Abstract
Downregulation of circ_0044226 has been demonstrated to reduce pulmonary fibrosis, but the role of circ_0044226 in liver fibrosis remains to be explored. In this work, we found that circ_0044226 expression was upregulated during liver fibrosis. Knockdown of circ_0044226 inhibited proliferation, promoted autophagy and apoptosis of hepatic stellate cell LX-2. Bioinformatic analysis and dual luciferase reporter assays confirmed the interaction between circ_0044226, miR-4677-3p and SEC61G. Mechanistically, knockdown of circ_0044226 suppressed SEC61G expression by releasing miR-4677-3p, thereby enhancing endoplasmic reticulum stress. Overexpression of SEC61G or endoplasmic reticulum stress inhibitor 4-phenylbutiric acid partially reversed the effect of knockdown circ_0044226 on LX-2 cell function. In vivo experiments showed that inhibition of circ_0044226 attenuated CCL4-induced liver fibrosis in mice. These imply that circ_0044226 may be a potential target for the treatment of liver fibrosis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver fibrosis, a chronic injury to the liver caused by various factors, is characterized by an excessive build-up of collagen and the accumulation of extracellular matrix (ECM) due to various molecular and cellular processes (Caligiuri et al. 2021). The activation of hepatic stellate cells plays a crucial role in liver fibrosis. Upon liver injury, hepatic stellate cells undergo a phenotypic transition from a static state to an activated state, which involves proliferation, contraction, and the production of a significant amount of ECM components and inflammatory factors (Tsuchida and Friedman 2017). TGF-β1, a vital cytokine, is known to drive fibrogenesis and is commonly utilized to stimulate hepatic stellate cells in laboratory studies (Bu et al. 2018; Dewidar et al. 2019). Numerous investigations have proposed that promoting the apoptosis or deactivation of activated hepatic stellate cells could potentially serve as a novel approach to promote the regression of fibrosis (Higashi et al. 2017). Autophagy dysregulation is closely associated with chronic liver disease and its effects vary depending on the cell type and the stage of the disease (Allaire et al. 2019). In hepatic stellate cells, autophagy has been suggested to hinder the release of extracellular vesicles, thereby mitigating liver fibrosis (Gao et al. 2020a).
The endoplasmic reticulum is an important organelle for protein synthesis, folding, and secretion in eukaryotic cells, and many factors can lead to homeostatic imbalance of endoplasmic reticulum function, forming endoplasmic reticulum stress (ERs) (Oakes 2020). ERs have been shown to regulate autophagy, thereby inducing cell death or survival (Fernández et al. 2015). To restore homeostasis, the core sensors PERK, IRE1 and ATF6 and the downstream transcription factor CHOP are induced, but they can also cause cell death (Iurlaro and Muñoz-Pinedo 2016). In addition, excessive stress response leads to the damage of endoplasmic reticulum, which will be phagocytosed and degraded by autophagic vesicles, and triggers intracellular apoptotic signals, prompting cell apoptosis. Autophagy appears to have become the last resort to restore endoplasmic reticulum homeostasis (Qi and Chen 2019). ERs have been shown to mediate autophagy through IRE1-α signaling, thereby weakening hepatic stellate cell viability and inducing apoptosis (Li et al. 2017). Further, enhanced ERs in hepatic stellate cells was demonstrated to promote apoptosis and accelerate the resolution of liver fibrosis (Huang et al. 2014).
CircRNA is a novel non-coding RNA with a covalent closed-loop structure. Some circRNA contain miRNA response elements and act as competitive endogenous RNA to regulate gene transcription by sponging microRNA (Liu et al. 2022). Many studies have shown that circRNA is involved in the fibrotic process and correlates with the severity of liver fibrosis (Zeng et al. 2021). For example, serum circMTO1 was negatively correlated with fibrosis stage in patients with chronic hepatitis B (Wang et al. 2019). Circ_0004018 as a sponge for miR-660-3p contributed to hepatic stellate cell activation and proliferation, and abated the progression of liver fibrosis in mice (Li et al. 2020). Circ_0044226 is a newly identified circRNA that was upregulated in idiopathic pulmonary fibrosis patients, and inhibition of circ_0044226 alleviated pulmonary fibrosis in vitro and in vivo (Qi et al. 2020). The parental gene for circ_0044226 is CDC27, which has been reported to be activated by TGF-β (Zhang et al. 2011) and involved in lung fibrosis progression (Qi et al. 2023). However, the role of circ_0044226 in liver fibrosis is not yet known.
