Abstract
Eutrophication and hypoxia are major problems affecting the health of coastal ecosystems throughout the world. Tokyo Bay, Japan, is a eutrophic coastal area where the abundance of the megabenthic community has been decreasing. To assess factors associated with the impaired biota, seasonal surveys of the megabenthic community and water and sediment quality were conducted in the bay. Cluster analysis showed a difference in the community structure between the northern and southern parts of the bay. The density of species and species diversity were high throughout the year in the southern part of the bay, whereas in the northern part of the bay species diversity was low and defaunation occurred in August. At this time, bottom hypoxia due to temperature and salinity stratification, and high concentrations of nutrients, chlorophyll a, and organic matter in the water column and/or sediment, dominated the northern part of the bay. In October, bottom hypoxia was less severe but was still present in the northern part of the bay, and recolonization by mobile fishes and sessile mussels occurred. Multivariate analyses of the megabenthic community and environmental parameters in August showed the spatial pattern of the community could be explained by concentrations of dissolved oxygen and particulate organic carbon in the bottom water, and total sulfide and total organic carbon in the sediments. In particular, impairment of the biota in the northern area could be explained by the threshold concentrations of dissolved oxygen <1.7 mL L−1 and total organic carbon >20.3 mg g−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Productivity in coastal waters is high due to high levels of primary production, which is supported by the supply of nutrients from terrestrial areas (Ryther 1969; Jennings et al. 2001). However, productivity has been impaired by various stressors from human activities, such as eutrophication due to excessive inputs of nutrients and organic matter (Boesch et al. 2001), discharge of toxic pollutants (Stein and Cadien 2009), suspended solids from dredging (Torres et al. 2009), loss of mud and sand flats from land reclamation (Furota 1997), and pressure from commercial or recreational fisheries (Jennings et al. 2001). In addition, bottom hypoxia (with dissolved oxygen [DO] concentration ≤2 mL L−1) caused by anthropogenic eutrophication is one of the major environmental stressors in coastal systems throughout the world and is a growing global concern (Diaz and Rosenberg 1995, 2008). Hypoxia is usually formed in eutrophic systems by excessive nutrient inputs leading to increased primary production, followed by an increase in deposition of organic matter on the bottom. Oxygen is used to decompose this bottom organic matter; however, the supply of oxygen from the surface is hindered when thermal/density stratification occurs, resulting in bottom hypoxia.
To date, various effects of hypoxia on aquatic organisms have been reported at the individual or population level in terms of mortality (Baden et al. 1990; Diaz and Rosenberg 1995), growth (Zhou et al. 2001), reproduction (Thomas et al. 2006, 2007; Thomas and Rahman 2009), settlement (Baker and Mann 1992; Furota 1996), spatial distribution (Pihl et al. 1991; Kodama et al. 2006, 2009), and species interaction (Breitburg et al. 1997; Shoji et al. 2005). Responses to hypoxia differ among taxa. For example, sedentary bivalve species are usually tolerant to hypoxia (Hochachka 1980), and some species can survive even in anoxic conditions for several to dozens of days (Tamai 1992; de Zwaan et al. 2001). Meanwhile, motile animals like fishes, crustaceans, and cephalopods, can evade hypoxic zones, although there are differences among species in the threshold DO level that is tolerated and/or the time when movement away from these conditions begins (Pihl et al. 1991; Das and Stickle 1994; Eby and Crowder 2002). Because coastal benthic communities comprise multiple species in a number of different taxonomic groups, studies of the effects of hypoxia on the health of the coastal system need to assess benthic animals not only at the species/population level but also at the community level. Although coastal macrobenthic and meiobenthic communities have been studied intensively (e.g., Diaz and Rosenberg 1995; Lim et al. 2006; Liu et al. 2008), information on the effects of hypoxia on the megabenthic community is limited.
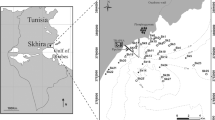
Tokyo Bay, on the east-central coast of Japan, is a semi-enclosed coastal system with a surface area of 960 km2 and an average depth of 15 m (Fig. 1). Water in the bay is exposed to the effects of various anthropogenic activities as a result of the population of about 26 million people in the surrounding catchment area (Furota 1997). Hypoxia was first observed in the bay in 1955 (Ishii et al. 2008) and is one of the major environmental issues affecting the quality of the bay water and sediment. Since 1955, the spatial extent and duration of the low oxygen conditions have been increasing (Ishii et al. 2008). Stock size of the megabenthic assemblage in the bay decreased dramatically in the late 1980s and has since remained low (Kodama et al. 2002). Multivariate analyses of long-term biotic and abiotic data suggest a decreasing trend in bottom DO concentration in the bay as one of the factors causing the stock-size decline of the megabenthic assemblage (Kodama et al. 2002).
The aim of this study was to describe the spatiotemporal patterns of environmental conditions and the megabenthic community in Tokyo Bay and to evaluate the effects of environmental factors in the bottom waters on disturbance of the megabenthic community structure.
Materials and methods
Field sampling
Bottom-trawl surveys were carried out in 2005 at 20 sampling sites across the bay to collect megabenthos. Sampling occurred four times on a seasonal basis: February 24 (winter), May 12 (spring), August 17 (summer), and October 26 (autumn) (Fig. 1). Surveys were conducted during the day by two 5-t trawlers, each using a trawl with a mouth 5.5 m wide and 0.6 m high. The trawls had a cod-end mesh size of 3 cm and were towed at each station for 10 min at a speed of 2 knots, resulting in a sampling area per station of 3.4 × 10−3 km2. The catch included fishes (elasmobranchs and teleosts), crustaceans (decapods and stomatopods), mollusks (bivalves, gastropods, and cephalopods), and echinoderms (echinoids and holothuroids), and these were collected and identified to species level. The number of individuals and the total wet weight (grams) for each species were recorded. Other taxa (e.g., polychaetes, asteroids, and hydroids) were not collected. The species density in both number of individuals and weight was calculated by dividing the number of individuals and weight, respectively, by the area swept by the trawl at each station.
