Abstract
Purinergic receptors are widespread in the human organism and are involved in several physiological functions like neurotransmission, nociception, platelet aggregation, etc. In the immune system, they may regulate the expression and release of pro-inflammatory factors as well as the activation and death of several cell types. It is already described the participation of some purinergic receptors in the inflammation and pathological processes, such as a few neglected tropical diseases (NTDs) which affect more than 1 billion people in the world. Although the high social influence those diseases represent endemic countries, most of them do not have an efficient, safe or affordable drug treatment. In that way, this review aims to discuss the current literature involving purinergic receptor and immune response to NTDs pathogens, which may contribute in the search for new therapeutic possibilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purinergic receptor roles in infections
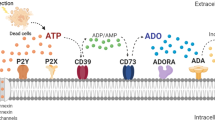
P2 receptors are cell-surface proteins responsive to purine and pyrimidine nucleotides and nucleosides that are divided into two types: the ionotropic P2X receptors (P2XR) and metabotropic P2Y receptors (P2YR) (Fig. 1) (Burnstock 2012). The P2XR are trimeric ATP-gated ion channels permeable to cation species that are composed of seven subtypes in mammalian tissues P2X1 – P2X7 (Khakh 2001). The P2YR are G protein-coupled receptors, with eight cloned subtypes in mammalian tissues P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 (Jacobson et al. 2012). The P2 receptors are involved in a diversity of physiological processes including neurotransmission, apoptosis, cytokine secretion, chemotaxis, inflammatory pain and activation of immune cells, etc. (Burnstock and Boeynaems 2014; Cekic and Linden 2016).
P2 receptors and purinergic signaling. ATP molecule is hydrolised into ADP and AMP molecules. P2X ion channels are activated by ATP whereas P2Y G-protein-coupled receptors are activated by either ATP and ADP molecules. Once activated, P2X receptors change their trimeric structural conformation into open ion channel, which alow small ions to pass, as sodium and potassium ions. As ATP or ADP activate P2Y receptors, it triggers intracellular signalings after G-protein hydrolisis into α and βγ subunits. Then, accordingly to G-protein subtype, there are distinct intracellular signaling pathways, as cyclic AMP and PLC/IP3/DAG, for example
Several mechanisms for ATP release have been described under normal and pathological conditions: vesicular transport, cell death (membrane damage), stretch-activated anion channels, volume-regulated channels, maxi-anion channels, pannexin and connexin hemichannels and P2X7 receptors (Fitz 2007; Dubyak 2012). In the context of innate immune response to infectious challenges, ATP and other mediators emitted by injured or dying cells can function as a “reinforcement signal” for the inflammatory response elicited by initial pathogen recognition. An important signaling pathway that illustrates this phenomenon is the caspase-1-dependent processing and release of mature pro-inflammatory cytokines IL-1β and IL-18. In fact, the P2X7 receptor is known as a potent activator of NOD-like receptor (NLRP3) inflammasome pathway (Schroder and Tschopp 2010; Gombault et al. 2012). In this regard, the recognition of microbial products would act a priming signal for the inflammatory cells, inducing transcription of biologically inactive forms of these cytokines and NLRP3 expression; and the P2X7 receptor stimulation would act as the final signal for NLRP3 activation and proteolytic maturation of IL-1β e IL-18 (Schroder and Tschopp 2010; Perregaux and Gabel 1994; Burnstock 2016). However, the exact molecular mechanism of P2X7-mediated inflammasome activation is still elusive.
