sediments is caused by changes in anoxic conditions and temperature of the bottom water, as described in the previous section.
4.3 Sediments for nutrient management
From the discussion, it established that nutrient fluxes from sediments have a range from the same to ~ 2 times higher for nitrogen or higher values (up to ~ 12 times) for phosphorus in comparison with river fluxes. In view of attempts to improvement a reversal of declining nutrient concentrations in the Seto Inland Sea (see Introduction), it is important to fully understand the levels and trends of nutrient fluxes from sediments as sources of DIN and DIP over appropriate time. However, effective nutrient management requires control over benthic nutrients, for which there are several factors to consider.
Firstly, it is important to consider the distribution of marine bottom sediments and position of rivers related to input sources of riverine nutrient. Class A rivers (rivers defined as economically important in Japan) flowing into Harima Nada are, from the Northwest, the rivers Asahi and Yoshii in Okayama Prefecture, and, from the Northeast, the Ibo and Kako rivers in Hyogo Prefecture. Thus, if these rivers are the main riverine source of nutrients, they are relatively easy to manage since there are just four locations. However, sedimentary pore water requires management of the entire ocean area because its sources are present throughout the entire ocean.
Secondly, the sources of organic particulate matter in bottom marine sediment are related. DIN in pore water is derived from degradation of particulate organic nitrogen in the sediment. The organic matter in the sediment consists mainly of detritus precipitation, including the dead remains of microalgae in the studied area. Indeed, the ratio of TOC to TN and/or their stable isotopic composition in sedimentary organic matter in Harima Nada investigated in previous studies indicates microalgae-like values (Asaoka et al. 2020; Yamaguchi et al. 2014), and it is estimated that more than 80% of the sediment organic matter is derived from microalgae (Nakakuni et al. 2022). Therefore, the TN content of sediments is the result of primary production in the water column and reflects its fluctuations. As an example of the N cycle between the water column and the sediment, it is estimated that, of the nitrogen particles produced by primary production (760 t day−1) in Osaka Bay, about 9% (67 t day−1) become sediment (Montani et al. 1991). This indicates that 90% of organic matter produced in the water column is decomposed or suspended in the water column rather than being transferred to sediment. Thus, in order to increase nitrogen supply to the bottom sediment, it is necessary to increase primary production (the conversion of inorganic to particulate nitrogen) and/or to induce strongly anoxic conditions in the water column to enhance preservation of organic matters. The former might result in a cause-effect reversal because the activation of primary production is a goal of nutrient management in the Seto Inland Sea. The latter cannot be artificially controlled. Therefore, when considering rivers, sediments, and the open sea as sources of nutrients, it appears that rivers present a more practical option for management compared to the others.
Certainly, then, this study illustrates that benthic nutrient fluxes contributes to nutrient sources in Harima Nada, and the importance of understanding the interactions involved is here reaffirmed. However, within the framework of nutrient management, it is also concluded that attempting to regulated nutrient fluxes from sediments is inherently complex. On the other hand, there is a need for continuous monitoring, as nutrient management strategies can modify biological nutrient cycling within the water column, potentially leading to changes in the concentration of particulate matter found in the sediments. Therefore, for successful nutrient management, estimation of fluctuations in different ecosystem compartments through continuous observation of nutrient fluxes from sediments is clearly important, and there are a number of different methods available (Tada 2015).
4.4 Comparison of field data and conventional models in nutrient profiles
Attempts have been made to understand the behavior of pore water nutrients in Japanese coastal areas including the Seto Inland Sea using empirical formulas (Muta et al. 2020; Tada et al. 2018; Ministry of the Environment 2017), which were estimated from limited seasonal data, coastal areas, and/or synthesis of findings from previous studies. Therefore, an attempt was made to determine if our data could be reproduced by these equations.