To monitor water quality, water sampling was conducted at the same 20 sites by two 10-t boats on the same dates as the trawl surveys (Fig. 1). Temperature, salinity, and DO concentrations were measured at the surface (at a depth that was 10% of the total water depth) and bottom layer (at a depth that was 90% of the total depth) using a conductivity, temperature, and depth (CTD) data logger with a DO sensor (model XR-420 CTD Marine, RBR Ltd., Ontario, Canada). DO measurements were not taken for sites 11–20 in February because of equipment problems. Water samples were collected with 8-L Go-Flo bottles (Model 1080, General Oceanics Inc., Miami, FL, USA) at each surface and bottom depth for a total of two samples per site. Water samples were brought back to the laboratory in 500-mL polypropylene bottles on ice.
To understand the spatial characteristics of the sediment during severe summer hypoxia as well as to examine the relationship between disturbance to the megabenthic community in summer and the ambient environment, a sediment sample was collected at each site with a Smith-McIntyre grab sampler (0.05 m2; Rigo, Tokyo, Japan) in August. After each collection, a subsample of the sediment was taken from the surface of the sample in the grab sampler and transported to the laboratory in a 280-mL stainless steel container on ice.
Determination of water and sediment quality
Water samples for chlorophyll a (chl a) analysis (100 mL) were filtered onto Whatman GF/F glass-fiber filters and the filtrates were collected for nutrient analysis. Pigments were then extracted by placing the filters in 10 mL dimethylformamide for 24 h at 35°C. Chl a concentrations were determined by fluorometry (model 10-AU, Turner Designs, Sunnyvale, CA, USA). Dissolved nitrite (NO2 −), nitrate (NO3 −), ammonium (NH4 +), and phosphorus (PO4 3−) concentrations were measured in the filtered water sample with an auto-analyzer (AACS-II; Bran + Luebbe, Norderstedt, Germany), using standard procedures (Japan Meteorological Agency 1999). Dissolved inorganic nitrogen (DIN) concentration was the sum of NO2 −, NO3 −, and NH4 + concentrations, and dissolved inorganic phosphorus (DIP) concentration was the PO4 3− concentration.
Water samples for measurements of particulate organic carbon (POC) and particulate organic nitrogen (PON) concentrations were separately filtered through Whatman GF/F glass-fiber filters and analyzed according to established procedures (UNESCO 1994), using a CHN-O analyzer (Finnigan Flash EA1112; Thermo Electron Corporation, Waltham, MA, USA).
Sediment samples collected in August were measured for grain size, loss on ignition (LOI), and concentrations of total sulfide (TS), total nitrogen (TN), and total organic carbon (TOC). Grain size was measured using the standard sieving and hydrometer procedure (Japan Industrial Standards Committee 2000), and relative percentages of sand (0.075 to <2 mm in diameter), silt (0.005 to <0.075 mm), and clay (<0.005 mm) were determined. LOI was determined according to the procedure by Bale and Kenny (2005) in which pre-weighed dry sediment samples (oven dried at 100°C) were combusted at 450°C for 8 h in a muffle furnace. Concentrations of TN and TOC were determined by a carbon–nitrogen analyzer (CHN-corder model MT-5; Yanaco, Kyoto, Japan), according to the procedure given by the Oceanographic Society of Japan (1986). The concentration of TS was measured by the iodometric titration method, according to the procedure given by the Environmental Agency of Japan (1996).
Data analysis
Prior to analyses, we excluded data on five pelagic species that were incidentally caught during field sampling but were not part of the megabenthic assemblage (three clupeoid fishes: Engraulis japonicus, Konosirus punctatus, and Sardinella zunasi; and two carangid fishes: Trachurus japonicus and Decapterus maruadsi).
Spatiotemporal patterns in the megabenthic assemblage were investigated through multivariate analyses according to Clarke (1993) and Clarke and Warwick (2001) with the PRIMER 6 software (PRIMER-E, Plymouth, UK). The biotic relationship, in terms of species composition and species density (by number of individuals or by weight), between any two stations was assessed with a zero-adjusted Bray-Curtis similarity index (Clarke et al. 2006). Prior to the calculation of similarity, species density by individuals and weight was logarithmically transformed to reduce the contributions of rare and very common species (Clarke 1993). Spearman’s rank correlation coefficient (ρ s ) in the zero-adjusted Bray-Curtis similarity matrices between densities in terms of numbers of individuals and weights within the same month was found to be significant (RELATE routine; ρ s = 0.99 in all months; P < 0.01), suggesting that the spatiotemporal pattern is very similar between densities in both number of individuals and in weight. Therefore, in the present study we examined species density by individuals only.
The spatial pattern of the megabenthic community was examined by hierarchical agglomerative clustering with group-average linking on the zero-adjusted Bray-Curtis coefficient (Clarke and Warwick 2001). We applied a similarity profile (SIMPROF) test for detecting significantly different site groups (Clarke and Gorley 2006; Clarke et al. 2008). Mean and standard deviation of species density, number of species, and the Shannon diversity index (H′; Clarke and Warwick 2001) were calculated for each site group separated by cluster analysis. We conducted analysis of similarity percentages (SIMPER) to identify dominant species within each site group (Clarke and Warwick 2001). We used two-way analysis of similarities (ANOSIM) to test spatial and temporal differences in assemblage structure (Clarke and Warwick 2001).
