Abstract
P2X7 is a member of the purinergic receptors family, with extracellular adenosine triphosphate (ATP) as the main agonist, promoting cations influx and membrane permeabilization that can lead to cell death. We previously proposed that extracellular ATP is involved in thymus atrophy induced by Trypanosoma cruzi infection through the induction of CD4+/CD8+ double-positive cell death and that P2X7 could be involved in this process. To further elucidate this possibility raised by in vitro assays, in this study, we used \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice and observed no difference in thymus atrophy or parasitemia when compared to C57Bl/6. We then decided to investigate other aspects of purinergic receptor interplay that could be better evidenced by the infection and observed that (1) thymocytes from infected and noninfected C57Bl/6 mice express P2X4 and P2X7 receptors (Western blotting), but ATP-induced membrane permeabilization only occurs in thymocytes from infected mice; (2) peritoneal macrophages from noninfected C57Bl/6 mice (\({\text{P}}2{\text{X}}_4^ + \) and \({\text{P}}2{\text{X}}_7^ + \)) are permeabilized by ATP. Although macrophages from infected C57Bl/6 mice are \({\text{P}}2{\text{X}}_7^ - \) but \({\text{P}}2{\text{X}}_4^ + \), they are resistant to ATP, either through permeabilization or Ca++ influx (fluorimetry); (3) using noninfected \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice, C57Bl/6 infected mice, and different agonistic stimuli, we observed interesting cross-talks among P2X and P2Y receptors (flow cytometry).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chagas’ disease is caused by the protozoan parasite Trypanosoma cruzi and has a widespread distribution in South America. Transmission to humans occurs primarily through blood-sucking reduvid bugs, but it may also occur, for example, through blood transfusion, organs transplant, and transplacentary infection (Moncayo 2003; WHO 2004). The disease is characterized by an initial acute phase, and it is generally accepted that patients with more severe acute infection may develop a more aggressive chronic phase (Coura 2007; Higuchi et al. 2003). A number of alterations are imposed by T. cruzi infection, and many relating to the immune system have been reported, such as polyclonal activation of B and T cells (Minoprio et al. 1989), splenomegaly, and Fas-dependent activation induced T cell death (AICD) of CD4+ T cells (Lopes et al. 1999). Moreover, phagocytosis of apoptotic cells by macrophages leads to declined proinflammatory cytokine expression and blockage of NO production by these cells, thus favoring the infection (Freire-de-Lima et al. 2000). Structural and functional alterations of the thymus are also observed in the acute phase of the pathology, with a severe atrophy of the organ characterized by CD4+/CD8+ double-positive (DP) cell loss (Leite-de-Moraes et al. 1992). Additionally, the thymic microenvironment is greatly altered, as shown by the increased production of extracellular matrix components (Cotta-de-Almeida et al. 2003), decreased epithelial thymic nurse cell (TNC) viability, and decreased number of thymocytes per TNC (Cotta-de-Almeida et al. 1997).
In the case of thymus atrophy, a potential role for trans-sialidase, a virulence factor shed by T. cruzi, has been reported. This enzyme induces apoptosis in TNC complexes, where immature DP cells undergo selection (Mucci et al. 2002). However, cellular death triggered by this pathway is observed only in males, suggesting the requirement of androgen hormones in this process (Mucci et al. 2005). Our group has published that the P2X7 receptor may also be involved in thymus atrophy induced by the acute phase of the infection. This receptor is a member of the purinergic receptor family, and its activation renders cell membranes permeable to molecules up to 900 Da in macrophages and 400 Da in lymphocytes, leading to cell death. Our previous results were obtained by in vitro assays and showed a positive correlation between thymus involution and extracellular adenosine triphosphate (ATPe)-induced pore opening with uptake of large solutes in thymocytes. DP cells from noninfected mice were refractory to ATPe-induced permeabilization or death, while DP thymocytes from T. cruzi-infected mice were susceptible to both. In addition, thymuses restored age-matched numbers of cells and structural architecture during the chronic phase, recovering low responsiveness to ATPe in vitro (Mantuano-Barradas et al. 2003).