SEC61G is a subunit of the SEC61 translocation complex responsible for importing newly synthesized polypeptides into endoplasmic reticulum. SEC61G plays an essential role in protein folding, modification and translocation, and in activating unfolded protein responses (Ma et al. 2021). Many studies have shown that SEC61G is increased in many tumors and serves to promote cancer progression (Meng et al. 2021). Silencing SEC61G impaired the proliferative capacity of hepatocellular carcinoma cells and induced apoptosis (Gao et al. 2020b). Notably, the downregulation of SEC61G activated ERs, thereby inhibiting the malignant development of lung adenocarcinoma (Zhang and Guo 2022). However, whether SEC61G participates in liver fibrosis and its regulation of ERs is unknown.
In this study, we explored the effects of circ_0044226 on proliferation, apoptosis and autophagy of hepatic stellate cells and investigated the molecular mechanisms based on SEC61G and ERs.
Materials and methods
Cell culture and treatment
Human LX-2 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The DMEM medium (Gibco, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and 100 U/mL penicillin and streptomycin was used to culture the cells. All the cells were incubated at 37℃ in an environment containing 5% CO2. In order to activate the LX-2 cells, TGF-β1 (Sigma-Aldrich, St. Louis, MO, USA) was introduced into the culture medium. To inhibit ERs, the addition of 4-Phenylbutyric acid (4-PBA) (Sigma-Aldrich) was employed.
Cell transfection
Lentivirus (titer: > 108 IU/mL) containing short hairpin RNA against circ_0044226 (sh-circ) and negative control (sh-NC) were acquired from GenePharma (Shanghai, China). LX-2 cells were plated in 6-well plates at a density of 1 × 105 cells per well. When the cells grew to 50% confluence, sh-NC or sh-circ lentivirus was used to infect them for 24 h. Detection of circ_0044226 by RT-qPCR to determine interference efficiency. MiR-4677-3p mimics (miR-mimic) and inhibitors (miR-inhibitor), and their negative controls (NC-mimic, NC-inhibitor), as well as the overexpression plasmid for SEC61G (ov-SEC61G) and negative control ov-NC) were provided by Ribobio (Guangzhou, China). Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) was used to transfect these oligonucleotides or plasmids into LX-2 cells.
CCK-8 assays
To evaluate the growth and proliferative potential of LX-2 cells, we utilized the CCK-8 method. LX-2 cells (1 × 104 cells/well) were placed into 96-well culture plates. At different intervals during the cultivation process, we added 10 μL of CCK-8 solution (Dojindo, Japan) into each well, allowing it to incubate for 4 h. Subsequently, we employed a microplate reader (Bio-Rad, USA) to determine the absorbance value at 450 nm for each individual well.
Western blot
Proteins extracted from LX-2 cells or mouse liver tissue were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Boston, MA, USA). The membranes blocked with 5% skim milk were incubated with primary and secondary antibodies in sequence. Then, the bands were visualized by electrochemiluminescence solution (Advansta, Menlo Park, CA, USA) and the relative protein expression was normalized according to GAPDH. Primary antibodies against α-SMA (ab5694, 1:3000); p62 (ab109012, 1:10000); Beclin-1 (ab207612, 1:2000); Bax (ab32503, 1:2000); Bcl2 (ab182858, 1:2000); cleaved-caspase3 (ab2302, 1:300); GRP78 (ab108613, 1:4000); p-IRE1 (ab48187, 1:1000); IRE1 (ab37073, 1:2000); ATF6 (ab37149, 1:3000); CHOP (ab11419, 1:1000); Collagen I (ab34710, 1:1000) and GAPDH (ab8245, 1:5000) were provided by Abcam (Cambridge, UK). Anti-SEC61G (11147–2-AP, 1:500) was obtained from ProteinTech Group, Inc (Wuhan, China). Anti-p-PERK (#3179, 1:1000) and anti-PERK (#3192, 1:1000) were purchased from Cell Signaling Technology (Massachusetts, USA).
Real-time quantitative PCR (RT-qPCR)
TRIzol reagent (Invitrogen) was used to obtain total RNA from LX-2 cells and mouse liver tissue. cDNA was synthesized by applying PrimeScript RT Reagent kit (Takara, Dalian, China). RT-qPCR was performed to determine the circ_0044226 and miR-4677-3p level, as well as SEC61G mRNA level by using SYBR Green PCR Kit (Takara). The data of RT-qPCR was calculated by the method of 2−ΔΔCt. The primer sequences were: circ_0044226, 5'-ATGCATGTACAACACCTCAGGTAT-3' (F) and 5'-CTTCTGAAATGATGGAAGAGTCC-3' (R). miR-4677-3p, 5'-CTGTGAGACCAAAGAACTACTCGC-3' (F) and 5'-CTCTACAGCTATATTGCCAGCCAC-3' (R). SEC61G, 5'-ATGGCAACAGCAATAGGAT-3' (F) and 5'-ACACTTGTTCACCAATCTCT-3' (R). GAPDH, 5'-GTTACCAGGGCTGCCTTCTC-3' (F) and 5'-GTGATGGCATGGACTGTGGT-3' (R). U6, 5'-CGCTTCGGCAGCACATATACTA-3' (F) and 5'-ATGGAACGCTTCACGAATTTGC-3' (R).