To identify water and sediment quality parameters showing a spatial pattern similar to that of the megabenthic assemblage, we applied the BIO-ENV analysis (Clarke and Gorley 2006). In the BIO-ENV analysis, a combination of water and sediment quality parameters is identified whose standardized Euclidean distance matrix shows the highest ρ s with the biotic Bray-Curtis similarity matrix (Clarke and Ainsworth 1993; Clarke and Warwick 2001). We used data collected in August for the BIO-ENV analysis because sediment data were available only in August. Pairs of variables with a significant Pearson’s correlation coefficient (P < 0.05) were represented by a single variable, and log(1 + x)-transformed variables of the bottom water or sediment were used for the BIO-ENV analysis (Clarke and Warwick 2001). To estimate the threshold of environmental variables for separation of site groups, linkage tree (LINKTREE) analysis, a nonparametric modification of De’ath’s multivariate regression trees (De’ath 2002), was performed using the subset of environmental variables identified by BIO-ENV analysis (Clarke and Gorley 2006; Clarke et al. 2008). An absolute measure of group difference (y-axis for the tree) is given by B%, which is the percentage of the average of the between-group rank similarity to the largest rank similarity in the original Bray-Curtis matrix for the megabenthic community data. Split of the tree was accepted if the SIMPROF test showed a significant difference between the divided site groups.
Differences in the monthly mean values of each water quality parameter were tested with nested analysis of variance (nested ANOVA), using R software (version 2.8.1; www.r-project.org). Site groups derived from cluster analysis of the megabenthic community were treated as subgroups within each month in the nested ANOVA. For DO concentrations at the surface and bottom, February data were excluded from the analysis due to the absence of data for sites 11–20 (see Field sampling).
Results
Spatiotemporal patterns in the megabenthic assemblage and hypoxia
Megabenthos was found throughout the bay in all seasons with the exception of two areas in the northern part of the bay where hypoxic bottom water was present: sites 1–5 and 7–9 in August and site 6 in October (Fig. 2). Cluster analysis delineated two site groups in February (groups a and b), five in May (c–g), three in August (h–j), and five in October (k–o) (SIMPROF test, P < 0.05; dendrogram not shown). These groupings generally showed a latitudinal gradient from north to south (Fig. 2). The differences in the spatial and temporal patterns of the megabenthic assemblage were supported by the results of the two-way ANOSIM test (P < 0.01 for differences among both seasons and sites).
Log(1 + x)-transformed individual densities of the megabenthic assemblage for each site in February, May, August, and October 2005 in Tokyo Bay. Hypoxic areas are displayed by shading (bottom dissolved oxygen [DO] concentration ≤1 mL L−1) and hatching (>1–2 mL L−1); a–o denote site groups delineated by the SIMPROF test on dendrograms from cluster analysis on the megabenthic community
Species density, species number, and species diversity (H′) did not indicate large differences between the northern (group a) and southern (group b) parts of the bay in February (Figs. 2 and 3), and both areas were dominated by various taxa. Fishes (Pseudopleuronectes yokohamae and Repomucenus valenciennei), crustaceans (Metapenaeus joyneri and Oratosquilla oratoria), and a mollusk (Fulvia mutica) were dominant in the northern part of the bay (Table 1). In the southern part of the bay, an echinoid Temnopleurus hardwickii was found in the highest density, and fishes (R. valenciennei, Pennahia argentata, and Sillago japonica), crustaceans (Pyromaia tuberculata and Trachysalambria curvirostris), and a mollusk (Loliolus japonica) were also abundant (Table 1).
Mean and standard deviation (vertical bars) of log(1 + x)-transformed individual density, number of species, and Shannon’s diversity index (H′) for the megabenthic community in Tokyo Bay; a–o denote site groups delineated by the SIMPROF test on dendrograms from cluster analysis on the megabenthic community
In May, although species densities were similar among site groups, species number and diversity showed a decreasing trend in the north-central part of the bay (Figs. 2 and 3). Only two species P. tuberculata and F. mutica occurred in high densities in the innermost part of the bay (group c; Table 2). Species composition of the dominant species in the north-central area of the bay in May (group d; Table 2) was similar to that found in the northern portion of the bay in February (group a; Table 1). In the mid-southern portion of the bay (group e), densities of echinoids (T. hardwickii and Temnopleurus toreumaticus) were high (Table 2). Species number and species diversity were high in the southwestern part of the bay (group f; Fig. 3), and the megabenthic community in this area was dominated by fishes (R. valenciennei, Apogon lineatus, and P. argentata), crustaceans (Crangon sp., P. tuberculata, T. curvirostris, O. oratoria, and Charybdis bimaculata), and a mollusk (Zeuxis castus) (Table 2). In the southeastern part of the bay (group g), echinoids (T. hardwickii and T. toreumaticus) were dominant and elasmobranchs Dasyatis akajei and Okamejei kenojei were also found in large numbers (Table 2).
In August, defaunation occurred in the northern parts of the bay where bottom hypoxia was present (Fig. 2). Species density, species number, and species diversity decreased substantially in group h (Fig. 3) where only one species (O. oratoria) was collected at site 6 (Fig. 2; Table 3). In the hypoxic edge (group i), species number and species diversity were low, and Scapharca broughtonii dominated the species composition (72.1%) (Table 3). Species density, species number, and species diversity were high in the southern part of the bay (group j; Fig. 3) where nine species dominated the megabenthic community, with the highest contribution by Repomucenus valenciennei (Table 3).