First, Tada et al. (2018) provided the following empirical formula for estimating DIN flux (\({J}_{DIN}\)) from sediment to the water column for Harima Nada and Osaka Bay:
where TN denotes total nitrogen per unit dry sediment weight. This formula was obtained from results of the DIN flux measured in core incubation experiments in different seasons at three sites in Harima Nada and five sites in Osaka Bay. The TN data were taken from the surface of 0–1 cm sediment. Through the application of this empirical formula, Tada et al. (2018) has confirmed a high coefficient of determination (r2 = 0.78) between measured NH4 flux values obtained by incubation experiments and from estimates.
A report by the (Ministry of the Environment 2017) included the following empirical formula for ammonium flux (\({J}_{N{H}_{4}}\)) for the Seto Inland Sea.
where DO and TOC are dissolved oxygen and total organic carbon per unit dry sediment weight. This equation was determined by multiple regression analysis using data on NH4 fluxes, TN, and DO reported in previous literature obtained from 13 locations in the Seto Inland Sea and two locations in freshwater lakes.
Lastly, Muta et al. (2020) proposed an empirical formula for estimating NH4 concentration of the surface sediment (\({C}_{{NH}_{4}}\)) in the seasonally hypoxic Omura Bay.
In that study, a total of six sampling points, one regular observation point and five sub-observation points were established in Omura Bay to measure nutrient concentrations in the pore water and TN content in the surface sediments (0–5 mm). At the regular observation point, monthly observations were conducted for one year. The equation for estimating the concentration of ammonia in surface sediments was then derived using TN content and water temperature as variables. Using this empirical formula, a coefficient of determination of 0.755 was obtained for the relationship between measured and estimated values for Omura Bay.
A comparison was made between observed values and values estimated using the above equations for NH4 flux, DIN flux and NH4 concentration. All estimated values showed significant correlations with the observed values (r > 0.4, p < 0.05), confirming that trend reproducibility can be obtained using these equations (Fig. 6). It is expected that these equations can be used to incorporate DIN dynamics from the bottom sediment into a model focused on nutrient cycles in Harima Nada. While another factors like bioturbation can change DIN flux from sediments (Gautreau et al. 2023; Tada et al. 2017), our results show that these factors (i.e., t, TOC, TN, and DO) largely explain the sediment fluxes observed at our sampling locations. This is confirmed also by the high coefficient of determination between the estimated and measured values obtained with the empirical formulae already proposed in previous studies (Muta et al. 2020; Tada et al. 2018). Furthermore, the calculations are based on TN and DO of the bottom sediment and water, suggesting that if these values are available for an objective area, it is possible to estimate DIN flux or its concentration at that time, even if it is in the past. Such an approach provides important knowledge for considering the transition history of marine areas such as the Seto Inland Sea, where nutrient environment and marine ecology are apparently different now from what they were in the past (Imai et al. 2006; Nishikawa et al. 2010, 2014; Umehara et al. 2022; Yamamoto 2003; Yoneda et al. 2022).