Flow cytometry for apoptosis
The apoptosis rate of LX-2 cells was detected by Annexin V-FITC Apoptosis Detection Kit (Beyotime, Shanghai, China). LX-2 cells were washed with PBS and collected through centrifugation. The cells were resuspended with 250 μL binding buffer containing 10 μL Annexin V-FITC and 5 μL propidium iodide. After incubation at dark for 15 min, cell fluorescence density was examined by flow cytometry (BD Biosciences), and the percentage of apoptotic cells was calculated.
Dual luciferase reporter assay
Binding sites for miR-4677-3p to circ_0044226 or SEC61G were predicted on the Starbase website (https://starbase.sysu.edu.cn/index.php). The luciferase reporter plasmids of circ_0044226 and SEC61G, including wild type (circ-WT, SEC61G-WT) and mutant type (circ-MUT, SEC61G-MUT), were constructed by GeneCopoeia (Guangzhou, China). According to the manufacturer’s instruction, LX-2 cells were co-transfected with NC-mimic or miR-mimic together with these reporter plasmids using Lipofectamine 2000 (Invitrogen). After 48 h, the luciferase activities were measured by Dual-luciferase Reporter Assay System (Promega, Madison, WI, USA), and normalized to renilla luciferase activity.
Animal model
7-week-old C57BL/6J male mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed at 24 ± 2°C with 55% ± 5% humidity and 12 h light/dark cycle. To induce liver fibrosis, mice (model group) received intraperitoneal injections of 10% CCL4 in olive oil (6 μL/g/mouse) twice a week for 6 weeks. Negative control mice (NC group) were injected intraperitoneally with an equal volume of olive oil. In addition, sh-circ lentivirus (1 × 109 pfu/100 μL) was injected into mice (model + sh-circ group) through the tail vein every two weeks for 6 weeks. Mice were anesthetized with 10% chloral hydrate, and after the livers were isolated, one part was fixed in 4% paraformaldehyde and the other part was stored at -80°C for pathological index detection. The experimental operations on mice were approved in advance by the Experimental Animal Care and Use Committee of The First Affiliated Hospital of Xi'an Jiaotong University.
Masson staining
Liver tissue isolated from mice was fixed in 4% paraformaldehyde and embedded in paraffin, then sectioned. Masson staining of liver tissue sections was performed using Trichrome Stain Kit (Abcam, ab150686). Sections were dewaxed and hydrated in distilled water and then incubated in preheated Bouin's Fluid for 1 h. Next, sections were stained with Weigert's Iron Hematoxylin for 5 min, and Biebrich Scarlet/Acid Fuchsin Solution and Phosphomolybdic/Phosphotungstic Acid Solution for 15 min each. After rinsing in distilled water, the slices were quickly dehydrated in 95% alcohol and absolute alcohol, cleared in xylene and sealed with synthetic resin. The stained sections were observed and photographed under microscope (Olympus).
Statistical analysis
All data were expressed as mean ± standard deviation and analyzed on GraphPad Prism software. Differences between two groups were assessed by Student's t-test. Differences between three and more groups were detected by ANOVA. P < 0.05 represents a statistically significant difference.
Results
Circ_0044226 is increased in TGF-β1-stimulated LX-2 cells
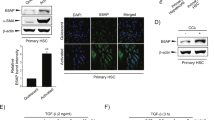
We first used TGF-β1 to induce LX-2 cell activation. Compared with untreated group, TGF-β1 resulted in significantly enhanced viability of LX-2 cells in a dose-time dependent manner (Fig. 1A and D). Meanwhile, a gradual elevation of α-SMA levels, a marker of hepatic stellate cell activation, was observed (Fig. 1B and E). The expression of circ_0044226 was markedly increased in TGF-β1-treated LX-2 cells (Fig. 1C and F). In the next experiments, LX-2 cells were treated with 10 ng/mL TGF-β1 for 48 h to establish a hepatic stellate cell activation model.