In October, hypoxia began to abate and recolonization by megabenthos took place in the northern part of the bay (Fig. 2). Some pockets of severe hypoxia (DO concentration less than 1 mL L−1) remained, however, and as a result, no megabenthos was found at site 6 and only P. yokohamae was collected at site 8 (group k; Fig. 2; Table 4). In other central and northern areas of the bay (groups l, m, and n), species density recovered from the August defaunation; however, species number and species diversity in these groups were still low compared with group o in the southern part of the bay. Mobile fishes (Lateolabrax japonicus and P. argentata) and a cephalopod (L. japonica) as well as sessile mussels (Perna viridis and Mytilus galloprovincialis) were dominant in the northern part of the bay (Table 4) where moderate hypoxia was present (Fig. 2). Meanwhile, the southern part of the bay (group o) was dominated by nine species from various taxa (Table 4).
Temporal changes in water and sediment quality parameters
Mean values of water quality parameters at the surface and bottom layers in each site group separated by cluster analysis on the megabenthic community are shown in Figure 4. All of the monthly mean values for each water quality parameter at both surface and bottom layers showed significant differences between certain times of the year (nested ANOVA; P < 0.01 for all water quality parameters). The highest mean surface water temperature occurred in August, as did the greatest difference between surface and bottom mean water temperatures (6.1°C in the southern part of the bay [group j] to 8.6°C in the northern part [group h]). Bottom salinity did not show a large temporal fluctuation, whereas salinity at the surface decreased in August. The difference in salinity between the surface and bottom water in August ranged from 6.1 in the southern part of the bay (group j) to 10.7 in the northern part (group h). Mean DO concentration remained at normoxic conditions at the surface throughout the year, whereas it decreased substantially at the bottom in the north-central part of the bay in August (groups h and i; Figs. 2 and 4). In October, hypoxia was still present at sites 5–10, although hypoxia abated at the innermost sites 1–4 (Fig. 2). The mean bottom DO concentration remained low in the north-central areas (groups k, l, m, and n; Fig. 4).
Mean values of temperature, salinity, and concentrations of dissolved oxygen (DO), chlorophyll a (chl a), dissolved inorganic nitrogen (DIN) and phosphorus (DIP), and particulate organic nitrogen (PON) and carbon (POC) in the surface (white circles) and bottom layer (solid triangles) of Tokyo Bay in 2005. Site groups are derived from cluster analysis on the megabenthic community. Vertical bars indicate standard deviations. No data were obtained for DO at site group b in February
Mean concentrations of chl a, DIN, DIP, PON, and POC were generally higher in the northern part of the bay than in the southern part (Fig. 4). The surface mean chl a concentration was highest in August, while that at the bottom decreased from February to October (Fig. 4). The surface mean DIN concentration was lower in August (Fig. 4). The mean DIP concentration was elevated at the surface in October, whereas it increased from May to October at the bottom, especially in the northern part of the bay (Fig. 4). Mean concentrations of PON and POC showed decreasing trends from February to October at the surface, whereas they peaked in August at the bottom (Fig. 4).
In August, the mean concentrations of sediment TS, TOC, and TN as well as LOI were high in the north-central part of the bay (groups h and i) where the sediment consisted primarily of silt and clay (Fig. 5). Mean concentrations of TS, TOC, and TN and LOI were low in the southern part of the bay (group j) where sand was the main constituent of the sediment (Fig. 5).
Mean values of concentrations of total sulfide (TS), total organic carbon (TOC), total nitrogen (TN), loss on ignition (LOI), and relative percentage of sand, silt, and clay in the sediment of Tokyo Bay in August 2005. Site groups are derived from cluster analysis on the megabenthic community. Vertical bars indicate standard deviations
Effect of water and sediment quality on the megabenthic assemblage
Two sets of environmental variables were used in the BIO-ENV analysis (the first set includes concentrations of DO and POC from the bottom water layer and TS from sediments and the second comprises concentrations of chl a from the bottom water layer and TOC from sediments) because these variables did not show a significant correlation with each other within the set (P > 0.05). In the BIO-ENV analysis for the first set, the combination of the three variables explained the spatial pattern in the megabenthic community in August (ρ s = 0.54, P < 0.01). The LINKTREE analysis of the megabenthic community based on concentrations of bottom DO, POC, and TS showed that sites could be separated into four groups (SIMPROF test, P < 0.01; Fig. 6a), and the grouping was similar to that by cluster analysis using the Bray-Curtis similarity index for the megabenthic community (Table 3). The first split separated sites 1–9 and 11 from sites 10 and 12–20 at B% = 94, with the threshold DO concentration less than 1.7 mL L−1 for the former site group and greater than 1.8 mL L−1 for the latter (Fig. 6a). The next division separated sites 1–9 from site 11 at B% = 73, with a higher POC concentration in the former sites (Fig. 6a). The southern part of the bay (sites 14–20) was separated from the central area (sites 10, 12, and 13) at B% = 72, with the former sites showing lower concentrations of POC and TS than the latter (Fig. 6a). In the second data set, only TOC concentration was selected by the BIO-ENV analysis (ρ s = 0.50, P < 0.01). The LINKTREE analysis of the megabenthic community based on TOC concentration split sites into three groups (SIMPROF test, P < 0.01; Fig. 6b). The first split at B% = 87 divided the central-northern part of the bay (sites 1–13 with the exception of site 2; corresponding to group h and i) from the southern part of the bay (sites 14–20; corresponding to group j) at a TOC level around 20 mg g−1 (Fig. 6b). The second split differentiated sites 1, 6, 7 and 11–13 (TOC concentration < 28.9 mg g−1) from sites 3–5 and 8–10 (>30.8 mg g−1) at B% = 50 (Fig. 6b).