Therefore, it is not a surprise that an increasing number of studies has demonstrated a direct involvement of purinergic receptors in the pathogenesis of some widespread infectious diseases such as tuberculosis, HIV, and malaria (Miller et al. 2011). Regarding the role of P2X receptors in Mycobacterium tuberculosis (MB) infection, a special attention has been given to P2X7 receptor. Indeed, some research groups have demonstrated through in vitro and in vivo studies association of the P2X7 receptor with MB infection (Santos Jr et al. 2013a; Placido et al. 2006; Fairbairn et al. 2001). For instance, treatment of infected macrophages or monocytes with ATP can lead to cell or mycobacterial death (Santos Jr et al. 2013a; Placido et al. 2006). Moreover, genetic studies have shown a strong association between a loss-of-function polymorphism in the human P2X7 receptor gene and increased susceptibility to tuberculosis (Lammas et al. 1997; Singla et al. 2012; Yim and Selvaraj 2010), which can be explained by a decreased capacity of macrophages in killing the bacillus (Fernando et al. 2007). On another hand, in vivo studies with P2X7 KO mice have shown both deleterious and protective effects, depending on mycobacterial strains or virulence (Santos Jr et al. 2013b; Amaral et al. 2014). In summary, these results suggest an important role of P2X7 receptor in modulating immune responses against mycobacterium infections.
There are also studies showing the involvement of P2 receptor in HIV infection (Hazleton et al. 2012; Séror et al. 2011; Pacheco et al. 2014). So far, P2X1, P2X7, P2Y1 and P2Y2 receptors have been implicated in the early stages of HIV-1 infection and replication. Also, ATP was shown to be released via pannexin-1 hemichannels from human cells upon infection and the pharmacological inhibition of purinergic receptors prevented HIV-1 replication in vitro (Hazleton et al. 2012; Orellana et al. 2011). In the case of Malaria, functional studies in infected erythrocytes showed ATP release from these cells (Swartz et al. 2015). The organic osmolyte permeability effect and the rectifying anion conductance observed in the intraerythrocytic development of P. falciparum also can be induced by ATP and P2 receptor agonists (Huber 2012). In addition, treatment with apyrase (an enzyme that catalysis the hydrolysis of ATP) and P2 antagonists was able to decrease the induction of the osmolyte permeability and Plasmodium infection and proliferation (Tanneur et al. 2006; Levano-Garcia et al. 2010).
Neglected tropical diseases: participation of purinergic signaling
According to World Health Organization (WHO), neglected tropical diseases (NTDs) are infectious diseases caused by microorganisms, such as viruses, bacteria, helminths and protozoa that can be transmitted directly or indirectly from person to person or via fomites, mosquitoes, tsetse flies, sandflies, blackflies, snails, fecal-oral route or via food products. NTDs include seventeen diseases (Huber 2012). They affect mostly marginalized populations without access to clean water and sanitation. NTDs have a huge social impact in terms of quality of life, loss of productivity and a high cost of the treatment.
Most of these diseases can be managed by controlling the vector and/or drug treatment. However, the number of effective, affordable and safe pharmaceuticals for NTDs is still scarce. The main reason is a low investment in drug research and development since NTDs are considered “low-return” and “non-profitable” by the pharmaceutical industry. Thus, there is an urgent necessity for the discovery of new drugs targets due the high mortality and morbidity of these diseases among poor people.
As mentioned before, ATP can be released to the extracellular milieu under infectious and inflammatory conditions. Interestingly, several NTDs exhibit inflammatory reactions in their symptomatology such as Chagas disease (Montgomery et al. 2014), Echinococcosis (Talvani and Teixeira 2011), Foodborne Trematodiases (Wang et al. 2014), Schistosomiasis (Keiser and Utzinger 2009), Human African trypanosomiasis (Barron and Wynn 2011), Leishmaniosis (MacLean et al. 2004), Soiltransmitted helminthiases (Souza et al. 2011), Dengue (Mascarini-Serra 2011), Chikungunya, Cysticercosis in combination with downregulation of ectonucleotidases (Sierra et al. 2010). In this context, P2 receptor activation may represent an attractive target in the studies aiming drug development for NTDs. Therefore, in this review, we present recent publications involving those receptors and some relevant NTDs.
Chagas’ disease
Chagas’ disease (or American trypanosomiasis) is an anthropozoonosis caused by the protozoan Trypanosoma cruzi (Chatelain 2017; Pérez-Molina et al. 2015; Pérez-Molina and Molina 2017). The disease comprises two clinical phases: an acute phase which is usually asymptomatic or unrecognized and a chronic phase which is characterized by organ dysfunction such as cardiomyopathy, inflammation, and destruction of parasympathetic neurons of the gastrointestinal tract, megaviscer and polyneuropathy (Pérez-Molina et al. 2015; Pérez-Molina and Molina 2017).