ATPe plays a variety of roles by interacting with P2 receptors, such as: the G protein-coupled P2Y receptors and the ligand-gated cation channel P2X receptors (Ralevic and Burnstock 1998). Besides ATPe, other agonists, such as adenosine diphosphate (ADP), uridine diphosphate (UDP), and uridine triphosphate (UTP) can activate some of the P2 receptors.
A complex network of intermolecular interactions within the family of purinergic receptors, called a “combinatorial receptor web,” has been suggested (Volonte et al. 2006). This set of interactions is possible due to: (1) similar molecular structures, allowing a certain agonist to interact with one or more receptors, altering their final responses; (2) the fact that P2X and P2Y subtypes can form homomers and heteromers, increasing the diversity in agonist and antagonist selectivity (Guo et al. 2007; Surprenant et al. 2000); and (3) cross-talk mechanisms among purinergic receptors and other families of receptors. Platelets, for example, share a reciprocal cross-talk between P2Y12 and P2Y1 (Hardy et al. 2004), and retinal pericyte cells couple P2Y and P2X7 activity (Sugiyama et al. 2005). P2 receptors can also interact with Cys-loop channels for acetylcholine, γ-amino butyric acid, glycine, and serotonin, glutamate-gated channels (kainite, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and N-methyl-d-aspartic acid), and opioid receptors (Volonte et al. 2006).
Other effects of P2X7 were proposed including the release of interleukin 1β from macrophages (Ferrari et al. 2006), CD62-L shedding (Gu et al. 1998), and maturation of T cells (Tsukimoto et al. 2006). Furthermore, innumerous loss-of-function polymorphisms (Adriouch et al. 2002; Shemon et al. 2006) and one that results in gain-of-function have been identified (Cabrini et al. 2005).
The activation of P2X4 receptor has also been reported to induce membrane permeability to cationic molecules (Virginio et al. 1999). However, in contrast to P2X7, few studies address its physiological properties and functional roles. It was only recently shown that P2X7 and P2X4 can be structurally and functionally associated on the cell surface, and this notion may unveil new approaches to target pharmacological studies (Guo et al. 2007; Dubyak 2007).
In the present study, we used P2X7-defficient \(\left( {{\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} } \right)\) mice to evaluate the role played by this receptor in in vivo thymic atrophy induced by T. cruzi infection. Although these experiments indicate that the receptor is not responsible for thymic involution, using \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice, we observed important interactions between P2X and P2Y receptors. These data further illustrate the complex modulatory web set up by purinergic receptors and that additional interactions can be imposed by pathological situations.
Materials and methods
Animals
Seven-week-old male C57Bl/6 \(\left( {{\text{P}}2{\text{X}}_7^{{ + \mathord{\left/ {\vphantom { + + }} \right. \kern-\nulldelimiterspace} + }} } \right)\) mice were purchased from the Breeding Laboratory Animal Center at Fundação Oswaldo Cruz, and \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice (C57Bl/6 background), derived from Pfizer (Groton, CT, USA) and generated by Solle et al. (2001), were kindly supplied by Dr. A Gabel and bred at the Transgenic Mice Laboratory at the Biophysics Institute Carlos Chagas Filho at the Federal University do Rio de Janeiro. Mice were housed for 7–10 days in the Laboratory of Cellular Biology, Division of Animal Experimentation, under environmental factors and sanitation conforming to the guide for the Care and Use of Laboratory Animals (DHEW publication no. [NIH] 80-23). This project was approved by the Fiocruz Committee of Ethics in Research (0308-06), according to resolution 196/96 of the National Health Council of Brazilian Ministry of Health. Experiments were carried out using C57Bl/6 mice, except when indicated as \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \).
Parasites and infection
Parasites were obtained from infected Swiss–Webster mice and isolated as previously described (Araújo-Jorge et al. 1989). Mice were intraperitoneally injected with 1 × 104 blood trypomastigote forms of T. cruzi Y strain in 200 μl of phosphate-buffered saline (PBS). Age-matched noninfected (control) mice received 200 μl of PBS and were treated under the same conditions. Individual parasitemia was scored in 5 μl of blood collected from tail snips.
Cell isolation
Thymocytes were isolated by mechanical dissociation, and peritoneal cells were harvested by injection of ice-cold medium RPMI 1640 (Sigma Chemical, St. Louis, MO, USA). Cells were washed, homogenized in RPMI/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10 mM (Sigma) pH 7.4, and maintained in ice until use. Samples were collected on days 11 or 14 post infection (dpi), as described in the text.