Results of the LINKTREE analysis from two data sets collected in August 2005 in Tokyo Bay; a dissolved oxygen (DO) and particulate organic carbon (POC) in bottom water and total sulfide (TS) in the sediment; and b chlorophyll a in bottom water (not retained in the LINKTREE analysis) and total organic carbon (TOC) in the sediment. Numbers to the right of each clade indicate sites. Threshold concentrations are shown to the left of each clade
Discussion
In eutrophic coastal systems, formation of hypoxia usually takes place during summer to autumn and is triggered by stratification of the water column, which reduces oxygen supply from the surface to the bottom (Diaz and Rosenberg 2008). In the present study, bottom hypoxia was present in August and October in the northern part of Tokyo Bay. Thermal and salinity stratification was observed in the summer and was prominent particularly in the northern part of the bay. This resulted in hypoxic conditions developing in the bottom layer of Tokyo Bay during this time. Thermal and salinity stratification abated in October; however, hypoxic conditions remained in the northern part of the bay, although the spatial extent of severe hypoxia (DO concentration ≤1 mL L−1) decreased. In the Neuse River Estuary (NC, USA), salinity stratification and high water temperature are the main factors causing bottom hypoxia, and hypoxia can occur with a difference in salinity of only 1–2 psu between the surface and bottom water when the temperature exceeds 20°C (Buzzelli et al. 2002). In Tokyo Bay, the bottom water temperature in October was still around 20°C (Fig. 4) and the difference in salinity between the surface and bottom layers was 3.1 psu in the hypoxic areas (sites 5–10; Figs. 2 and 4). In addition, concentrations of sediment organic matter in the northern part of the bay were higher than in the southern areas (Figs. 2 and 5), suggesting that microbial decomposition of organic matter could have caused the persisting bottom hypoxia, as reported in other coastal systems (Diaz and Rosenberg 2008). The high concentrations of nutrients and sediment organic matter in the northern part of the bay can be attributed to the combination of anthropogenic activities in the adjacent terrestrial areas and to rivers emptying into the bay (Furota 1997). These high nutrient levels most likely caused the increase in phytoplankton production, as suggested by the high chl a concentrations that were also found in this area (Fig. 4). Dead phytoplankton that eventually accumulate and decay on the bottom would be one of the major sources of the high concentrations of sediment organic matter in the northern part of the bay. In contrast, bottom hypoxia was not evident in the southern part of the bay in August, although thermal and salinity stratification was observed in the southwestern area. This absence of hypoxic conditions in the southern part of the bay can be attributed to the low concentration of organic matter in the sediment compared to the northern areas and to the exchange of sea water between the southern part of the bay and the ocean, which provides oxygen-saturated water to the bay and helps prevent the waters from becoming hypoxic (Furota 1997). Similar bottom DO gradients (low in the inner part and high in the outer part) have been reported in other semi-enclosed coastal systems; e.g., Chinhae Bay, Korea (Lim et al. 2006) and Pensacola Bay, FL, USA (Thomas et al. 2007).
Bottom hypoxia affects species density and spatial distribution of megabenthic animals. In the Kattegat, Sweden, species density of demersal fishes decreased substantially when the bottom DO concentration was reduced to less than 2 mL L−1 (Baden et al. 1990). In addition, threshold DO levels needed to support megabenthic species have been estimated to be around 1.4 mL L−1 in the Neuse River Estuary (Eby and Crowder 2002) and the Gulf of Mexico shelf (Craig and Crowder 2005), and both studies showed contraction of the spatial distribution of demersal species due to bottom hypoxia. In the present study, cluster analysis of the megabenthic community separated sampling sites into 2–5 groups on the basis of differences in species density and species composition. In general, species density, species number, and species diversity were higher in the southern part of the bay than in the northern part. The bottom hypoxia that prevailed in the northern half of the bay in August would have caused a substantial reduction in species density and species diversity; no organisms were found in severely hypoxic areas (DO concentration ≤1 mL L−1). In contrast, species density and species diversity of the megabenthos in the southern part of the bay, where a high DO concentration and low POC and TS concentrations were observed in the bottom layer and/or sediment, were high throughout the year. The BIO-ENV analysis for August data showed that concentrations of DO and POC in the bottom water and TS in the sediments were significant factors affecting spatial structure in the megabenthic community. The LINKTREE analysis separated the bay into northern and southern sections at the first split with DO levels between 1.7 and 1.8 mL L−1, suggesting that the presence of megabenthos in the bay was strongly regulated by this threshold DO concentration.
Mortality and behavioral response to hypoxia differ among taxa, with some taxa surviving in hypoxic conditions for a certain period of time. In particular, some bivalve species are tolerant of low DO concentrations, and this tolerance is suggested to be linked to enhanced anaerobic ATP yield as well as a depressed metabolic rate (Hochachka 1980). In the present study, S. broughtonii, a hypoxic-tolerant sessile bivalve species (Watanabe et al. 2005), dominated the hypoxic central area of the bay in August. In October, however, the density of this species decreased substantially and it was no longer dominant, suggesting that S. broughtonii could not survive the prolonged exposure to hypoxic conditions, as also reported for other hypoxia-tolerant bivalves (Theede et al. 1969; Altieri 2006; Holmes and Miller 2006). In August, mantis shrimp O. oratoria were still present in the northern hypoxic area (site 6), although species density was very low. In a previous study, Pihl et al. (1991) showed that mantis shrimp Squilla empusa in the York River, Chesapeake Bay, USA, remained in hypoxic areas until their tolerance was exceeded, at which point they migrated to normoxic water. Pihl et al. (1991) suggested that increased food availability under hypoxic conditions, during which infaunal macrobenthic species come to the sediment surface, would be attractive to hypoxia-tolerant S. empusa. In Tokyo Bay, however, the vast majority of O. oratoria probably either die or leave the severely hypoxic areas in summer because (1) defaunation of macrobenthic prey species also occurs (Ohkoshi and Furota 1997) resulting in a lack of food for survival, (2) behavioral abnormality (i.e., immobilization) occurs in adult O. oratoria at DO concentrations < 3.1 mL L−1, although the lethal DO concentration is 0.6 mL L−1 (Hamano and Yamamoto 2005), and (3) tagged O. oratoria released in the northern part of the bay in May were recaptured only in the southern part during the summer (Ohtomi et al. 1989), suggesting that individuals either migrate to the southern part of the bay or die in the northern part. Indirect effects of hypoxia on megabenthic community structure, such as predator-prey interaction, as suggested by Pihl et al. (1991), have not been investigated in Tokyo Bay but should be addressed in future studies.