TGF-β1 promotes LX-2 cell activation and circ_0044226 expression. LX-2 cells were stimulated by TGF-β1 (5, 10, 15 ng/mL) for different times (24, 48, 72 h). A, D LX-2 cell viability was assayed by the CCK-8 method. B, E Western blot analysis of the activation marker α-SMA. C, F The expression level of circ_0044226 was determined by RT-qPCR. *P < 0.05 vs. untreated group
Knockdown of circ_0044226 inhibits LX-2 cell proliferation, promote autophagy and apoptosis
In order to investigate the role of circ_0044226 in activated hepatic stellate cells, lentiviruses that inhibit circ_0044226 expression (sh-circ) and negative control lentiviruses (sh-NC) were used to infect LX-2 cells. RT-qPCR results showed that the expression level of circ_0044226 in LX-2 cells was successfully reduced by sh-circ, and sh-circ-2 had the highest efficiency, which was used for subsequent experiments (Fig. 2A). Compared with the untreated group, TGF-β1 significantly enhanced the proliferation of LX-2 cells, which was attenuated by circ_0044226 inhibition (Fig. 2B). TGF-β1 induced LC3-II/I and Beclin-1 expression (Fig. 2C, D, and F) and inhibited p62 expression in LX-2 cells (Fig. 2C and E), indicating that TGF-β1 promoted autophagy. However, infection with sh-circ lentivirus further enhanced autophagy in TGF-β1-treated LX-2 cells (Fig. 2C-F). In addition, inhibition of circ_0044226 increased apoptosis rate, Bax/Bcl2 ratio and cleaved-caspase3 protein levels in TGF-β1-treated LX-2 cells (Fig. 2G-J). These data suggest that silencing circ_0044226 reduces the proliferation of activated hepatic stellate cells and facilitates their autophagy and apoptosis.
Effects of knockdown circ_0044226 on proliferation, autophagy and apoptosis of activated LX-2 cells. A RT-qPCR was performed to detect the interference efficiency of circ_0044226. B After TGF-β1 stimulation, LX-2 cell proliferation capacity was evaluated by CCK-8 assays. C-F Autophagy proteins were analyzed by Western blot. G Apoptosis rate was measured by flow cytometry. H-J Analysis of apoptosis-associated proteins by Western Blot. *P < 0.05 vs. untreated group; #P < 0.05 vs. control group
Knockdown of circ_0044226 enhances ERs in LX-2 cell
Given the importance of ERs in cell proliferation, autophagy and survival, we next explored the effect of circ_0044226 on ERs in LX2 cells. The results showed that treatment with TGF-β1 increased GRP78, ATF6 and CHOP, and raised phosphorylation levels of PERK and IRE1 (Fig. 3A-F), implying that TGF-β1 induced ERs in LX-2 cells. Furthermore, we found that deletion of circ_0044226 further promoted ERs in TGF-β1-treated LX-2 cells (Fig. 3A-F). It has been reported that SEC61G is involved in regulating ERs (Zhang and Guo 2022). Our RT-qPCR and Western blot experiments revealed that SEC61G was markedly upregulated after transfection with its overexpression plasmid (supplementary figure). Overexpression of SEC61G suppressed ERs in TGF-β1-treated LX-2 cells (Fig. 3A-F). Since down-regulating circ_0044226 and overexpressing SEC61G showed opposite effects on ERs, we hypothesized whether circ_0044226 modulates ERs through SEC61G. Rescue experiments showed that upregulation of SEC61G partially reversed the effects of circ_0044226 inhibition on ERs (Fig. 3A-F). It is thus clear that circ_0044226 is able to regulate ERs which may be related to the expression of SEC61G.
Circ_0044226 regulates ERs via SEC61G. A The protein expression of ERs-related proteins (GRP78, p-PERK, PERK, p-IRE1, IRE1, ATF6 and CHOP) was analyzed by Western blot after LX-2 cells were treated with interfering lentivirus of circ_0044226, overexpression plasmid of SEC61G or TGF-β1. B The protein level of GRP78. C The phosphorylation level of PERK. D The phosphorylation level of IRE1. E The protein level of ATF6. F The protein level of CHOP. *P < 0.05 vs. untreated group; #P < 0.05 vs. control group; &P < 0.05 vs. sh-circ group
Circ_0044226 regulates SEC61G expression by sponging miR-4677-3p
CircRNAs often regulate the expression of target genes by competitively binding microRNAs, so we predicted microRNAs that potentially bind to circ_0044226 and SEC61G with the Starbase website. miR-4677-3p was found to have common motifs with both circ_0044226 and SEC61G. In order to confirm the binding of miR-4677-3p to circ_0044226 and SEC61G, the wild and mutant luciferase reporter plasmids of circ_0044226 and SEC61G were constructed (Fig. 4A). miR-4677-3p mimics and inhibitors were applied to up- and down-regulate miR-4677-3p expression in LX-2 cells, respectively (Fig. 4B). The results of the dual luciferase reporter experiment showed that miR-mimic diminished the luciferase activity in circ-WT and SEC61G-WT groups compared with NC-mimic group, while there was no significant change in circ-MUT and SEC61G-MUT groups (Fig. 4C and D). Further experiments showed that knockdown of circ_0044226 promoted miR-4677-3p expression and inhibited SEC61G expression in LX-2 cells (Fig. 4E-G). And inhibiting miR-4677-3p promoted SEC61G expression in LX-2 cells, and reversed the effect of deleting circ_0044226 on SEC61G (Fig. 4E-G). It indicates that circ_0044226 regulates SEC61G expression by sponging miR-4677-3p.