The first evidence of purinergic receptors relation with T. cruzi infection came from a study that identified an increased susceptibility of double positive (DP) thymocytes obtained from infected mice to extracellular ATP-induced permeabilization or cell death during thymus atrophy which could be related to P2X7 receptor activation (Mantuano-Barradas et al. 2003). In a second study, the same research group found no difference in the thymic atrophy in P2X7-knockout mice when compared to a wild type. Despite these results, the authors pointed out that other approaches than knockout mice might be explored to study the role of individual purinergic receptors in thymus atrophy due to compensatory effects that can mask their primary physiological activities. Moreover, using this knockout mice system, they were also able to identify interactions and modulations involving P2X and P2Y receptors (Cascabulho et al. 2008). Meuser-Batista and collaborators found a reduction in cardiac and peritoneal mast cells (MC) numbers during the early acute phase in infected mice. Although the authors observed that the infection induced the transcription of P2X7 mRNA in heart and peritoneal cavity, only for the latter, the P2X7 receptor expression could be correlated with the decrease in MC numbers, indicating a possible participation of this receptor in cell death in vivo (Meuser-Batista et al. 2011).
Dengue
Dengue is a systemic disease caused by a single-stranded RNA virus of the genus Flavivirus and transmitted by the female mosquitoes of several species, as Aedes aegypti, with an estimated global incidence of in 96 million people, plus 294 million subclinical infections. Currently, the difficulty of a specific treatment is due to the lack of a robust animal model with appropriate resemblance to human susceptibility (Katzelnick et al. 2017; Castro et al. 2017; Whitehorn et al. 2014).
Immune cells are the main affected cells by Dengue virus. As they express the P2 receptor, it is conceivable to propose that purinergic signaling may participate somehow in the infection process. Notably, monocyte/macrophage and dendritic cells are main examples of cellular models that express P2X7R. Nevertheless, only a few works tried to study the relation between P2X7 receptor and Dengue virus (DENV). Correa et al. (2016) described a direct involvement of P2X7 receptor in modulation of the antiviral and inflammatory response to DENV in vitro. Pre-treatment of monocytes with 1 mM ATP resulted in significantly reduced NS1 secretion and DENV-Ag positive cell frequencies. Moreover, pre-incubation with ATP or BzATP re-established monocytes production of nitric oxide upon infection. Finally, P2X7 receptor activation impaired the production of the cytokines TNF-α and IL-8, and chemokines CCL2 and CXCL10 that are associated with severity of dengue disease. On the other hand, Tsai et al. (2015) demonstrated that the IFN-γ response of γδT cells to Dengue virus-infected dendritic cells is dependent on the P2X7 receptor pathway, demonstrating that this antiviral action of the P2X7 receptor may be present in different immune cells. Obviously, more studies concerning purinergic signaling in the Dengue infection processes need to be developed, but these results might indicate that P2 receptors can pose as novel therapeutic targets for Dengue fever.
Schistosomiasis
Schistosomiasis is a chronic intravascular disease caused by a different species of the genus Schistosoma trematode helminth (Colley et al. 2014; Mutapi et al. 2017). Most of the clinical features of the disease result from a granulomatous response against schistosome eggs and released antigens that can lead to fibrogenesis in host tissues (Burke et al. 2009; Barsoum et al. 2013).
