Abstract
In this work, 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramics were prepared through a traditional solid-state process. The effects of Li2O–B2O3–SiO2–CaO–Al2O3 (LBSCA) glass addition on phase formation, microstructure, sintering characteristic and microwave dielectric properties of the ceramics were investigated. A small amount of LBSCA glass addition significantly reduced sintering temperature of the ceramics. X-ray diffraction analysis revealed that Li2ZnTi3O8 and Li2TiO3 phases coexisted without producing any other crystal phases in the sintered ceramics. Dielectric constant and Qf values were related to the amount of LBSCA addition and sintering temperatures. The specimens obtained near-zero temperature coefficient (τf) values through the compensation on the positive τf of Li2TiO3 and the negative τf of Li2ZnTi3O8. The 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramic with 0.75 wt% LBSCA addition and sintered at 900 °C for 3 h exhibited excellent microwave dielectric properties of ɛr = 23.907, Qf = 63050 GHz and τf = 1.2 ppm/°C, which was very suitable for LTCC (low temperature co-fired ceramics) applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of wireless communication technology, an increasing number of studies have focused on materials with brilliant microwave dielectric properties. At the same time, to achieve the miniaturisation and integration of microwave devices, many studies have investigated the LTCC technology, which plays an important role in microelectronic applications [1,2,3]. Ag is widely used as internal-electrode material in LTCC devices to ensure their low cost and high conductivity. Considering that Ag has a melting point of approximately 961 °C, the typical sintering temperature of LTCC materials must be reduced to approximately 900 °C or lower to better co-fire with Ag internal electrodes [4,5,6]. Low-melting-point glasses were usually used as sintering aids in LTCC materials, which reduced the sintering temperature and achieved optimal microwave dielectric properties. Furthermore, near-zero τf of LTCC materials are important to obtain stable LTCC microwave components [7,8,9,10,11,12]. Near-zero τf LTCC materials can be obtained by properly compounding the materials with negative and positive τf.
Li2ZnTi3O8 ceramics had excellent microwave dielectric properties with ɛr values of ~ 25.8, a Qf value of ~ 74,200 GHz and a τf value of − 13 ppm/°C [13]. However, the τf value of Li2ZnTi3O8 ceramics was negative. On the other hand, Li2TiO3 had attracted considerable attention in microwave dielectric ceramics because of its positive τf value and relatively outstanding microwave dielectric properties [14]. In the present work, we combined Li2ZnTi3O8 with Li2TiO3 to obtain materials with near-zero τf values. And LBSCA glass was adopted as sintering aid to lower the sintering temperature of Li2ZnTi3O8–Li2TiO3 compound ceramics because it had very low softening temperature point and efficient help-melting effect [15, 16]. The effects of LBSCA glass addition on the sintering behaviour, microstructure and microwave dielectric properties of the ceramics were investigated and discussed.
2 Experimental procedure
Li2ZnTi3O8–Li2TiO3 ceramic samples were prepared by the conventional solid-state reaction. High-purity Li2CO3 (99%), ZnO (99.5%) and TiO2 (99%) were used as starting materials. Li2ZnTi3O8 calcined powders were produced by ball-milling Li2CO3, ZnO and TiO2 in a 1:1:3 molar ratio. Then the mixed powders were dried and calcined at 900 °C for 3 h. Li2TiO3 calcined powders were produced by ball-milling Li2CO3 and TiO2 in a 1:1 molar ratio. Then the mixed powders were dried and calcined at 820 °C for 3 h. LBSCA glass was prepared by the quenching technology. The oxide raw materials were mixed and melted at 1000 °C for 2 h using an alumina crucible at a Li2O:B2O3:SiO2:CaO:Al2O3 molar ratio of 52.45:31.06:11.99:2.25:2.25 [15, 16]. The solution was removed from the furnace and then poured into cold water to obtain the glass. Then, approximately 0–1.5 wt% of LBSCA glass was added into 0.73Li2ZnTi3O8–0.27Li2TiO3 compounds respectively (0.73:0.27 was the weight ratio of Li2ZnTi3O8 and Li2TiO3 calcined powders, which was calculated by their respective τf to obtain near-zero τf in the compound materials). The mixtures were milled in nylon pots with zirconia balls. After drying and granulation, the powders were pressed into cylinders, which were final sintered at 875–950 °C for 3 h.
Crystalline phase structures were analysed through X-ray diffraction (XRD:DX-2700) using Cu Kα radiation. Bulk densities of the sintered samples were measured using the Archimedes method, and the densities were obtained by the ratio of mass and volume. Sample micrographs were examined by scanning electron microscopy (SEM:JEOL JSM6490LV). The microwave dielectric properties of the sintered ceramics in microwave frequency were measured using the Hakki–Coleman method and Agilent N5230A network analyser (300 MHz–20 GHz) in a resonant cavity. τf values were measured at a temperature range of 20–80 °C. The values were calculated from the following formula:
where \({f}_{T}\) and \({f}_{0}\) are the resonant frequencies at 80 and 20 °C, respectively.