Circ_0044226 regulates SEC61G by sponging miR-4677-3p. A Sequence information of miR-4677-3p with circ_0044226 and SEC61G. B Expression of miR-4677-3p in LX-2 cells after treatment with mimics or inhibitors of miR-4677-3p. C Luciferase activity after transfecting circ_0044226 wild or mutated reporter plasmids. D Luciferase activity after transfecting SEC61G wild or mutated reporter plasmids. E Expression of miR-4677-3p was detected by RT-qPCR after treating LX-2 cells with the lentivirus of interfering circ_0044226 or/and inhibitor of miR-4677-3p. F The mRNA level of SEC61G was detected by RT-qPCR. G SEC61G protein expression was determined by western blot. *P < 0.05 vs. control group; #P < 0.05 vs. NC-mimic group; &P < 0.05 vs. sh-circ group
Circ_0044226 affects LX-2 cell function through SEC61G and ERs
To further determine the molecular mechanism by which circ_0044226 regulates LX-2 cell function, SEC61G overexpression plasmid and ERs inhibitor (4-PBA) were used to perform cell function rescue experiments. CCK-8 assay showed that SEC61G overexpression or 4-PBA significantly enhanced the proliferation of TGF-β1-treated LX-2 cells (Fig. 5A). Meanwhile, SEC61G overexpression or 4-PBA inhibited LC3-II/I and Beclin-1 expression, and promoted p62 expression (Fig. 5B-E). Furthermore, overexpression of SEC61G or 4-PBA decreased cell apoptosis rate (Fig. 5F) and Bax/Bcl2 ratio (Fig. 5G and H), inhibited the expression of cleaved-caspase3 (Fig. 5I). More importantly, SEC61G upregulation or 4-PBA diminished the effect of circ_0044226 on LX-2 cell proliferation, survival and autophagy (Fig. 5A-I). The above results illustrate that circ_0044226 is involved in the regulation of hepatic stellate cell function through SEC61G and ERs.
Circ_0044226 affects LX-2 cell function through SEC61G and ERs. A Effect of SEC61G overexpression plasmid or ERs inhibitor 4-PBA on TGF-β1-treated LX-2 cell proliferation. B-E Autophagy-associated proteins were detected by Western blot after LX-2 cells were treated with overexpression plasmid of SEC61G, 4-PBA and TGF-β1. F Flow cytometry was used to analyze cell apoptosis. G-I Apoptosis-related proteins were analyzed. *P < 0.05 vs. control group; #P < 0.05 vs. sh-circ group
Inhibition of circ_0044226 attenuates CCL4-induced liver fibrosis in mice
For further validating the role and molecular mechanism of circ_0044226 in vivo, we injected sh-circ lentivirus into CCL4-induced liver fibrosis mice via tail vein. As shown by Masson staining, inhibition of circ_0044226 reduced the accumulation of collagen in model group (Fig. 6A). The protein level of Collagen I was also significantly reduced in the liver tissue of the model + sh-circ group (Fig. 6B and C). This suggests that inhibition circ_0044226 mitigate CCL4-induced liver fibrosis in vivo. Administration of sh-circ lentivirus greatly reduced circ_0044226 and SEC61G levels and increased miR-4677-3p levels in vivo (Fig. 6D-G). ERs indicators GRP78 and CHOP were found to be highly expressed in the model group, and their expression was further increased after removing circ_0044226 (Fig. 6H and I). In addition, eliminating circ_0044226 also increased the levels of autophagy protein LC3-II/I and apoptotic protein cleaved-caspase3 (Fig. 6J-L). All the above results indicate that deleting circ_0044226 could alleviate liver fibrosis by enhancing ERs through miR-4677-3p/SEC61G axis.