Evidence from murine experimental schistosomiasis have shown an association of a strong CD4+ Th2 response and the granuloma formation, involving several cell populations and immune mediators (Burke et al. 2009). Regarding purinergic signaling, Oliveira et al. observed a reduced P2X7 receptor-mediated permeabilization effect on mesenteric endothelial cells from infected mice when compared to the control (Oliveira et al. 2013). Similar results were found when the authors evaluated endothelial nitric oxide (NO) production. Western blot and immunocytochemistry assays demonstrated a reduced expression of the P2X7 receptor in these mesenteric endothelial cells (Barsoum et al. 2013). Additionally, Oliveira et al. (2014) also demonstrated a critical reduction of P2X7 receptor activity (dye uptake assay and intracellular calcium mobilization) in murine peritoneal macrophage cells upon S. mansoni infection. Interestingly, the authors found that this impairment of the P2X7 receptor function in the infected group was correlated to an effect of the anti-inflammatory cytokine TGF-β1. In fact, they observed higher TGF-β1 levels in the peritoneal cavity of infected mice. Moreover, pretreatment of peritoneal macrophages from uninfected animals with TGF-β1 resulted in a reduction of ATP-induced dye uptake. Although this phenomenon was not associated to changes in P2X7 protein expression and mRNA levels between the uninfected, infected, and TGF-β1-treated group, they found a diminished cell surface localization of P2X7 receptors in macrophages from infected mice. The authors also demonstrated that P2X7 receptor seems to be crucial in the tolerance and survival of the infected mice since there was a high lethally pattern in P2X7-knockout mice (Oliveira et al. 2014).
Muniz and collaborators identified a role for P2Y12 receptor in the modulation of eosinophil activation in S. mansoni-induced inflammation. The authors found that P2Y12 receptor blockade with clopidogrel reduced granulomatous inflammatory area and the number of eosinophils in mice liver during S. mansoni infection (Muniz et al. 2015).
Schistosomiasis was shown to modulate the leukocyte adhesion to endothelial cells by affecting P2Y1 receptor signaling. Oliveira et al. (2016) demonstrated that mesenteric endothelial cells from infected mice possessed an increased expression of NTPDases 2 and 3 what was correlated to higher ATP hydrolysis to ADP when compared to control groups (Oliveira et al. 2016). They also observed higher basal adhesion values in the infected group and that the pre-treatment with MRS2179, a P2Y1 receptor antagonist, reduced this effect. In the control group, the same treatment had no effect on basal leukocyte adhesion. Together, these data indicated that Schistosoma mansoni infection induced pro-inflammatory phenotype in mesenteric cells characterized by an up-regulation of NTPDases 2 and 3, which in turn could favor ADP accumulation and leukocyte adhesion. Interestingly, intravascular stages of the parasite also exhibit ATP-hydrolyzing activity by expression of ATP diphosphohydrolase (SmATPDase1) what can enhance ADP accumulation in vivo (Da’dara et al. 2014).
Leishmaniasis
Leishmaniasis is a parasitic infection transmitted to vertebrate hosts by the bite of different phlebotomine sand flies (genus Phlebotomus in Europe, Asia and Africa, and Lutzomya in Americas and Oceania) (Stockdale and Newton 2013; Gupta et al. 2014). The causative agent is an obligatory unicellular protozoan of the genus Leishmania that targets mainly phagocytic cells. The parasite had developed several strategies to survive within the host, evading the immune response such as prevention of the lysosomal enzymes activity, inhibition of pro-inflammatory cytokines production, alteration of Toll-like receptors signaling pathways, attenuation T cell-mediated immune responses, etc. (Loría-Cervera and Andrade-Narváez 2014).
Recent publications have emphasized a direct involvement of some purinergic receptors in the immune response to Leishmania (Chaves et al. 2016; de Figueiredo et al. 2016). One of the first evidences was a study showing modulation of P2X7 purinergic receptor in macrophages by Leishmania amazonensis infection (Chaves et al. 2009). Infected macrophages exhibited higher expression of P2X7 receptor, what was correlated to an increased ATP-induced membrane permeabilization and apoptosis. Treatment with ATP also reduced the parasite load. In a second work, the same group demonstrated a differential dye uptake in infected macrophages after treatment with ATP (Marques-da-Silva et al. 2011a). While the authors observed an enhanced uptake of anionic dyes, such as Lucifer Yellow and carboxyfluorescein, the uptake of cationic dyes was drastically reduced. Interestingly, this differential modulation of dye uptake showed to be dependent on viable L. amazonensis parasites, since macrophages showed increased uptake of both anionic and cationic dyes when they used parasites killed by freezing-and-thawing or fixed with paraformaldehyde. Still concerning the role of P2X7 receptor, Chaves and collaborators (2014) showed that P2X7 receptor activation by ATP induced Leukotriene B4 (LTB4) production and release from uninfected and infected macrophages (Chaves et al. 2014). Additionally, they observed that infected macrophages stimulated with ATP exhibited higher LTB4 release than both infected and uninfected no stimulated macrophages. This effect was dependent on P2X7 receptor activation since knockout mice had a significant reduction in LTB4 release. Another evidence that corroborates the role of P2X7 receptor as a resistance factor to Leishmania infection came from a study in which knockout mice exhibited increased susceptibility to infection by L. amazonensis and presented larger lesion size and parasite load when compared to wild type mice. Furthermore, these alterations were associated to a pronounced pro-inflammatory profile compatible with a TH1 response in infected tissues (Figliuolo et al. 2017).