Permeabilization assay
Peritoneal cells (a population with high susceptibility to ATPe used as a positive control) and thymocytes were prewarmed for 5 min in RPMI 1640/HEPES 10 mM at 37°C and then incubated for 10 min in the presence or absence of 10 μM, 100 μM, 1 mM, or 5 mM of ATPe, adenosine monophosphate (AMP), adenosine, UTP, or ATP plus UTP (all purchased from Sigma). In some cases, cells were preincubated for 5 min in 100 μM ZnCL2, 50 μM CuCl2, 5 mM UTP, or 35 μM before Brilliant Blue G Coomassie (BBG) before exposure to ATPe. To evaluate agonist-induced cell permeabilization, one of the following membrane-impermeant deoxyribonucleic acid-staining fluorescent dyes was added during the last 5 min of incubation, as mentioned in the text: propidium iodide (Sigma) 2.5 μM or TO-PRO-3 (Molecular Probes, Eugene, OR, USA) 1 μM. Samples were analyzed using a FACScalibur flow cytometer (Becton & Dickinson, San Jose, CA, USA).
Permeabilization of each subpopulation of thymocytes was achieved by labeling the cells for 30 min in ice with phycoerythrin (PE)-conjugated anti-CD4 and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 mAb (SouthernBiotech, Birminghan, AL, USA) before exposure to the agonist. Using these fluorochromes, permeabilization assays were carried out using TO-PRO-3. In most experiments, we used total peritoneal cells, except in permeabilization assays where macrophages were gated by a combination of forward scatter × side scatter and MAC-1+ labeling. Flow cytometry data were analyzed using CellQuest software version 3.2 (Becton & Dickinson).
Cell sorting
DP thymocytes were incubated with bead-conjugated anti-CD8 (Miltenyi Biotec, Auburn, CA, USA) at 4°C for 15 min, centrifuged (25 × g/10 min), homogenized in 500 μl of PBS, and applied to a MiniMACS column (Miltenyi Biotec) in a magnetic field. Sort purity of CD8+ cells was determined by labeling the cells with anti-CD8 FITC and anti-CD4 PE mAb for flow cytometry analysis. We used samples of at least 95% enrichment of DP cells.
Intracellular calcium measurements
Freshly collected intraperitoneal cells or enriched DP thymocytes were loaded with Fura 2-AM (Molecular Probes) 5 μM for 45 min at room temperature in RPMI-1640/HEPES 20 mM/probenecid 2.5 mM (Sigma). Cells were then left to decant for 10 min at room temperature on glass coverslips coated with poly-l-lysin 0.001% and placed in a three-compartment superfusion chamber. The central chamber containing cells was continuously perfused at a rate of 1 ml/min with RPMI-1640/HEPES 10 mM at 37°C until stabilization of background signal. Intracellular calcium was monitored using a fluorescence photometer (Photon Technology, Princeton, NJ, USA) throughout perfusion, initially with medium only and then with medium containing ATPe 5 mM. All traces recorded were representative of 30–60 cells preincubated or not with BBG. Saponin 0.01% was used to control Fura-2 loading.
Western blotting
Intraperitoneal cells or thymocytes were lysed with extraction buffer (Tris–HCl 50 mM, NP-40 1%, leupeptin 1 mM, phenylmethylsulfonyl fluoride (PMSF) 100 mM, pepstatin A 1 mM, ethylenediamine tetraacetic acid (EDTA) 100 mM); (Sigma), and total proteins (50 μg) were resolved using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) 12%. Proteins on nitrocellulose membranes were incubated with anti-P2X7 Ab (Alomone, Jerusalem, Israel) for 2 h, rinsed in blocking buffer, and incubated with alkaline phosphatase-conjugated anti-rabbit IgG (SouthernBiotech) for 1 h. The detection was performed using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium solution (BCIP/NBT) (SouthernBiotech). Thymocytes from \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice were used as negative controls.