The result of the LINKTREE analysis showed that bottom TS concentration is one of the factors affecting the megabenthic community structure between hypoxic and normoxic areas in the bay, and this may be related to defaunation in the hypoxic areas as a result of hydrogen sulfide (H2S) production. H2S is produced during decomposition of organic matter by anaerobic bacteria, and the production is dependent on temperature and pH (Boyd and Tucker 1998). In August 2005 in the north-central part of Tokyo Bay, bottom water temperatures ranged from 18.5 to 19.5°C in the hypoxic areas (groups h and i; Fig. 4), and pH ranged from 7.7 to 8.6 (National Institute for Environmental Studies, www.nies.go.jp/igreen/index.html). Under these conditions, 3–26% of bottom TS exists as H2S (Boyd and Tucker 1998). H2S is toxic to aquatic organisms, inhibiting the electron transport chain in aerobic respiration (Torrans and Clemens 1982). The survival rate of the prawn Macrobrachium nipponense under hypoxic conditions has been shown to decrease in the presence of H2S compared to prawn exposed to equivalent hypoxic conditions without H2S (Kang et al. 1995). However, tolerance to hypoxia combined with sulfide differs between species (Diaz and Rosenberg 1995). The bivalve S. broughtonii was dominant in the central part of the bay in August, suggesting that this species is tolerant of hypoxic conditions when H2S is present, as reported for other bivalve species (Theede et al. 1969). In this study, however, it was not possible to estimate the H2S concentrations in the water from the TS measurements in the sediment, and, additionally, the threshold lethal concentration for H2S under hypoxic conditions is unknown for the megabenthic animals in the bay. In situ monitoring of H2S concentrations in the bottom water of Tokyo Bay as well as laboratory experiments to determine lethal H2S concentrations under hypoxic conditions for each megabenthic species are required to better understand how defaunation occurs in the bay during hypoxic periods.
Sediment TOC concentration represents the amount of organic matter (Magni et al. 2008) and correlates with factors causing ecological stress, such as hypoxia, high ammonia and sulphide, and chemical contamination of sediments (Hyland et al. 2005). Magni et al. (2008) proposed that TOC can be used as a general screening-level indicator for evaluating the likelihood of reduced sediment quality and associated effects on the macrobenthic community in coastal areas. Hyland et al. (2005) have shown impaired macrobenthic communities from seven coastal systems around the world and have suggested that the risk of reduced species richness of the macrobenthic community should be high at TOC values > 35 mg g−1. In the present study, the spatial pattern of the megabenthic community in Tokyo Bay was correlated with that of TOC concentration in the sediment. The LINKTREE analysis divided the central-northern part of the bay (group h and i) from the southern part of the bay (group j) at a TOC level around 20 mg g−1 (Fig. 6), where species density, species number, and species diversity of the megabenthic community in the former sites were lower than in the latter in August. Our results suggest that the critical TOC level may differ among different trophic levels or taxa, and this deserves further examination in order to develop sound management procedures for coastal ecosystems.
Recolonization in defaunated areas occurs after bottom hypoxia abates. The first stage of recolonization, however, does not involve all species of the natural community for the area but rather species that are mobile and/or settlement of pelagic larvae. Recolonization by immigration of mobile demersal species to areas where bottom water hypoxia has abated has been reported in other coastal systems (e.g., Pihl et al. 1991; Eby and Crowder 2002). We attribute the autumn recolonization of the megabenthic assemblage in the northern part of Tokyo Bay to an immigration of the mobile fish L. japonicus, P. argentata, and P. yokohamae and the cephalopod L. japonica and to recruitment of young-of-the-year mussels P. viridis and M. galloprovincialis. High tolerance of larvae to hypoxic conditions may increase reproductive success and result in rapid recruitment to the northern part of the bay soon after abatement of hypoxia, which may explain the increase in young-of-the-year juveniles of P. viridis and M. galloprovincialis (mean individual body weights, 1.5 and 2.5 g, respectively). In a closely related mussel (Mytilus edulis), larval settlement is not hindered by a low DO concentration of 0.9 mL L−1 (Wang and Widdows 1991).
In conclusion, summer hypoxia in the central-north part of Tokyo Bay caused defaunation of the megabenthos, possibly because of mass mortality and/or migration toward normoxic areas of the bay. In addition to causing defaunation of adult megabenthos, hypoxic conditions may disturb larval settlement of the dominant megabenthic species in the bay. Although extended spawning periods have been reported for the dragonet (R. valenciennei; spring to autumn) and mantis shrimp (O. oratoria; spring to summer), the settlement of larvae from the spring spawning does not take place, probably because of the persistence of bottom hypoxia in the settlement grounds (Ikejima and Shimizu 1999; Kodama et al. 2006, 2009). Comprehensive action is needed to reduce hypoxia and to further the recovery of the megabenthic community in the bay. Measures to decelerate eutrophication and hypoxia could include conservation and enlargement of tidal flats, reduction of the influx of inorganic nutrients and organic substances into the bay from terrestrial areas, and removal of organic substances and sludge accumulating on the bay bottom (Council for Renaissance of Tokyo Bay 2003). Implementation of these measures would at least partially facilitate the recovery in stock size of the megabenthic community in the bay.