Other purinergic receptors have been also implicated in protective response against Leishmania. Marques-de-Silva et al. found that L. amazonensis infection upregulated the expression of P2Y2 and P2Y4 receptor in infected peritoneal macrophages. Moreover, UTP treatment reduced the parasite load and induced apoptosis and intermediate reactive oxygen and nitrogen species production in these cells. They also identified damage of intracellular parasites after UTP treatment (Marques-da-Silva et al. 2011b).
Conclusion
Neglected tropical diseases (NTDs) still represent a serious public health problem affecting mainly poor populations in Africa, Asia, and Latin America. However, in consequence of intense migratory flows, some of these diseases can also be found in developed regions of the world (Tam et al. 2016). Due to the high direct cost of disease prevention and treatment and indirect cost with the reduction of worker productivity, NTDs also represent a serious economic burden for low-income countries (Conteh et al. 2010). According to World Health Organization reports more than one billion of people suffer from one or more NTDs in the developing countries (Who 2012). Despite the high mortality and morbidity, there is still a lack of effective and affordable drugs for the treatment of these diseases. Since drug discovery is costly and risky, the search for new therapeutic targets is highly desirable. In this sense, this review aims to present purinergic receptors as important targets in the study of NTDs immunopathogenesis. As mentioned before, recent studies have implicated P2 receptor in immune responses against microbes. However, only a few numbers of studies have been performed to understand the role of these receptors in the immune response to causative pathogens of NTDs. Although these receptors may not work as a general molecular target candidate for all these diseases, the understanding of their action or modulation during the course of the infection can help to identify essential immune mediators and pathways that can be targeted for NTDs management.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- ATP:
-
Adenosine Triphosphate
- cAMP:
-
3′-5′-cyclic adenosine monophosphate
- COX:
-
cyclooxygenase
- DCs:
-
dendritic cells
- IP3:
-
inositol triphosphate
- MT:
-
Mycobacterium tuberculosis
- NK cells:
-
natural killer
- NLRs:
-
NOD-like receptors
- NO:
-
nitric oxide
- NTDs:
-
Negleted Tropical Diseases
- P2R:
-
P2 rec1eptors
- PAMPs:
-
pathogen-associated molecular patterns
- PRRs:
-
pattern recognition receptors
- RLRs:
-
RIG-I–like receptors
- TcEcto-NTPDases:
-
ecto-nucleoside thriphosphate diphosphohydrolase
- TLRs:
-
Toll-Like receptors
- TM:
-
transmembrane domains
- WHO:
-
World Health Organization
References
Amaral EP, Ribeiro SC, Lanes VR, Almeida FM, de Andrade MR, Bomfim CC (2014) Pulmonary infection with hypervirulent Mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog 10:e1004188. https://doi.org/10.1371/journal.ppat.1004188
Barron L, Wynn T (2011) Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol 41:2509–2514
Barsoum RS, Esmat G, El-Baz T (2013) Human schistosomiasis: clinical perspective: review. J Adv Res 4:433–444
Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, Mcmanus DP (2009) Immunopathogenesis of human schistosomiasis. Parasite Immunol 31:163–176
Burnstock G (2012) Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays 34:218–225
Burnstock G (2016) P2X ion channel receptors and inflammation. Purinergic Signal 12:59–67
Burnstock G, Boeynaems J-M (2014) Purinergic signalling and immune cells. Purinergic Signal 10:529–564
Cascabulho CM, Menna-Barreto RFS, Coutinho-Silva R, Persechini PM, Henriques-Pons A (2008) P2X7 modulatory web in Trypanosoma cruzi infection. Parasitol Res 103:829–838