Transmission electron microscopy
DP-enriched thymocytes were collected from noninfected mice, washed in cold PBS, and fixed using paraformoldehyde 4% and glutaraldehyde 0.1% in 0.1 M of Na-cacodylate buffer (pH 7.2) plus 0.01% of saponine at 4°C for 30 min. Cells were washed in washing buffer (PBS containing bovine serum albumin 4% and saponin 0.05%) at 20°C and incubated with anti-P2X7 Ab diluted in washing buffer for 1 h. After washing, samples were incubated with gold-conjugated goat anti-rabbit mAb (Sigma), washed in washing buffer, followed by saponine-free washing buffer and finally Na-cacodylate buffer 0.1 M. Samples were fixed with glutaraldehyde 2.5% in Na-cacodylate buffer 0.1 M (pH 7.2) at room temperature for 40 min and postfixed with a solution of OsO4 1%. Cells were dehydrated in an ascending acetone series and embedded in PolyBed 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Zeiss EM10C (Zeiss, Germany) transmission electron microscope.
Statistical analysis
Using the software SPSS version 8.0, Student’s t test was used to compare two sets of data. p values are indicated in figure legends.
Results
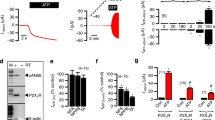
Given that macrophages are important host cells for T. cruzi life cycle and express high levels of P2X7, we evaluated blood parasitemia as a basic parasitological parameter in \({\text{P}}2{\text{X}}_7^{{ + \mathord{\left/ {\vphantom { + + }} \right. \kern-\nulldelimiterspace} + }} \) and \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice. Using a T. cruzi strain that induces an early acute peak, we observed similar results in both groups of mice (Fig. 1a). Thymus atrophy was also similar in infected \({\text{P}}2{\text{X}}_7^{{ + \mathord{\left/ {\vphantom { + + }} \right. \kern-\nulldelimiterspace} + }} \) or \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice (Fig. 1b). In addition, we observed that noninfected \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice maintained normal distribution of thymocyte subpopulations, when compared to \({\text{P}}2{\text{X}}_7^{{ + \mathord{\left/ {\vphantom { + + }} \right. \kern-\nulldelimiterspace} + }} \) (Fig. 1c,d). Importantly, the DP subpopulation was equally affected in both groups of infected mice (Fig. 1e,f). Therefore, contrary to what we might expect based on our previous in vitro experiments (Mantuano-Barradas et al. 2003), these in vivo results indicate that P2X7 is not a central molecule in thymus atrophy induced by T. cruzi infection. Therefore, we decided to investigate whether other aspects of P2 receptors were altered by the infection.
Thymus atrophy and P2X7. Blood parasitemia (a) and total thymocytes (b) were counted at indicated time points. Thymocyte subpopulations were labeled with anti-CD4 and anti-CD8, and quadrant numbers indicate individual percentages from: noninfected C57Bl/6 (c) or \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) (d); infected (dpi 14) C57Bl/6 (e) or \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) (f) mice. Cellular extracts of: thymocytes from noninfected (g—lane 1) and infected (2), peritoneal cells from noninfected (3) and infected (4), all from C57Bl/6 mice and thymocytes from noninfected \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice (5, negative control) were used in Western blotting. Arrowhead indicates 68 kDa. Results are representative of five independent experiments with ten mice per experiment. Ultrastructural immunocytochemical labeling indicated P2X7 labeling (arrows in h). Bar = 10 μm. Asterisk means p≤0.05 when compared to noninfected mice
Western blotting analyses were performed on dpi 11, and we found P2X7 labeling in total thymocytes from noninfected (Fig. 1g arrowhead—lane 1) and infected mice (Fig. 1g—lane 2), as well as in peritoneal cells from noninfected mice (Fig. 1g—lane 3). However, peritoneal cells from infected mice did not express the receptor (Fig. 1g—lane 4) as well as negative control (Fig. 1g—lane 5). The same results were obtained using DP-enriched thymocytes (data not shown).
Since thymocytes from noninfected mice expressed P2X7 but were resistant to ATPe-induced permeabilization, we used electron microscopy to determine the receptor localization, as it can be sequestered within cytoplasmic granules. We observed P2X7 labeling in chromatin, nuclear membrane, cytoplasmic vesicles, and plasma membrane (Fig. 1h), suggesting that the resistance to permeabilization is not due to the lack of P2X7 expression on the cell surface.