References
Altieri AH (2006) Inducible variation in hypoxia tolerance across the intertidal–subtidal distribution of the blue mussel Mytilus edulis. Mar Ecol Prog Ser 325:295–300
Baden SP, Loo LO, Pihl L, Rosenberg R (1990) Effects of eutrophication on benthic communities including fish: Swedish West Coast. Ambio 19:113–122
Baker SM, Mann R (1992) Effects of hypoxia and anoxia on larval settlement, juvenile growth, and juvenile survival of the oyster Crassostrea virginica. Biol Bull 182:265–269
Bale AJ, Kenny AJ (2005) Sediment analysis and seabed characterization. In: Eleftheriou A, McIntyre A (eds) Methods for the study of marine benthos, 3rd edn. Blackwell Science, Oxford, pp 43–86
Boesch DF, Brinsfield RB, Magnien RE (2001) Chesapeake Bay eutrophication: scientific understanding, ecosystem restoration, and challenges for agriculture. J Environ Qual 30:303–320
Boyd CE, Tucker CS (1998) Pond aquaculture water quality management. Kluwer, Boston
Breitburg DL, Loher T, Pacey CA, Gerstein A (1997) Varying effects of low dissolved oxygen on trophic interactions in an estuarine food web. Ecol Monogr 67:489–507
Buzzelli CP, Luettich RA, Powers SP, Peterson CH, McNinch JE, Pinckney JL, Paerl HW (2002) Estimating the spatial extent of bottom-water hypoxia and habitat degradation in a shallow estuary. Mar Ecol Prog Ser 230:103–112
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian J Ecol 18:117–143
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Clarke KR, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80
Clarke KR, Somerfield PJ, Gorley RN (2008) Testing of null hypotheses in explanatory community analyses: similarity profiles and biota-environment linkage. J Exp Mar Biol Ecol 366:56–69
Council for Renaissance of Tokyo Bay (2003) Action plan for recovery of Tokyo Bay (final summary). Council for Renaissance of Tokyo Bay, Tokyo
Craig JK, Crowder LB (2005) Hypoxia-induced habitat shifts and energetic consequences in Atlantic croaker and brown shrimp on the Gulf of Mexico shelf. Mar Ecol Prog Ser 294:79–94
Das T, Stickle WB (1994) Detection and avoidance of hypoxic water by juvenile Callinectes sapidus and C. similis. Mar Biol 120:593–600
de Zwaan A, Cattani O, Vitali G, Cortesi P (2001) Influence of incubation conditions on the anoxic survival of marine bivalves: static and semi-static incubations. Mar Ecol Prog Ser 211:169–179
De’ath G (2002) Multivariate regression trees: a new technique for modeling species environment relationships. Ecology 83:1105–1117
Diaz RJ, Rosenberg R (1995) Marine benthic hypoxia: a review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanogr Mar Biol Annu Rev 33:245–303
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Eby LA, Crowder LB (2002) Hypoxia-based habitat compression in the Neuse River Estuary: context-dependent shifts in behavioral avoidance thresholds. Can J Fish Aquat Sci 59:952–965
Environmental Agency of Japan (1996) Methods and explanation for sediment research. Maruzen, Tokyo (in Japanese)
Furota T (1996) Life cycle studies on the introduced spider crab Pyromaia tuberculata (Lockington) (Brachyura: Majidae). II. Crab stage and reproduction. J Crust Biol 16:77–91
Furota T (1997) Present status of ecosystem and environmental conditions in Tokyo Bay. In: Numata M, Furota T (eds) Biology in Tokyo Bay. Tsukiji Shyokan, Tokyo, pp 2–23 (in Japanese)
Hamano T, Yamamoto K (2005) Rheotaxis and tolerance to oxygen deficiency as factors affecting the spatial pattern and the stock of the Japanese mantis shrimp Oratosquilla oratoria in fishing grounds. J Natl Fish Univ 53:117–129 (in Japanese with English abstract)
Hochachka PW (1980) Living without oxygen. Harvard University Press, Cambridge
Holmes SP, Miller N (2006) The hypoxic tolerance of the protobranch bivalve Nucula sulcata Bronn. J Shellfish Res 25:865–867
Hyland J, Balthis L, Karakassis I, Magni P, Petrov A, Shine J, Vestergaard O, Warwick R (2005) Organic carbon content of sediments as an indicator of stress in the marine benthos. Mar Ecol Prog Ser 295:91–103
Ikejima K, Shimizu M (1999) Disappearance of a spring cohort in a population of the dragonet, Repomucenus valenciennei, with spring and autumn spawning peaks in Tokyo Bay, Japan. Ichthyol Res 46:331–339
Ishii M, Hasegawa K, Kakino J (2008) Long-term fluctuations of the water quality in Tokyo Bay judged from a data set of Chiba Prefecture. Bull Jpn Soc Fish Oceanogr 72:189–199 (in Japanese with English abstract)
Japan Industrial Standards Committee (2000) Test method for particle size distribution of soils (JIS A1204). Japan Industrial Standards Committee, Tokyo (in Japanese)
Japan Meteorological Agency (1999) Mannual on oceanographic observation part 1. Japan Meteorological Business Support Center, Tokyo
Jennings S, Kaiser MJ, Reynolds JD (2001) Marine fisheries ecology. Blackwell Science, Oxford
Kang JC, Matsuda O, Imamura N (1995) Effects of hypoxia and hydrogen sulfide on survival of the prawn Macrobrachium nipponense in Lake Kojima, Japan. Nippon Susan Gakkaishi 61:821–826 (in Japanese with English abstract)