Comparison of nutrient fluxes from pore water to the water column (NH4 or DIN fluxes) and nutrient concentration (NH4) in the pore water of surface sediment samples (0‒1 cm) obtained in this study with their values as estimated by empirical equations proposed by previous studies. A Measured and estimated DIN fluxes from pore water to the water column. The empirical equation for (A [\({J}_{DIN}=1.802\times {e}^{0.1277\bullet t}\times \left(TN-1.301\right)\)]) was obtained from TN content in surface sediment (0‒1 cm) and overlying water temperature as variables, using experimental data from core incubations conducted from Osaka Bay to Harima Nada (Tada et al. 2018). B Measured and estimated NH4 fluxes from pore water to the water column. The empirical equation for (B [\({J}_{N{H}_{4}}=-0.36\times {1.09}^{t-25}\times \left(DO-12.09\right)\times \left(TOC-5.93\right)\)]) was obtained using the flux data from a total of 15 sediment sites, including the Seto Inland Sea. The variables in this equation are the TN content in surface sediment (0‒1 cm), overlying water temperature, and the dissolved oxygen in the overlying water (Ministry of the Environment 2017). C Measured and estimated NH4 concentrations of pore water in surface sediment (0‒1 cm); the empirical equation for (C [\({C}_{{{\text{NH}}}_{4}}=35.9\times \left(TN/100\right)+2.9\times t-37.6\)]) was proposed using the TN content in the surface sediments and the temperature of the overlying water as variables, based on two years of observations in Omura Bay, Nagasaki Prefecture, Japan (Muta et al. 2020)
However, the difference in absolute concentration between the observed and estimated values (the value of the slope) should be noted. Specifically, both NH4 flux and DIN flux were estimated to be about double the values of the observed data (Fig. 6). This may be due to differences in the values obtained from different flux measurement methods. For benthic fluxes, the methods of flux estimation (the chamber methods of Forja and Gómez-Parra (1998); Reay et al. (1995); the incubation methods of Cowan et al. (1996); Kamiyama et al. (1997); Srithongouthai et al. (2003a, b)and calculations using the diffusive flux equations of Koriyama et al. (2013); Yamamoto et al. (1998) and differences in sediment samplers (e.g., diameter of the corer tube) have been reported to result in different values (Tada 2015; Tada et al. 2018). These differences are of the order of a factor of ~ 2–3, for example for different corers (a KK type core sampler, Φ 4.2 cm; a G.S. type core sampler ‘Asyura’, Φ 8.2 cm; and an HR type core sampler, Φ 11 cm; Tada (2015). Such differences may be one of the reasons for the large differences observed between the values reported here and the estimated values, although the estimated absolute concentrations in the pore water were in relatively good agreement with the measured values.
Also, it should be considered that TOC and TN collected only in January and February were used for the estimation. Because TOC and TN in surface sediments fluctuate, the correlation might be higher if the monthly TOC and TN fluctuations were included in data sets for the estimation. However, TOC and TN variations in surface sediments observed in the surrounding area of Harima Nada are certainly not large: Koomklang et al. (2018) measured surface TN content (0–1 cm) over a 20-month period in Shido Bay, Harima Nada, and reported a mean variation of only 2.49 ± 0.17 mg-N g−1. Therefore, it is unlikely that these variations would cancel the two-fold difference in DIN or NH4 flux data observed between the observation and the estimation. Alternatively, it has been proposed that the top layer of surface sediment, just ~ 1 mm deep, may experience significant variation in organic content, with consequent effects on nutrient fluxes (Koomklang et al. 2018; Sohma et al. 2008). However, the organic materials in this layer degrade easily and are difficult to collect by easy resuspension, making research into this aspect challenging.
It is also necessary to recognize that the degree of bioturbation is a factor that alters nutrient fluxes from sediments (Callender and Hammond 1982). Tada et al. (2017) added living Polychaeta and the bivalve Musculista (= Arcuatula) senhousia to sediments from Shido Bay, adjacent to Harima Nada, at densities approximately 6–18 times higher than the on-site population density, and conducted incubation experiments to determine changes in nutrient fluxes from the sediments. The results showed that nutrient fluxes increased in experiments where the M. senhousia was added, but no effect was observed in the presence of Polychaeta compared to the control. From this, it is concluded that in the Seto Inland Sea, changes in nutrient fluxes from sediments by bioturbation should be considered in sediments with containing large number of bivalves but, at in-situ population densities, the rate of nutrient leaching due to bioturbation is not largely affected. The dominant benthic facies in the vicinity of the sampling point of this study (Site H) has been identified as Polychaeta (50–60%) followed by Mollusca at about 10% (Tsujino 2018). Therefore, it is likely that the benthic biota at this site do not have a marked impact on nutrient fluxes at Site H, and this may also be a reason why nutrient fluxes could be explained by simple factors (i.e., t, TOC, TN, and DO). However, if empirical formulae are proposed that also incorporate these factors (i.e., bioturbation, benthic biota), it is expected that more accurate estimates can be obtained.
Data availability
Data will be made available on request.
Change history
08 May 2024
Alignment of values were incorrect in Table 1 and corrected in this version.