Inhibition of circ_0044226 attenuates CCL4-induced liver fibrosis in mice. A Collagen accumulation in mouse liver tissue was assessed by Masson staining. B and C Protein levels of Collagen I were detected by Western blot in isolated mouse liver tissue. D and E The expression levels of circ_0044226 and miR-4677-3p were analyzed by RT-qPCR in mouse liver tissue. F-I Western blot analysis of SEC61G, ERs proteins GRP78 and CHOP. J-L Detection of autophagy protein LC3-II/I and apoptotic protein cleaved-caspase3. *P < 0.05 vs. NC group; #P < 0.05 vs. model group
Discussion
Liver fibrosis is a prevalent pathological phenomenon in various chronic liver diseases, and the initiation of hepatic stellate cells represents a key event in the development of liver fibrosis (Higashi et al. 2017). In the present study, we used TGF-β1 to stimulate LX-2 cell activation with a subsequent increase of the marker α-SMA. The activated hepatic stellate cells actively contribute to the progression of liver fibrosis and facilitate the reestablishment of intrahepatic architecture by extensive cell proliferation and ECM secretion, notably collagen I. Circ_0044226 is a newly identified circRNA associated with the progression of pulmonary fibrosis (Qi et al. 2020). It was observed that knockdown of circ_0044226 led to diminished proliferation of TGF-β1-stimulated LX-2 cells. In a mouse model of CCL4-induced liver fibrosis, Collagen I was reduced in mouse liver tissue after tail vein injection of a lentivirus that inhibited circ_0044226 expression, and improved liver histopathology was clearly observed by Masson staining. Studies have shown that promoting apoptosis of activated hepatic stellate cells improves the pathological status of liver fibrosis mice (Cai et al. 2020). Our findings suggest that downregulation of circ_0044226 induced apoptosis in activated LX-2 cells. These results indicate that circ_0044226 could potentially be targeted for the treatment of liver fibrosis.
The involvement of ERs in the pathogenesis of fibrosis has been observed in various organs such as the kidney, heart, liver, gastrointestinal tract, and lung (Tanjore et al. 2013). Jiang S et al. demonstrated that ERs contribute to the differentiation of synovial myofibroblasts in joint capsule fibrosis and may serve as a potential therapeutic target for joint contracture (Jiang et al. 2018). Furthermore, inhibition of ERs has shown to improve renal fibrosis, suggesting a novel approach for preventing renal fibrosis in chronic kidney diseases (Liu et al. 2018). Similarly, ERs have been implicated in the progression of liver fibrosis (Koo et al. 2016). Recent studies have shown that ERs mediated hepatic stellate cell necroptosis, thereby ameliorating liver fibrosis (Sun et al. 2022). Our results showed that downregulation of circ_0044226 enhanced ERs. And inhibition of ERs promoted the proliferation and survival of activated LX-2 cells. These data suggest that circ_0044226 plays a role in liver fibrosis progression by regulating ERs. In our work, it is additionally important to note that TGF-β1 promoted ERs in LX-2 cells, which may result from protein synthesis overload after cell activation (Oakes and Papa 2015). Similarly, TGF-β1 was found to induce autophagy in LX-2 cells. This may be an adaptive change of activated LX-2 cells to achieve their own metabolic needs and renewal of certain organelles (Mizushima and Komatsu 2011). Knockdown of circ_0044226 was followed by enhanced ERs and autophagy in activated LX-2 cells, which may be associated with increased apoptosis, as mentioned in other articles (Hu et al. 2018; Zhu et al. 2022).
Mechanistically, circ_0044226 binds to miR-4677-3p as a competitive endogenous RNA and disarms the inhibitory effect of miR-4677-3p on SEC61G expression. Knockdown of circ_0044226 resulted in reduced binding of circ_0044226 to miR-4677-3p, leading to suppression of SEC61G expression. Previous studies reported that SEC61G was overexpressed in hepatocellular carcinoma and that knockdown of SEC61G inhibited cell proliferation and induced apoptosis (Gao et al. 2020b). Here, we explored the role of SEC61G in hepatic stellate cells for the first time. SEC61G expression is increased in CCL4-induced liver fibrosis mice. And SEC61G expression was suppressed after knockdown of circ_0044226. Down-regulation of SEC61G in lung cancer cells activated ERs and attenuated the malignant behavior of lung cancer cells (Zhang and Guo 2022). In this study, overexpression of SEC61G inhibited ERs and promoted LX-2 cell proliferation and survival, as well as inhibited autophagy, reversing the effect of knockdown circ_0044226 on LX-2 cell function.