Kodama K, Aoki I, Shimizu M, Taniuchi T (2002) Long-term changes in the assemblage of demersal fishes and invertebrates in relation to environmental variation in Tokyo Bay, Japan. Fish Manag Ecol 9:303–313
Kodama K, Horiguchi T, Kume G, Nagayama S, Shimizu T, Shiraishi H, Morita M, Shimizu M (2006) Effects of hypoxia on early life history of the stomatopod Oratosquilla oratoria in a coastal sea. Mar Ecol Prog Ser 324:197–206
Kodama K, Oyama M, Lee JH, Akaba Y, Tajima Y, Shimizu T, Shiraishi H, Horiguchi T (2009) Interannual variation in quantitative relationships among egg production and densities of larvae and juveniles of the Japanese mantis shrimp Oratosquilla oratoria in Tokyo Bay, Japan. Fish Sci 75:875–886
Lim HS, Diaz RJ, Hong JS, Schaffner LC (2006) Hypoxia and benthic community recovery in Korean coastal waters. Mar Pollut Bull 52:1517–1526
Liu XS, Xu WZ, Cheung SG, Shin PKS (2008) Subtropical meiobenthic nematode communities in Victoria Harbour, Hong Kong. Mar Pollut Bull 56:1486–1512
Magni P, De Falco G, Como S, Casu D, Floris A, Petrov AN, Castelli A, Perilli A (2008) Distribution and ecological relevance of fine sediments in organic-enriched lagoons: the case study of the Cabras lagoon (Sardinia, Italy). Mar Pollut Bull 56:549–564
Oceanographic Society of Japan (1986) Manual for research on coastal environment (sediment and organisms). Kouseisha-kouseikaku, Tokyo, Japan (in Japanese)
Ohkoshi W, Furota T (1997) Benthic animals. In: Numata M, Furota T (eds) Biology in Tokyo Bay. Tsukiji Shokan, Tokyo, pp 45–114 (in Japanese)
Ohtomi J, Park JS, Shimizu M (1989) The distribution of the Japanese mantis shrimp Oratosquilla oratoria and its relationship to fishing grounds in Tokyo Bay. Nippon Suisan Gakkaishi 55:1529–1538 (in Japanese with English abstract)
Pihl L, Baden SP, Diaz RJ (1991) Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar Biol 108:349–360
Ryther JH (1969) Relationships of photosynthesis to fish production in the sea. Science 166:72–76
Shoji J, Masuda R, Yamashita Y, Tanaka M (2005) Predation on fish larvae by moon jellyfish Aurelia aurita under low dissolved oxygen concentrations. Fish Sci 71:748–753
Stein ED, Cadien DB (2009) Ecosystem response to regulatory and management actions: the southern California experience in long-term monitoring. Mar Pollut Bull 59:91–100
Tamai K (1992) Tolerance of Theora fragilis (Bivalvia: Semelidae) to low concentrations of dissolved oxygen. Nippon Suisan Gakkaishi 59:615–620 (in Japanese with English abstract)
Theede H, Ponat A, Hiroki K, Schlieper C (1969) Studies on the resistance of marine bottom invertebrates to oxygen-deficiency and hydrogen sulphide. Mar Biol 2:325–337
Thomas P, Rahman MS (2009) Chronic hypoxia impairs gamete maturation in Atlantic croaker induced by progestins through nongenomic mechanisms resulting in reduced reproductive success. Environ Sci Technol 43:4175–4180
Thomas P, Rahman MS, Kummer JA, Lawson S (2006) Reproductive endocrine dysfunction in Atlantic croaker exposed to hypoxia. Mar Environ Res 62:S249–S252
Thomas P, Rahman MS, Khan IA, Kummer JA (2007) Widespread endocrine disruption and reproductive impairment in an estuarine fish population exposed to seasonal hypoxia. Proc R Soc B 274:2693–2701
Torrans EL, Clemens HP (1982) Physiological and biochemical effects of acute exposure of fish to hydrogen sulfide. Comp Biochem Physiol 71C:183–190
Torres RJ, Abessa DMS, Santos FC, Maranho LA, Davanso MB, do Nascimento MRL, Mozeto AA (2009) Effects of dredging operations on sediment quality: contaminant mobilization in dredged sediments from the Port of Santos, SP, Brazil. J Soil Sediment 9:420–432
UNESCO (1994) Protocols for the joint global ocean flux study (JGOFS) core measurements: intergovernmental oceanographic commission, scientific committee on oceanic research, manual and guides 29. UNESCO, Paris
Wang WX, Widdows J (1991) Physiological responses of mussel larvae Mytilus edulis to environmental hypoxia and anoxia. Mar Ecol Prog Ser 70:223–236
Watanabe T, Shibata K, Kera Y, Takahashi S, Yamada R (2005) Effects of hypoxic and osmotic stress on the free D-aspartate level in the muscle of blood shell Scapharca broughtonii. Amino Acids 28:291–296
Zhou BS, Wu RSS, Randall DJ, Lam PKS (2001) Bioenergetics and RNA/DNA ratios in the common carp (Cyprinus carpio) under hypoxia. J Comp Physiol B 171:49–57
Acknowledgments
We thank the fishermen of Chiba and Kanagawa Prefectures as well as the staff at Japan NUS Co., Ltd. and the National Institute for Environmental Studies for their help with field sampling and water quality determination.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodama, K., Oyama, M., Kume, G. et al. Impaired megabenthic community structure caused by summer hypoxia in a eutrophic coastal bay. Ecotoxicology 19, 479–492 (2010). https://doi.org/10.1007/s10646-009-0438-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0438-7