Castro MC, Wilson ME, Bloom DE (2017) Disease and economic burdens of dengue. Lancet Infect Dis 17:e70–e78
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16:177–192
Chatelain E (2017) Chagas disease research and development: is there light at the end of the tunnel? Comput Struct Biotechnol J 15:98–103
Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B (2009) Modulation of P2X7 purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect 11:842–849
Chaves MM, Marques-da-Silva C, Monteiro APT, Canetti C, Coutinho-Silva R (2014) Leukotriene B4 modulates P2X7 receptor-mediated Leishmania amazonensis elimination in murine macrophages. J Immunol 192:4765–4773
Chaves MM, Canetti C, Coutinho-Silva R (2016) Crosstalk between purinergic receptors and lipid mediators in leishmaniasis. Parasit Vectors 9:489
Colley DG, Bustinduy AL, Secor WE, King CH (2014) Human schistosomiasis. Lancet 383:2253–2264
Conteh L, Engels T, Molyneux DH (2010) Socioeconomic aspects of neglected tropical diseases. Lancet 375:239–247
Corrêa G, de A Lindenberg C, Fernandes-Santos C, Gandini M, Petitinga Paiva F, Coutinho-Silva R, Kubelka CF (2016) The purinergic receptor P2X7 role in control of dengue virus-2 infection and cytokine/chemokine production in infected human monocytes. Immunobiology 221:794–802
Da’dara AA, Bhardwaj R, Skelly PJ (2014) Schistosome apyrase SmATPDase1, but not SmATPDase2, hydrolyses exogenous ATP and ADP. Purinergic Signal 10:573–580
de Figueiredo AB, Souza-Testasicca MC, Afonso LCC (2016) Purinergic signaling and infection by Leishmania: a new approach to evasion of the immune response [figure presented]. Biom J 39:244–250
Dubyak GR (2012) P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol 14:1697–1706
Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA (2001) ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol 167:3300–3307
Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB et al (2007) A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med 175:360–366. https://doi.org/10.1164/rccm.200607-970OC
Figliuolo VR, Chaves SP, Savio LEB, Thorstenberg MLP, Machado Salles É, Takyia CM, D'Império-Lima MR, de Mattos-Guedes HL, Rossi-Bergmann B, Coutinho-Silva R (2017) The role of the P2X7 receptor in murine cutaneous leishmaniasis: aspects of inflammation and parasite control. Purinergic Signal 13:143–152
Fitz JG (2007) Regulation of cellular ATP release. Trans Am Clin Climatol Assoc 118:199–208
Gombault A, Baron L, Couillin I (2012) ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol 3:414
Gupta G, Oghumu S, Satoskar AR (2014) Mechanisms of immune evasion in Leishmaniasis. Adv Appl Microbiol 82:1–23
Hazleton JE, Berman JW, Eugenin E (2012) Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol 188:4488–4495
Huber SM (2012) Purinoceptor signaling in malaria-infected erythrocytes. Microbes Infect 14:779–786
Jacobson KA, Jayasekara MPS, Costanzi S (2012) Molecular structure of P2Y receptors: mutagenesis, modeling, and chemical probes. Wiley Interdiscip Rev Membr Transp Signal 12:1–19
Katzelnick LC, Coloma J, Harris E (2017) Dengue: knowledge gaps, unmet needs, and research priorities. Lancet Infect Dis 17:e88–e100
Keiser J, Utzinger J (2009) Food-borne trematodiases. Clin Microbiol Rev 22:466–483
Khakh BS (2001) Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2:165–174
Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS (1997) ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433–444
Levano-Garcia J, Dluzewski AR, Markus RP, Garcia CRS (2010) Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal 6:365–372
Loría-Cervera EN, Andrade-Narváez FJ (2014) Animal models for the study of leishmaniasis immunology. Rev Inst Med Trop Sao Paulo 56:1–11