References
Abo K, Nakagawa N, Abe K (2015) Nutrient dynamics and nutrient level management for Nori (Pyropia) culture in the eastern Seto Inland Sea. Aquabiology 37:274–279 (In Japanese with English abstract)
Arndt S, Jørgensen BB, LaRowe DE, Middelburg JJ, Pancost RD, Regnier P (2013) Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth-Sci Rev 123:53–86. https://doi.org/10.1016/j.earscirev.2013.02.008
Asahi T, Ichimi K, Yamaguchi H, Tada K (2014) Horizontal distribution of particulate matter and its characterization using phosphorus as an indicator in surface coastal water, Harima-Nada, the Seto Inland Sea, Japan. J Oceanogr 70:277–287. https://doi.org/10.1007/s10872-014-0230-z
Asaoka S, Jadoon WA, Umehara A, Takeda K, Otani S, Ohno M, Fujitake N, Sakugawa H, Okamura H (2020) Organic matter degradation characteristics of coastal marine sediments collected from the Seto Inland Sea. Japan Mar Chem 225:103854. https://doi.org/10.1016/j.marchem.2020.103854
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton Univ Press. https://doi.org/10.2307/j.ctvx8b6p2
Boesch DF (2019) Barriers and bridges in abating coastal eutrophication. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00123
Callender E, Hammond DE (1982) Nutrient exchange across the sediment-water interface in the potomac river estuary. Estuar Coast Shelf Sci 15:395–413. https://doi.org/10.1016/0272-7714(82)90050-6
Cavalcante H, Araújo F, Noyma NP, Becker V (2018) Phosphorus fractionation in sediments of tropical semiarid reservoirs. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.11.204
Cowan JLW, Pennock JR, Boynton WR (1996) Seasonal and interannual patterns of sediment-water nutrient and oxygen fluxes in Mobile bay, Alabama (USA): regulating factors and ecological significance. Mar Ecol Prog Ser 141:229–245. https://doi.org/10.3354/meps141229
Forja JM, Gómez-Parra A (1998) Measuring nutrient fluxes across the sediment-water interface using benthic chambers. Mar Ecol Prog Ser 164:95–105. https://doi.org/10.3354/meps164095
Fujiwara N, Unotsu N, Tada M, Nakatsuji K, Kasai A, Sakamoto W (1997) Load of nitrogen and phosphorus flowing into the Seto Inland Sea from the Pacific Ocean. Proc Coast Eng. https://doi.org/10.2208/proce1989.44.1061
Gächter R, Müller B (2003) Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol Oceanogr 48:929–933. https://doi.org/10.4319/lo.2003.48.2.0929
Gautreau E, Volatier L, Nogaro G, Gouze E, Marmonier P, Mermillod-Blondin F (2023) Interactions between microbial activity and bioturbation modes of benthic invertebrates determine nutrient releases from reservoir sediments. Freshw Biol 68:245–259. https://doi.org/10.1111/fwb.14021
Hashiguchi S, Nishikawa T, Uozumi K, Furusawa K, Mori A, Imao K, Tanda M (2021) Causal factors of interannual decrease in stomach content index SCI of juvenile western sand lance Ammodytes japonicus in Harima-Nada, eastern Seto Inland Sea, Japan. Bull Jpn Soc Fish Oceanogr 85:24–32. https://doi.org/10.34423/jsfo.85.1_24
Hayami Y, Usui S, Takeoka H (2004) The stock and long term variation in nitrogen and phosphorus in the Seto Inland Sea. Umi Sora (sea Sky) 80:75–78 (In Japanese)
Hedges JI, Stern JH (1984) Carbon and nitrogen determinations of carbonate-containing solids. Limnol Oceanogr 29:657–663. https://doi.org/10.4319/lo.1984.29.3.0657