To further investigate the modulation of P2 receptors during the infection, we investigated ATPe-triggered Ca++ influx. In peritoneal cells from noninfected mice, ATPe induced a biphasic Ca++ response, consisting of a rapid transient signal, peaking within 20–30 s and followed by a plateau, compatible with a permeabilization response (Fig. 2a). Importantly, the second component of the signal (plateau) was blocked by BBG, an antagonist of P2X7 (Eschke et al. 2002; Fig. 2b). When peritoneal cells from infected mice (dpi 11) were treated with ATPe (Fig. 2c), neither component of the response was observed. This result is in agreement with our data showing that the infection downregulates the expression of the P2X7 receptor in macrophages and suggests that besides P2X7, other P2 receptors are absent or not functional in these cells.
Calcium influx of peritoneal cells. Calcium influx of Fura-2-loaded C57Bl/6 peritoneal cells was evaluated in: noninfected mice/untreated cells (a) or BBG-treated (b) and infected mice/untreated cells (c). ATPe 5 mM was added (first arrow), and saponin was used as a positive control (second arrow). Results are representative of four independent experiments with eight mice per experiment (30 to 60 cells were recorded per microscopic field per mouse)
DP thymocytes from noninfected mice showed only the rapid component of the signal and then declined to basal levels after 100 s (Fig. 3a). Pretreatment with BBG did not block this signal (Fig. 3b), further suggesting that although expressed on the membrane, P2X7 receptor is functionally downregulated in these cells. On the other hand, DP thymocytes from infected mice showed a continuous response induced by the agonist, reaching a plateau after 100 s (Fig. 3c). After pretreatment with BBG, we observed only the rapid peak within 30–40 s, declining to basal levels in 80–90 s (Fig. 3d). In conclusion, we observed that the infection induces a gain of function of P2X7 in thymocytes but a downregulation of P2 receptors in peritoneal cells.
Calcium influx of thymocytes. Calcium influx of Fura-2-loaded C57Bl/6 thymocytes was evaluated in: noninfected mice/untreated cells (a) or BBG-treated (b), infected mice/untreated cells (c) or BBG-treated (d). ATPe 5 mM was added (first arrow), and saponin was used as a positive control (second arrow). Results are representative of four independent experiments with eight mice per experiment (30 to 60 cells were recorded per microscopic field per mouse)
As there was no response induced by ATPe in noninfected thymocytes and P2X receptors may share extracellular domains and agonists due to heteromerization, we evaluated whether other agonists could trigger cellular permeabilization. However, we still observed no cellular response using adenosine, AMP, or UTP (data not shown).
Since ATPe-induced permeabilization can also be mediated by P2X2 and P2X4 (Virginio et al. 1999), we evaluated whether P2X7 modulations could affect other purinegic receptors (cross-talks). We observed that the preincubation with ZnCl2, a condition that favors P2X4 activity (Coddou et al. 2003), rendered peritoneal cells from noninfected C57Bl/6 mice more susceptible to permeabilization with lower concentrations of ATPe (100 μM), when compared to ATPe only (1 mM; Fig. 4a). In addition, incubation with CuCl2, a condition that prevents P2X4 activity (Coddou et al. 2003), induced significant permeabilization only with 5 mM of ATPe, and there was no response in the presence of BBG. All incubations using \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) cells induced no response, even in the presence of ZnCl2, where we expected to observe membrane permeabilization through P2X4 (Fig. 4a). Using peritoneal cells from infected (Fig. 4b) and DP thymocytes from noninfected mice (Fig. 4c), we observed no response under any condition tested. Thymocytes from infected mice were permeabilized in the presence of ATPe or ZnCl2/ATPe (Fig. 4d), and once more, there was no permeabilization of DP thymocytes from infected \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice preincubated with ZnCl2, suggesting P2X4 functional downregulation also in this cell type.