In conclusion, we found that inhibition of circ_0044226 reduced SEC61G expression by releasing miR-4677-3p, which enhanced ERs, inhibited proliferation in activated hepatic stellate cells and promoted their autophagy and apoptosis. In addition, circ_0044226 downregulation markedly alleviated CCL4-induced liver fibrosis in mice. Therefore, circ_0044226 could be a hopeful therapeutic target for liver fibrosis.
Data availability
No datasets were generated or analysed during the current study.
References
Allaire M, Rautou PE, Codogno P, Lotersztajn S (2019) Autophagy in liver diseases: time for translation? J Hepatol 70(5):985–998. https://doi.org/10.1016/j.jhep.2019.01.026
Bu FT, Chen Y, Yu HX, Chen X, Yang Y, Pan XY et al (2018) SENP2 alleviates CCl(4)-induced liver fibrosis by promoting activated hepatic stellate cell apoptosis and reversion. Toxicol Lett 289:86–98. https://doi.org/10.1016/j.toxlet.2018.03.010
Cai X, Wang J, Wang J, Zhou Q, Yang B, He Q et al (2020) Intercellular crosstalk of hepatic stellate cells in liver fibrosis: new insights into therapy. Pharmacol Res 155:104720. https://doi.org/10.1016/j.phrs.2020.104720
Caligiuri A, Gentilini A, Pastore M, Gitto S, Marra F (2021) Cellular and molecular mechanisms underlying liver fibrosis regression. Cells 10(10). https://doi.org/10.3390/cells10102759
Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN (2019) TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells 8(11). https://doi.org/10.3390/cells8111419
Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL (2015) Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res 59(3):292–307. https://doi.org/10.1111/jpi.12264
Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S et al (2020a) Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol 73(5):1144–1154. https://doi.org/10.1016/j.jhep.2020.04.044
Gao H, Niu W, He Z, Gao C, Peng C, Niu J (2020b) SEC61G plays an oncogenic role in hepatocellular carcinoma cells. Cell Cycle 19(23):3348–3361. https://doi.org/10.1080/15384101.2020.1843816
Higashi T, Friedman SL, Hoshida Y (2017) Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 121:27–42. https://doi.org/10.1016/j.addr.2017.05.007
Hu H, Tian M, Ding C, Yu S (2018) The C/EBP Homologous Protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 9:3083. https://doi.org/10.3389/fimmu.2018.03083
Huang Y, Li X, Wang Y, Wang H, Huang C, Li J (2014) Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways. Mol Cell Biochem 394(1–2):1–12. https://doi.org/10.1007/s11010-014-2073-8
Iurlaro R, Muñoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283(14):2640–2652. https://doi.org/10.1111/febs.13598
Jiang S, He R, Zhu L, Liang T, Wang Z, Lu Y et al (2018) Endoplasmic reticulum stress-dependent ROS production mediates synovial myofibroblastic differentiation in the immobilization-induced rat knee joint contracture model. Exp Cell Res 369(2):325–334. https://doi.org/10.1016/j.yexcr.2018.05.036
Koo JH, Lee HJ, Kim W, Kim SG (2016) Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2. Gastroenterology 150(1):181-193.e188. https://doi.org/10.1053/j.gastro.2015.09.039
Li Y, Chen Y, Huang H, Shi M, Yang W, Kuang J et al (2017) Autophagy mediated by endoplasmic reticulum stress enhances the caffeine-induced apoptosis of hepatic stellate cells. Int J Mol Med 40(5):1405–1414. https://doi.org/10.3892/ijmm.2017.3145
Li S, Song F, Lei X, Li J, Li F, Tan H (2020) hsa_circ_0004018 suppresses the progression of liver fibrosis through regulating the hsa-miR-660-3p/TEP1 axis. Aging 12(12):11517–11529. https://doi.org/10.18632/aging.103257
Liu Y, Wang Y, Ding W, Wang Y (2018) Mito-TEMPO alleviates renal fibrosis by reducing inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress. Oxid Med Cell Longev 2018:5828120. https://doi.org/10.1155/2018/5828120
Liu R, Zhang L, Zhao X, Liu J, Chang W, Zhou L et al (2022) circRNA: regulatory factors and potential therapeutic targets in inflammatory dermatoses. J Cell Mol Med 26(16):4389–4400. https://doi.org/10.1111/jcmm.17473
Ma J, He Z, Zhang H, Zhang W, Gao S, Ni X (2021) SEC61G promotes breast cancer development and metastasis via modulating glycolysis and is transcriptionally regulated by E2F1. Cell Death Dis 12(6):550. https://doi.org/10.1038/s41419-021-03797-3