Imai I, Yamaguchi M, Hori Y (2006) Eutrophication and occurrences of harmful algal blooms in the Seto Inland Sea, Japan. Plankton Benthos Res 1:71–84. https://doi.org/10.3800/pbr.1.71
Kamiyama T, Tamai K, Tsujino M (1997) Effects of sediment conditions and dissolved oxygen concentration of bottom seawater on nutrient regeneration processes from marine sediments. Bull Nansei Reg Fish Res Lab 30:209–218 (In Japanese with English abstract)
Kamiyama T, Tsujino M, Tamai K (1998) Estimation of bottom fluxes of several nutrients in Harima Nada, the Seto Inland Sea of Japan. Bull Nansei Natl Fish Res Inst 31:33–43 (In Japanese with English abstract)
Karim MR, Sekine M, Ukita M (2002) Simulation of eutrophication and associated occurrence of hypoxic and anoxic condition in a coastal bay in Japan. Mar Pollut Bull 45:280–285. https://doi.org/10.1016/S0025-326X(02)00098-X
Koomklang J, Yamaguchi H, Ichimi K, Tada K (2018) A role for a superficial sediment layer in upward nutrient fluxes across the overlying water–sediment interface. J Oceanogr 74:13–21. https://doi.org/10.1007/s10872-017-0434-0
Koriyama M, Hayami Y, Koga A, Yamamoto K, Isnasetyo A, Hamada T, Yoshino K, Katano T, Yamaguchi S (2013) Seasonal variations of water column nutrients in the inner area of Ariake Bay, Japan: the role of muddy sediments. Environ Monit Assess 185:6831–6846. https://doi.org/10.1007/s10661-013-3068-8
Kubo A, Kanda J (2017) Seasonal variations and sources of sedimentary organic carbon in Tokyo Bay. Mar Pollut Bull 114:637–643. https://doi.org/10.1016/j.marpolbul.2016.10.030
Leng Q, Guo X, Zhu J, Morimoto A (2023) Contribution of the open ocean to the nutrient and phytoplankton inventory in a semi-enclosed coastal sea. Biogeosciences 20:4323–4338. https://doi.org/10.5194/bg-20-4323-2023
Lerman A (1979) Geochemical Processes: water and sediment environments. Wiley, New York
Li YH, Gregory S (1974) Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38:703–714. https://doi.org/10.1016/0016-7037(74)90145-8
Matsuoka S, Yoshimatsu S, Ono A, Ichimi K, Fujiwara M, Honda K, Tada K (2005) Discoloration of nori Porphyra and characteristics of water quality in eastern Bisan Seto. Bull Coast Oceanogr 43:77–84. https://doi.org/10.32142/engankaiyo.43.1_77
Ministry of the Environment (2015) Report on an investigation for total reduction of water quality and examination of measures to reduce pollution load. (In Japanese)
Ministry of the Environment (2017) Seto Inland Sea environmental information basic survey. (In Japanese)
Montani S, Mishima Y, Okaichi T (1991) Nitrogen and phosphorus circulation and eutrophic feature related with the marine organisms in Osaka Bay. Bull Coast Oceanogr 29:13–27. https://doi.org/10.32142/engankaiyo.29.1_13. (In Japanese with English abstract)
Muta N, Umezawa Y, Yamaguchi A, Suzaki H, Wada M, Nakata H, Kawamoto K, Matsuoka K (2020) Estimation of spatiotemporal variations in nutrient fluxes from sediments in the seasonally hypoxic Omura Bay, Japan. Limnology 21:341–356. https://doi.org/10.1007/s10201-019-00591-1
Nakakuni M, Loassachan N, Ichimi K, Nagao S, Tada K (2022) Biophilic elements in core sediments as records of coastal eutrophication in the Seto Inland Sea. Jpn Reg Stud Mar Sci 50:102093. https://doi.org/10.1016/j.rsma.2021.102093
Nakakuni M, Obo T, Koomklang J, Yamaguchi H, Ichimi K, Tada K (2023) Impact of previous aquaculture (yellowtail), as persistent phosphorus, remains for decades in deeper sediments. Aquacult Int. https://doi.org/10.1007/s10499-023-01239-9