3 Results and discussion
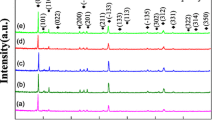
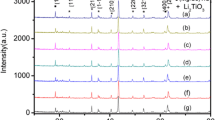
Figure 1 shows the XRD patterns of 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramics with different LBSCA glasses and sintered at 900 °C for 3 h. All samples contained Li2ZnTi3O8 and Li2TiO3 phases, no other phases were detected. All peaks were indexed in terms of Li2ZnTi3O8 (PDF #44-1037) and Li2TiO3 (•, #33–0831). Li2ZnTi3O8 is a cubic structure which belongs to the P4332 space group. Zn2+ and 1/2 of the Li+ are located in the centre of [ZnO4] and [LiO4] tetrahedral units [17]. Ti4+ and the remaining 1/2 Li+ are located in the [TiO6] and [LiO6] octahedron centre. Li2TiO3 is a monoclinic structure which belongs to the C2/c(15) space group [18]. Ti4+ is located in the centre of [TiO6] octahedron, and the octahedrons are connected to each other through six-coordinated Li+. Due to the large crystal structure difference between Li2ZnTi3O8 and Li2TiO3, the Li2TiO3 phase beneficially coexisted with the Li2ZnTi3O8 phase in the 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramics. LBSCA addition was undetectable, which indicated that LBSCA existed in the amorphous phase.
Figure 2 shows the SEM micrographs of 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramics with different LBSCA glasses. LBSCA addition significantly influenced the densification and average grain size of the compound ceramics. Figure 2a presented a porous microstructure with many intergranular pores. Figure 2b, c showed that the intergranular pores decreased, the grain size increased and the samples became dense with the increase of LBSCA glass content. This phenomenon was due to that LBSCA glass formed a liquid phase during sintering, which accelerated mass transfer and promoted sintering [19]. Figure 2d showed that the sample obtained a dense and uniform microstructure, which was doped with 0.75 wt% LBSCA glass. Figure 2e–g showed that the microstructure of the samples did not change significantly with further increasing LBSCA glass content.
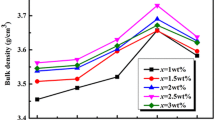
Figure 3 presents the variation in sintered densities of the samples with different LBSCA contents. The sintered densities initially increased and reached their maximum with x = 0.75 wt% and then gradually decreased. The first increase in densities was due to the liquid phase of LBSCA formed during sintering effectively promoted the densification of the materials [20]. The densities monotonously decreased with further increasing LBSCA content, which could be attributed to two reasons. One reason was that excessive grain boundary amorphous LBSCA glass hindered the further densification of the materials. The other reason was that the content ratio of low-density glass addition contributed to the decrease in densities of the samples.
Figure 4 shows the variation in ɛr values of the ceramics with different LBSCA glass contents and sintered under different temperatures. Obviously, the tendency of ɛr variation was consistent with that of density. As LBSCA content increased, ɛr gradually increased and reached the maximum with 0.75 wt% LBSCA. After that, ɛr slightly decreased with further increasing LBSCA content. Similar to the variation of density, this trend remained the same under different sintering temperatures. Therefore, sintered density was the main factor determining the ɛr value of the ceramics.
Figure 5 shows the Qf values of 0.73Li2ZnTi3O–0.27Li2TiO3 ceramics with different LBSCA contents and sintered at 875–950 °C. The Qf values initially increased and then decreased with increasing LBSCA content. The maximal Qf values were obtained with 0.75 wt% LBSCA content, in spite of different sintering temperatures. After that, Qf values gradually decreased with further increasing LBSCA content. This phenomenon was very close to the variation of density. The microwave dielectric losses can be divided into internal and external losses [21]. Internal losses are related to the internal crystal structure of the dielectric material and are mainly caused by the lattice vibration modes, whereas extrinsic losses are associated with many factors, such as second phases, oxygen vacancies, grain size and densification. Therefore, the initial increase in Qf values might be attributed to the increase in densification and average grain size. The subsequent decrease in Qf values was mainly due to the reduced density. Furthermore, the relatively high loss of glassy phase was also responsible for the decrease of Qf values.