P2X7 and P2X4 interaction. Peritoneal macrophages from noninfected (a) or infected mice (b) or thymocytes from noninfected (c) or infected mice (d) were collected from C57Bl/6 and \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice (as indicated) and preincubated or not with ZnCl2, CuCl2, or BBG. ATP was then added in indicated concentrations (μM), and propidium iodide incorporation was measured by flow cytometry. Results are representative of six independent experiments with ten mice per experiment. @ means p < 0.05 when comparing Zn/ATP 5 mM with Zn/ATP 10 μM; # when comparing Zn/ATP 100 μM and 1 mM with other parameters at the same concentration; * when comparing Cu/ATP 5 mM with ATP 5 mM;  when comparing Zn/ATP 5 mM with Cu/ATP 5 mM
when comparing Zn/ATP 5 mM with Cu/ATP 5 mM
Since previous data from literature (Sugiyama et al. 2005) and from our laboratory indicated a possible cross-interaction between P2X7 and P2Y, we evaluated if pre- and/or coincubation of UTP with ATPe could modulate the permeabilization response. We observed that pre-exposure to UTP blocked ATPe-induced pore opening only in peritoneal cells from noninfected mice (Figs. 5a,b). Regarding DP thymocytes, this effect was observed only in cells from infected mice (Figs. 5c,d). Taken together, these data indicate a highly complex group of functional interactions not only involving P2X receptors but also P2X and P2Y receptors.
P2X7 and P2Y interaction. Peritoneal macrophages from noninfected (a) or infected mice (b) or thymocytes from noninfected (c) or infected mice (d) were collected from C57Bl/6 and \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice (as indicated) and were incubated, preincubated (UTP ⇒ ATP), or coincubated with UTP (UTP/ATP) (5 mM). ATPe was then added in indicated concentrations (μM), and propidium iodide incorporation was measured by flow cytometry. Results are representative of six independent experiments with ten mice per experiment. @ means p < 0.05 when comparing ATP 5 mM with UTP ⇒ ATP 5 mM; # when comparing ATP 5 mM with UTP/ATP 5 mM; * when comparing UTP/ATP 5 mM with UTP ⇒ ATP 5 mM
Discussion
Our previous in vitro data indicated a role for ATPe-induced cell death in thymus atrophy induced by T. cruzi infection, raising the possibility that the P2X7 receptor is involved in this phenomenon (Mantuano-Barradas et al. 2003). However, in vivo assays using \({\text{P}}2{\text{X}}_7^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice indicated that the receptor is not a central molecule in the process. On the other hand, knockout mice may not be an appropriate tool to evaluate this possibility, since alternative pathways may take place and substitute the deleted molecule or interdependent intracellular pathways of signal transduction could be masking the relevance of P2X7 in the atrophy (cross-talk). Therefore, we suggest that this interdependence among P2 receptors may make purinergic knockout mice a two-edged sword, and we should be aware of this possibility. Accordingly, in this work we observed important interactions and modulations concerning not only P2X7 function and expression but also P2X4 and P2Y. These data illustrate the complexity of roles played by members of P2 purinergic receptors family and the wide range of possible immune modulatory interplays.
Our previous data using C57Bl/6 mice showed that DP thymocytes from noninfected mice are resistant to ATPe-induced permeabilization and cell death (Mantuano-Barradas et al. 2003). In the present work, we extended these observations by demonstrating that although the P2X7 receptor is expressed on the plasma membrane of these cells, ATPe did not induce Ca++ influx compatible to membrane permeabilization. One possibility could be that the receptor and the component(s) of the pore itself are different entities (Coutinho-Silva and Persechini 1997) and, although the receptor is present in DP cells, the molecules that compose the pore are missing. It has recently been proposed that pannexin hemichannels participate in the pore structure (Pelegrin and Surprenant 2006), and it could be possible that this molecule was not being expressed or even not coupled to the signaling cascade of permeabilization or death before infection. It would be interesting to examine the expression of this molecule in thymocytes.
During the phase of thymus atrophy induced by T. cruzi infection, DP thymocytes become responsive to ATPe-induced permeabilization and Ca++ signaling through P2X7 activity. This could be due to cellular phenotypic modulation and induction of pannexin expression, for example, completing the panel of required molecules to induce P2X7 response. It could also be possible that the infection, through cytokines for example, induced the coupling of signaling machinery to pre-existing surface molecules.