Nishikawa T, Hori Y, Nagai S, Miyahara K, Nakamura Y, Harada K, Tanda M, Manabe T, Tada K (2010) Nutrient and phytoplankton dynamics in Harima-Nada, eastern Seto Inland Sea, Japan during a 35-year period from 1973 to 2007. Estuaries Coast 33:417–427. https://doi.org/10.1007/s12237-009-9198-0
Nishikawa T, Hori Y, Nagai S, Miyahara K, Nakamura Y, Harada K, Tada K, Imai I (2014) Long-term (36-year) observations on the dynamics of the fish-killing raphidophyte Chattonella in Harima-Nada, eastern Seto Inland Sea, Japan. J Oceanogr 70:153–164. https://doi.org/10.1007/s10872-014-0219-7
Nishikawa T, Nakamura Y, Okamoto S, Ueda H (2020) Interannual decrease in condition factor of the western sand lance Ammodytes japonicus in Japan in the last decade: Evidence for food-limited decline of the catch. Fish Oceanogr 29:52–55. https://doi.org/10.1111/fog.12448
Nixon SW (1982) Nutrient dynamics, primary production and fisheries yields of lagoons. Oceanol Acta 4 (Special issue):357–371
Patrick WH, Khalid RA (1974) Phosphate release and sorption by soils and sediments: effect of aerobic and anaerobic conditions. Science 186:53–55. https://doi.org/10.1126/science.186.4158.53
Reay WG, Gallagher DL, Simmons GM (1995) Sediment-water column oxygen and nutrient fluxes in nearshore environments of the lower Delmarva Peninsula, USA. Mar Ecol Prog Ser 118:215–227. https://doi.org/10.3354/meps118215
Rebreanu L, Vanderborght JP, Chou L (2008) The diffusion coefficient of dissolved silica revisited. Mar Chem 112:230–233. https://doi.org/10.1016/j.marchem.2008.08.004
Robador A, Brüchert V, Jørgensen BB (2009) The impact of temperature change on the activity and community composition of sulfate-reducing bacteria in arctic versus temperate marine sediments. Environ Microbiol 11:1692–1703. https://doi.org/10.1111/j.1462-2920.2009.01896.x
Sayama M, Sohma A, Takasugi Y (2002) Experimental analysis of the effect of current velocity on oxygen dynamics adjacent to the water-sediment interface in coastal waters. Proc Coast Eng, JSCE 49:996–1000. https://doi.org/10.2208/proce1989.49.996. (In Japanese)
SetoUchi Net (2023) Fisheries transition. https://www.env.go.jp/water/heisa/heisa_net/setouchiNet/seto/g2/g2cat02/suisangyou/index.html (In Japanese)
Sohma A, Sekiguchi Y, Kuwae T, Nakamura Y (2008) A benthic–pelagic coupled ecosystem model to estimate the hypoxic estuary including tidal flat—Model description and validation of seasonal/daily dynamics. Ecol Modell 215:10–39. https://doi.org/10.1016/j.ecolmodel.2008.02.027
Srithongouthai S, Sonoyama YI, Tada K, Montani S (2002) Annual cycle of ammonium and phosphate fluxes across the sediment-water interface in coastal marine environment (the Seto Inland Sea). Fish Sci 68:600–603. https://doi.org/10.2331/fishsci.68.sup1_600
Srithongouthai S, Sonoyama YI, Tada K, Montani S (2003a) The influence of environmental variability on silicate exchange rates between sediment and water in a shallow-water coastal ecosystem, the Seto Inland Sea, Japan. Mar Pollut Bull 47:10–17. https://doi.org/10.1016/S0025-326X(02)00406-X
Srithongouthai S, Sonoyama Y, Tada K, Montani S (2003b) Annual cycle of ammonium and phosphate fluxes across the sediment water interface in coastal marine environment (the Seto Inland Sea). Fish Sci 68:600–603. https://doi.org/10.2331/fishsci.68.sup1_600