MacLean L, Chisi JE, Odiit M, Gibson WC, Ferris V, Picozzi K, Sternberg JM (2004) Severity of human african trypanosomiasis in East Africa is associated with geographic location, parasite genotype, and host inflammatory cytokine response profile. Infect Immun 72:7040–7044
Mantuano-Barradas M, Henriques-Pons A, Araújo-Jorge TC, Di Virgilio F, Coutinho-Silva R, Persechini PM (2003) Extracellular ATP induces cell death in CD4+/CD8+ double-positive thymocytes in mice infected with Trypanosoma cruzi. Microbes Infect 5:1363–1371
Marques-da-Silva C, Chaves MM, Rodrigues JC, Corte-Real S, Coutinho-Silva R, Persechini PM (2011a) Differential modulation of ATP-induced P2X7-associated permeabilities to cations and anions of macrophages by infection with Leishmania amazonensis. PLoS One 6:e25356
Marques-da-Silva C, Chaves MM, Chaves SP, Figliuolo VR, Meyer-Fernandes JR, Lameu C, Corte-Real S, Ulrich H, Ojcius DM, Rossi-Bergmann B, Coutinho-Silva R (2011b) Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host-cell susceptibility to UTP-mediated apoptosis. Cell Microbiol 13:1410–1428
Mascarini-Serra L (2011) Prevention of soil-transmitted helminth infection. J Global Infect Dis 3:175–182
Meuser-Batista M, Corrêa JR, Carvalho VF, de Carvalho BCFDP, da Cruz MO, Batista MM, Filho FAF, Silva PMR, Soares MJ, Lannes-Vieira J, Coutinho-Solva R, Henrique-Pons A (2011) Mast cell function and death in Trypanosoma cruzi infection. Am J Pathol 179:1894–1904
Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC (2011) The role of the P2X7 receptor in infectious diseases. PLoS Pathog 7:e1002212
Montgomery SP, Starr MC, Cantey PT, Edwards MS, Meymandi SK (2014) Neglected parasitic infections in the United States: Chagas disease. Am J Trop Med Hyg 90:814–818
Muniz VS, Baptista-Dos-reis R, Benjamim CF, Mata-Santos HA, Pyrrho AS, Strauch MA, Melo PA, Vicentino ARR, Silva-Paiva J, Bandeira-Melo C, Weller PF, Figueiredo RT, Neves JS (2015) Purinergic P2Y12 receptor activation in eosinophils and the schistosomal host response. PLoS One 10:1–21
Mutapi F, Maizels R, Fenwick A, Woolhouse M (2017) Human schistosomiasis in the post mass drug administration era. Lancet Infect Dis 17:e42–e48
Oliveira SDDS, Coutinho-Silva R, Silva CLM (2013) Endothelial P2X7 receptors’ expression is reduced by schistosomiasis. Purinergic Signal 9:81–89
Oliveira SDS, Nanini HF, Savio LEB, Waghabi MC, Silva CLM, Coutinho-Silva R (2014) Macrophage P2X7 receptor function is reduced during schistosomiasis: putative role of TGF-β1. Mediat Inflamm 2014:134974
Oliveira SDS, Oliveira NF, Meyer-Fernandes JR, Savio LEB, Ornelas FGI, Ferreira ZS, Coutinho-Silva R, Silva CLM (2016) Increased expression of NTPDases 2 and 3 in mesenteric endothelial cells during schistosomiasis favors leukocyte adhesion through P2Y1 receptors. Vasc Pharmacol 82:66–72
Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Sáez JC (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118:826–840
Pacheco PA, Faria RX, Ferreira LG, Paixão IC (2014) Putative roles of purinergic signaling in human immunodeficiency virus-1 infection. Biol Direct 9:21–33
Pérez-Molina JA, Molina I (2017) Chagas disease. Lancet 6736:1–13
Pérez-Molina JA, Perez AM, Norman FF, Monge-Maillo B, López-Vélez R (2015) Old and new challenges in Chagas disease. Lancet Infect Dis 15:1347–1356
Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203
Placido R, Auricchio G, Falzoni S, Battistini L, Colizzi V, Brunetti E, Di Virgilio F, Mancino G (2006) P2X(7) purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell Immunol 244:10–18