A different type of P2 receptors modulation was observed in peritoneal cells. We observed that these cells from infected mice downregulate the expression of P2X7, making them no longer susceptible to ATPe. Similarly, it has been shown that J774 cells infected with Chlamydia also reduce ATPe-induced cell membrane permeabilization, but sustain Ca++ influx induced by ATPe (Coutinho-Silva et al. 2001).
Although it has been reported that interferon-γ and tumor necrosis factor-α upregulate the expression of P2X7 in monocytes (Humphreys and Dubyak 1998) and these cytokines are abundant in the acute phase of the infection, other stimuli certainly took place, abolishing the expression of this receptor in peritoneal cells. Since there was no detectable Ca++ signal triggered by ATPe in these peritoneal cells from infected mice, other P2 receptors are also probably not functional or expressed at very low levels. At least in the case of P2X4, Western blotting assays indicated that these cells express the receptor (data not shown). However, in this case, due to the absence of P2X7, the P2X4, for example, can mostly be sequestered in intracellular vesicles, not exposed to agonistic stimulation (Guo et al. 2007).
Our present data indicate normal expression and function of P2X4 and P2X7 in peritoneal cells from noninfected mice. However, our findings with cells from noninfected P2X7−/− mice evidenced an interesting cross-talk between both receptors, with no permeabilization even in the presence of ZnCl2, which indicates no P2X4 response. In addition, the preincubation of macrophages from noninfected C57Bl/6 mice with BBG (a blocker of P2X7 activity) showed no P2X7 or P2X4 activity after incubation with ATPe. These results were reproduced using thymocytes from infected mice, indicating a phenomenon conserved in cells from myeloid and lymphoid lineages. These data are in agreement with the literature that shows both receptors forming heteromeric structures that ultimately affect their final function (Dubyak 2007; Guo et al. 2007).
Although we had no indication of the possible relevance of P2Y in thymus atrophy, our present data concerning P2X cross-talks, previous results from our laboratory, and related literature (Sugiyama et al. 2005) motivated the investigation of P2Y/P2X7 interaction. To this regard, we observed that preincubation with 5 mM of UTP blocked ATPe-dependent permeabilization both in peritoneal cells from noninfected mice and DP thymocytes from infected mice. Similar results have been obtained with retinal pericyte cells, where the incubation of isolated cells with 30 μM of UTP for 30 min before exposure to 100 μM of benzoylbenzoyl-ATP blocks P2X7-dependent permeabilization (Sugiyama et al. 2005)
We thus consider that additional experiments based, for example, on electrophysiology and pharmacological approaches are necessary to re-evaluate P2X7 receptor relevance in thymus atrophy induced by T. cruzi infection in vivo. Approaches other than those using knockout mice should be adopted, since compensatory mechanisms may take control and mask primary physiological roles of individual purinergic receptors during the atrophy. However, these mice, together with the infection, revealed interesting interactions that can help us to understand the role of P2 receptors in immunomodulations of protozoan infections.
References
Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F (2002) Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol 169:4108–4112
Araujo-Jorge TC, Sampaio EP, De Souza W, Meirelles N (1989) Trypanosoma cruzi: the effect of variations in experimental conditions on the levels of macrophage infection in vitro. Parasitol Res 75:257–263
Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, Cuneo A, Castoldi G, Baricordi OR, Di Virgilio F (2005) A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol 175:82–89
Coddou C, Morales B, Huidobro-Toro JP (2003) Neuromodulator role of zinc and copper during prolonged ATP applications to P2X4 purinoceptors. Eur J Pharmacol 472:49–56
Cotta-de-Almeida V, Bertho AL, Villa-Verde DM, Savino W (1997) Phenotypic and functional alterations of thymic nurse cells following acute Trypanosoma cruzi infection. Clin Immunol Immunopathol 82:125–132
Cotta-de-Almeida V, Bonomo A, Mendes-da-Cruz DA, Riederer I, De Meis J, Lima-Quaresma KR, Vieira-de-Abreu A, Villa-Verde DM, Savino W (2003) Trypanosoma cruzi infection modulates intrathymic contents of extracellular matrix ligands and receptors and alters thymocyte migration. Eur J Immunol 33:2439–2448
Coura JR (2007) Chagas disease: what is known and what is needed-a background article. Mem Inst Oswaldo Cruz 102(Suppl 1):113–122