Meng H, Jiang X, Wang J, Sang Z, Guo L, Yin G et al (2021) SEC61G is upregulated and required for tumor progression in human kidney cancer. Mol Med Rep 23(6). https://doi.org/10.3892/mmr.2021.12066
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. https://doi.org/10.1016/j.cell.2011.10.026
Oakes SA (2020) Endoplasmic reticulum stress signaling in cancer cells. Am J Pathol 190(5):934–946. https://doi.org/10.1016/j.ajpath.2020.01.010
Oakes SA, Papa FR (2015) The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10:173–194. https://doi.org/10.1146/annurev-pathol-012513-104649
Qi Z, Chen L (2019) Endoplasmic reticulum stress and autophagy. Adv Exp Med Biol 1206:167–177. https://doi.org/10.1007/978-981-15-0602-4_8
Qi F, Li Y, Yang X, Wu Y, Lin L, Liu X (2020) Hsa_circ_0044226 knockdown attenuates progression of pulmonary fibrosis by inhibiting CDC27. Aging 12(14):14808–14818. https://doi.org/10.18632/aging.103543
Qi F, Lv ZD, Huang WD, Wei SC, Liu XM, Song WD (2023) LncRNA TUG1 promotes pulmonary fibrosis progression via up-regulating CDC27 and activating PI3K/Akt/mTOR pathway. Epigenetics 18(1):2195305. https://doi.org/10.1080/15592294.2023.2195305
Sun S, Huan S, Li Z, Yao Y, Su Y, Xia S et al (2022) Curcumol alleviates liver fibrosis by inducing endoplasmic reticulum stress-mediated necroptosis of hepatic stellate cells through Sirt1/NICD pathway. PeerJ 10:e13376. https://doi.org/10.7717/peerj.13376
Tanjore H, Lawson WE, Blackwell TS (2013) Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochem Biophys Acta 1832(7):940–947. https://doi.org/10.1016/j.bbadis.2012.11.011
Tsuchida T, Friedman SL (2017) Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14(7):397–411. https://doi.org/10.1038/nrgastro.2017.38
Wang W, Dong R, Guo Y, He J, Shao C, Yi P et al (2019) CircMTO1 inhibits liver fibrosis via regulation of miR-17-5p and Smad7. J Cell Mol Med 23(8):5486–5496. https://doi.org/10.1111/jcmm.14432
Zeng X, Yuan X, Cai Q, Tang C, Gao J (2021) Circular RNA as an epigenetic regulator in chronic liver diseases. Cells 10(8). https://doi.org/10.3390/cells10081945
Zhang Q, Guo Z (2022) SEC61G participates in endoplasmic reticulum stress by interacting with CREB3 to promote the malignant progression of lung adenocarcinoma. Oncol Lett 24(1):233. https://doi.org/10.3892/ol.2022.13316
Zhang L, Fujita T, Wu G, Xiao X, Wan Y (2011) Phosphorylation of the anaphase-promoting complex/Cdc27 is involved in TGF-beta signaling. J Biol Chem 286(12):10041–10050. https://doi.org/10.1074/jbc.M110.205518
Zhu S, Li X, Dang B, Wu F, Wang C, Lin C (2022) Lycium Barbarum polysaccharide protects HaCaT cells from PM2.5-induced apoptosis via inhibiting oxidative stress, ER stress and autophagy. Redox Rep 27(1):32–44. https://doi.org/10.1080/13510002.2022.2036507
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Shanshan Yuan and Jiaming Liu: Conceptualization and Writing – original draft; Li Yang and Xin Zhang: Formal analysis, Methodology and Writing – review & editing; Kun Zhuang: Data curation and Software; Shuixiang He: Conceptualization, Project administration and Writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the ethics committee of The First Affiliated Hospital of Xi'an Jiaotong University. And all animal experiments complied with ARRIVE guidelines, and were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shanshan Yuan and Jiaming Liu contribute equally to this study and regarded as co-first author.
Highlights
1. Knockdown of circ_0044226 inhibits the proliferation of activated hepatic stellate cells.
2. Knockdown of circ_0044226 promotes autophagy and apoptosis in activated hepatic stellate cells.
3. Circ_0044226 regulates SEC61G-mediated ERs by sponging miR-4677-3p.
4. Inhibition of circ_0044226 attenuates CCL4-induced liver fibrosis in mice.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, S., Liu, J., Yang, L. et al. Knockdown of circ_0044226 promotes endoplasmic reticulum stress-mediated autophagy and apoptosis in hepatic stellate cells via miR-4677-3p/SEC61G axis. J Bioenerg Biomembr 56, 261–271 (2024). https://doi.org/10.1007/s10863-024-10007-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-024-10007-0