Tada K (2015) Upward nutrient fluxes from coastal sediments. Kaiyo to Seibutsu (aquabiology) 37:217–221 (In Japanese with English abstract)
Tada K (2021) Primary production, nutrients and nutrient release from bottom sediments in coastal water. J Jan Soc Water Environ 44:137–141 (In Japanese)
Tada K, Monaka K, Morishita M, Hashimoto T (1998) Standing stocks and production rates of phytoplankton and abundance of bacteria in the Seto Inland Sea, Japan. J Oceanogr 54:285–295. https://doi.org/10.2116/bunsekikagaku.59.945
Tada K, Fujiwara M, Honjo T (2010) Water quality and Nori (Porphyra) culture in the Seto Inland Sea. Bunseki Kagaku 59:945–955. https://doi.org/10.2116/bunsekikagaku.59.945. (In Japanese with English abstract)
Tada K, Koomklang J, Ichimi K, Yamaguchi H (2017) Negligible effect of the benthic fauna on measuring the nutrient upward fluxes from coastal sediments. J Oceanogr 73:397–402. https://doi.org/10.1007/s10872-016-0406-9
Tada K, Nakajima M, Yamaguchi H, Asahi T, Ichimi K (2018) The nutrient dynamics and bottom sediment in coastal water. Bull Coast Oceanogr 55:113–124. https://doi.org/10.32142/engankaiyo.55.2_113. (In Japanese with English abstract)
Tada K, Nakakuni M, Koomklang J, Yamaguchi H, Ichimi K (2023) The impact of fish farming on phosphorus loading of surface sediment in coastal complex aquaculture. Fish Sci 89:375–386. https://doi.org/10.1007/s12562-022-01666-2
Tada K, Nakakuni M, Yamaguchi H, Ichimi K (2024) Changes in nutrients and their effects on fisheries after the introduction of land-based nutrient loading regulations in the Seto Inland Sea since 1973 a review. La mer 61:175–187. https://doi.org/10.32211/lamer.61.3-4_175
Takeoka H (2006) Methods to estimate amounts of ocean−origin nutrients in coastal seas and their difficulties. Bull Coast Oceanogr 43:105–111. https://doi.org/10.32142/engankaiyo.43.2_105. (In Japanese with English abstract)
Tanaka K (1994) Effects of soil loading on the phosphorus cycle in estuarine and coastal marine environments. Bull Nansei Natl Fish Res Inst 28:73–119 (In Japanese with English abstract)
Tanda M, Akashige S, Ariyama H, Yamanoi H, Kimura H, Dan H, Sakamoto H, Saiki Y, Ishida Y, Kotobuki H, Yamada T (2014) Nutrient environment and fisheries in the Seto Inland Sea. J Fish Tech 7:37–46 (In Japanese with English abstract)
Tsuboi S, Yamamura S, Imai A, Satou T, Iwasaki K (2014) Linking temporal changes in bacterial community structures with the detection and phylogenetic analysis of neutral metalloprotease genes in the sediments of a hypereutrophic lake. Microbes Environ 29:314–321. https://doi.org/10.1264/jsme2.ME14064
Tsujino M (2018) Biomass and production of macrobenthos in the Seto Inland Sea Japan. Nippon Suisan Gakkaishi 84:211–220. https://doi.org/10.5179/benthos.63.35. (In Japanese with English abstract)
Tu L, Jarosch KA, Schneider T, Grosjean M (2019) Phosphorus fractions in sediments and their relevance for historical lake eutrophication in the Ponte Tresa basin (Lake Lugano, Switzerland) since 1959. Sci Total Environ 685:806–817. https://doi.org/10.1016/j.scitotenv.2019.06.243
Umehara A, Borja Á, Ishida A, Nakai S, Nishijima W (2022) Responses of the benthic environment to reduction in anthropogenic nutrient loading in the Seto Inland Sea (Japan), based on M-AMBI assessment. Mar Environ Res 173:105509. https://doi.org/10.1016/j.marenvres.2021.105509