Santos AA Jr, Rodrigues-Junior V, Zanin RF, Borges TJ, Bonorino C, Coutinho-Silva R, Takyia CM, Santos DS, Campos MM, Morrone FB (2013a) Implication of purinergic P2X7 receptor in M. tuberculosis infection and host interaction mechanisms: a mouse model study. Immunobiology 218:1104–1112
Santos AA Jr, Rodrigues-Junior V, Zanin RF, Borges TJ, Bonorino C, Coutinho-Silva R (2013b) Implication of purinergic P2X7 receptor in M. tuberculosis infection and host interaction mechanisms: a mouse model study. Immunobiology 218:1104–1112. https://doi.org/10.1016/j.imbio.2013.03.003
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832
Séror C, Melki M, Subra F, Raza SQ, Bras M, Saïdi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Métivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet J, Ojcius DM, Piacentini M, Gougeon M, Kroemer G (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med 208:1823–1834
Sierra B, Perez AB, Vogt K, Garcia G, Schmolke K, Aguirre E, Alvarez M, Kern F, Kourí G, Volk H, Guzman MG (2010) Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell Immunol 262:134–140
Singla N, Gupta D, Joshi A, Batra N, Singh J (2012) Genetic polymorphisms in the P2X7 gene and its association with susceptibility to tuberculosis. Int J Tuberc Lung Dis 16:224–229
Souza VL, Veras PS, Welby-Borges M, Silva TM, Leite BR, Ferraro RB, Meyer-Fernandes JR, Barral A, Costa JM, de Freitas LA (2011) Immune and inflammatory responses to Leishmania amazonensis isolated from different clinical forms of human leishmaniasis in CBA mice. Mem Inst Oswaldo Cruz 106:23–31
Stockdale L, Newton R (2013) A review of preventative methods against human leishmaniasis infection. PLoS Negl Trop Dis 7:e2278
Swartz TH, Dubyak GR, Chen BK (2015) Purinergic receptors: key mediators of HIV-1 infection and inflammation. Front Immunol 6
Talvani A, Teixeira MM (2011) Inflammation and Chagas disease some mechanisms and relevance. Adv Parasitol 76:171–194
Tam CC, Khan MS, Legido-Quigley H (2016) Where economics and epidemics collide: migrant workers and emerging infections. Lancet 388:1374–1376
Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, Kasinathan RS, Gachet C, Sluyter R, Barden JA, Wiley JS, Lang F, Huber SM (2006) Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J 20:133–135
Tsai C-Y, Liong KH, Gunalan MG, Li N, Lim DSL, Fisher DA, MacAry PA, Leo YS, Wong S, Puan KJ, Wong SBJ (2015) Type I IFNs and IL-18 regulate the antiviral response of primary human γδ T cells against dendritic cells infected with dengue virus. J Immunol 194:3890–3900
Wang H, Li J, Pu H, Hasan B, Ma J, Jones MK, Zheng K, Zhang X, Ma H, McManus DP, Lin R, Wen H (2014) Echinococcus granulosus infection reduces airway inflammation of mice likely through enhancing IL-10 and down-regulation of IL-5 and IL-17A. Parasit Vectors 7:522
Whitehorn J, Van VCN, Simmons CP (2014) Dengue human infection models supporting drug development. J Infect Dis 209(Suppl):S66–S70
World Health Organization (2012) Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis. World Health Organ Tech Rep Ser: v–xii, 1-100. Available: https://www.ncbi.nlm.nih.gov/pubmed/23484340
Yim J-J, Selvaraj P (2010) Genetic susceptibility in tuberculosis. Respirology 15:241–256
Author information
Authors and Affiliations
Contributions
PAFP – wrote P2R section and P2Rs and diseases, LPD – wrote P2R section and P2Rs and diseases, LGBF – wrote P2R section and P2Rs and diseases, RXF – planned and reviewed P2R section and P2Rs and diseases. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pacheco, P.A.F., Dantas, L.P., Ferreira, L.G.B. et al. Purinergic receptors and neglected tropical diseases: why ignore purinergic signaling in the search for new molecular targets?. J Bioenerg Biomembr 50, 307–313 (2018). https://doi.org/10.1007/s10863-018-9761-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-018-9761-0