Coutinho-Silva R, Persechini PM (1997) P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol 273:C1793–C1800
Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM (2001) Modulation of P2Z/P2X(7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol 280:C81–C89
Dubyak GR (2007) Go it alone no more-P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol 72:1402–1405
Eschke D, Wust M, Hauschildt S, Nieber K (2002) Pharmacological characterization of the P2X(7) receptor on human macrophages using the patch-clamp technique. Naunyn-Schmiedeberg’s Arch Pharmacol 365:168–171
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:3877–3883
Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF (2000) Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403:199–203
Gu B, Bendall LJ, Wiley JS (1998) Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood 92:946–951
Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD (2007) Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72:1447–1456
Hardy AR, Jones ML, Mundell SJ, Poole AW (2004) Reciprocal cross-talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood 104:1745–1752
Higuchi ML, Benvenuti LA, Martins Reis M, Metzger M (2003) Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovasc Res 60(1):96–107
Humphreys BD, Dubyak GR (1998) Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 64:265–273
Leite-de-Moraes MC, Hontebeyrie-Joskowicz M, Dardenne M, Savino W (1992) Modulation of thymocyte subsets during acute and chronic phases of experimental Trypanosoma cruzi infection. Immunology 77:95–98
Lopes MF, Nunes MP, Henriques-Pons A, Giese N, Morse HC, Davidson WF, Araujo-Jorge TC, DosReis GA (1999) Increased susceptibility of Fas ligand-deficient gld mice to Trypanosoma cruzi infection due to a Th2-biased host immune response. Eur J Immunol 29:81–89
Mantuano-Barradas M, Henriques-Pons A, Araujo-Jorge TC, Di Virgilio F, Coutinho-Silva R, Persechini PM (2003) Extracellular ATP induces cell death in CD4+/CD8+ double-positive thymocytes in mice infected with Trypanosoma cruzi. Microbes Infect 5:1363–1371
Minoprio P, Itohara S, Heusser C, Tonegawa S, Coutinho A (1989) Immunobiology of murine Trypanosoma cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol Rev 112:183–207
Moncayo A (2003) Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz 98(5):577–591
Mucci J, Hidalgo A, Mocetti E, Argibay PF, Leguizamon MS, Campetella O (2002) Thymocyte depletion in Trypanosoma cruzi infection is mediated by trans-sialidase-induced apoptosis on nurse cells complex. Proc Natl Acad Sci USA 99:3896–3901
Mucci J, Mocetti E, Leguizamon MS, Campetella O (2005) A sexual dimorphism in intrathymic sialylation survey is revealed by the trans-sialidase from Trypanosoma cruzi. J Immunol 174:4545–4550
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS (2006) A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 281:2079–2086
Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA (2001) Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276:125–132
Sugiyama T, Kawamura H, Yamanishi S, Kobayashi M, Katsumura K, Puro DG (2005) Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am J Physiol 288:C568–576
Surprenant A, Schneider DA, Wilson HL, Galligan JJ, North RA (2000) Functional properties of heteromeric P2X(1/5) receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J Auton Nerv Syst 81:249–263
Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M (2006) P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol 177:2842–2850
Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A (1999) Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2:315–321
Volonte C, Amadio S, D’Ambrosi N, Colpi M, Burnstock G (2006) P2 receptor web: complexity and fine-tuning. Pharmacol Ther 112:264–280
WHO (2004) Online newsletter: TDRNews. Available at: www.who.int/tdr/publications/tdrnews/default.htm. Access: May 2007
Acknowledgments
This work was supported by Fiocruz, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and PRONEX—Fundação de Amparo à Pesquisa do Rio de Janeiro (FAPERJ). We are grateful to Dr. James Mobley (PGRD, Pfizer, Groton, CT, USA) for kindly providing the P2X7R−/− mice. We thank Dr. Luis Anastácio Alves for invaluable assistance and Mr. Marcos Meuser Batista for excellent technical assistance. We declare that the experiments comply with the current laws of the country, as stated in “Materials and methods.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cascabulho, C.M., Menna-Barreto, R.F.S., Coutinho-Silva, R. et al. P2X7 modulatory web in Trypanosoma cruzi infection. Parasitol Res 103, 829–838 (2008). https://doi.org/10.1007/s00436-008-1063-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1063-8