Viktorsson L, Ekeroth N, Nilsson M, Kononets M, Hall POJ (2013) Phosphorus recycling in sediments of the central Baltic Sea. Biogeosciences 10:3901–3916. https://doi.org/10.5194/bg-10-3901-2013
Watanabe Y, Tsunogai S (1984) Adsorption—desorption control of phosphate in anoxic sediment of a coastal sea, Funka Bay, Japan. Mar Chem 15:71–83. https://doi.org/10.1016/0304-4203(84)90039-2
Yamaguchi H, Hirade N, Kayama M, Ichimi K, Tada K (2014) Total organic carbon and nitrogen contents in surface sediments of Harima Nada, eastern Seto Inland Sea, Japan: a comparison under two different trophic states. J Oceanogr 70:355–366. https://doi.org/10.1007/s10872-014-0235-7
Yamaguchi H, Koga N, Ichimi K, Tada K (2020) Seasonal variations in phytoplankton productivity in a shallow cove in the eastern Seto Inland Sea, Japan. Fish Sci 86:1067–1078. https://doi.org/10.1007/s12562-020-01468-4
Yamamoto T (2003) The Seto Inland Sea––eutrophic or oligotrophic? Mar Pollut Bull 47:37–42. https://doi.org/10.1016/S0025-326X(02)00416-2
Yamamoto T, Matsuda O, Hashimoto T, Imose H, Kitamura T (1998) Estimation of benthic fluxes of dissolved inorganic nitrogen and phosphorus from sediments of the Seto Inland Sea. Oceanogr Jpn 7:151–158. https://doi.org/10.5928/kaiyou.7.151. (In Japanese with English abstract)
Yanagi T (2015) Eutrophication and Oligotrophication in Japanese Estuaries. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9915-7_3
Yanagi T, Ishii D (2004) Open ocean originated phosphorus and nitrogen in the Seto Inland Sea, Japan. J Oceanogr 60:1001–1005. https://doi.org/10.1007/s10872-005-0008-4
Yasuhara M, Yamazaki H (2005) The impact of 150 years of anthropogenic pollution on the shallow marine ostracode fauna, Osaka Bay, Japan. Mar Micropaleontol 55:63–74. https://doi.org/10.1016/j.marmicro.2005.02.005
Yasui S, Kanda J, Usui T, Ogawa H (2016) Seasonal variations of dissolved organic matter and nutrients in sediment pore water in the inner part of Tokyo Bay. J Oceanogr 72:851–866. https://doi.org/10.1007/s10872-016-0382-0
Yoneda M, Fujita T, Yamamoto M, Tadokoro K, Okazaki Y, Nakamura M, Takahashi M, Kono N, Matsubara T, Abo K, Xinyu G, Yoshie N (2022) Bottom-up processes drive reproductive success of Japanese anchovy in an oligotrophic sea: a case study in the central Seto Inland Sea Japan. Prog Oceanogr 206:102860. https://doi.org/10.1016/j.pocean.2022.102860
Acknowledgements
We would like to extend our heartfelt appreciation to Dr. Siraporn Tong-U-Dom (Ehime University) and Prof. Akihiko Morimoto (Ehime University) for their invaluable contribution to this paper by providing us with data on DIN and DIP fluxes from rivers. Also, we are most grateful for the invaluable contributions of two anonymous referees in reviewing the manuscript. This study was performed using grants from The Toyota Foundation (D19-R-0050), the Environment Research and Technology Development Fund (JPMEERF20205005) of the Environmental Restoration and Conservation Agency, provided by the Ministry of Environment of Japan, and the Kagawa University Research Promotion Program 2022 (22K1B002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakakuni, M., Yamaguchi, H., Ichimi, K. et al. Seasonal variation in pore water nutrients and their fluxes from the bottom sediments in Harima Nada, Seto Inland Sea. J Oceanogr 80, 219–232 (2024). https://doi.org/10.1007/s10872-024-00719-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-024-00719-7