Figure 6 shows the τf values of 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramics sintered at 900 °C for 3 h. τf can be tuned by the mixtures of dielectrics with opposite τf values. A previous report showed that Li2ZnTi3O8 has a negative τf value of − 13.75 ppm/°C, whereas Li2TiO3 had a positive τf value of 35.78 ppm/°C. Therefore, the expected near-zero τf values could be achieved by the compensation of the positive τf of Li2TiO3 and the negative τf of Li2ZnTi3O8 in 0.73Li2ZnTi3O8–0.27Li2TiO3. Furthermore, τf altered from − 6 to 7 ppm/°C with increasing LBSCA content in this study. This fact might due to the influence of the glass’s τf and the variation of sintered densities. The specimen with 0.75 wt% LBSCA and sintered at 900 °C could obtain very high Qf value (which was 63,050 GHz) and near-zero τf of 1.2 ppm/°C, which was considered suitable for LTCC applications.
4 Conclusion
In this study, the effects of LBSCA addition on the phase formation, sintering characteristic, microstructure and microwave dielectric properties of the 0.73Li2ZnTi3O8–0.27Li2TiO3 ceramics were investigated. Proper LBSCA glass addition could effectively densify the samples and improve the microwave dielectric properties. No chemical reaction occurred between the Li2ZnTi3O8 and Li2TiO3 ceramics. A near-zero τf value was obtained through the compensation of the positive τf of Li2TiO3 and the negative τf of Li2ZnTi3O8. The 0.73Li2ZnTi3O8–0.27Li2TiO3 sample with 0.75 wt% LBSCA glass addition and sintered at 900 °C presented excellent dielectric properties with ɛr = 23.907, Qf = 63,050 GHz and τf = 1.2 ppm/°C. The proposed ceramic was a perfect candidate material for LTCC applications.
References
P. Kumari, P. Tripathi, O. Parkash, D. Kumar, Low temperature sintering and characterization of MgO-B2O3-SiO2 glass-ceramics for LTCC substrate applications. Trans. Indian Ceram. Soc. 75, 229–233 (2016)
Y. Lai, C. Hong, L. Jin, X. Tang, H. Zhang, X. Huang, J. Li, H. Su, Temperature stability and high-Qf of low temperature firing Mg2SiO4-Li2TiO3 microwave dielectric ceramics. Ceram. Int. 43, 16167–16173 (2017)
C.C. Xia, G.H. Chen, C.L. Yuan, C.R. Zhou, Low-temperature co-fired LiMnPO4–TiO2 ceramics with near-zero temperature coefficient of resonant frequency. J. Mater. Sci.: Mater. Electron. 28, 13970–13975 (2017)
H. Zhou, J. Huang, X. Tan, N. Wang, G. Fan, X. Chen, Compatibility with silver electrode and microwave dielectric properties of low firing CaWO4-2Li2WO4 ceramics. Mater. Res. Bull. 89, 150–153 (2017)
A. Sayyadi-Shahraki, E. Taheri-Nassaj, S.A. Hassanzadeh-Tabrizi, H. Barzegar-Bafrooei, Microwave dielectric properties and chemical compatibility with silver electrode of Li2TiO3 ceramic with Li2O–ZnO–B2O3 glass additive. Physica B 457, 57–61 (2015)
N.-X. Xu, J.-H. Zhou, H. Yang, Q.-L. Zhang, M.-J. Wang, L. Hu, Structural evolution and microwave dielectric properties of MgO–LiF co-doped Li2TiO3 ceramics for LTCC applications. Ceram. Int. 40, 15191–15198 (2014)
J.-X. Xu, X.Y. Zhang, C. High-Isolation, LTCC Diplexer using common stub-loaded resonator with controllable frequencies and bandwidths. IEEE Trans. Microw. Theory Tech. 65, 4636–4644 (2017)
Y. Li, Y. Xie, R. Chen, L. Han, D. Chen, H. Su, A multilayer power inductor fabricated by cofirable ceramic/ferrite materials with LTCC technology. IEEE Trans. Compon. Packag. Manuf. Technol. 7, 1402–1409 (2017)
X.Y. Zhang, X.-F. Liu, Y.C. Li, W.-L. Zhan, Q.Y. Lu, J.-X. Chen, LTCC out-of-phase filtering power divider based on multiple broadside coupled lines. IEEE Trans. Compon. Packag. Manuf. Technol. 7, 777–785 (2017)
W. Feng, X. Gao, W. Che, W. Yang, Q. Xue, LTCC wideband bandpass filters with high performance using coupled lines with open/shorted stubs. IEEE Trans. Compon. Packag. Manuf. Technol. 7, 602–609 (2017)
D. Zhou, L.-X. Pang, D.-W. Wang, C. Li, B.-B. Jin, I.M. Reaney, High permittivity and low loss microwave dielectrics suitable for 5G resonators and low temperature co-fired ceramic architecture. J. Mater. Chem. C 5, 10094–10098 (2017)
D. Zhou, D. Guo, W.-B. Li, L.-X. Pang, X. Yao, D.-W. Wang, I.M. Reaney, Novel temperature stable high-εr microwave dielectrics in the Bi2O3–TiO2–V2O5 system. J. Mater. Chem. C 4, 5357–5362 (2016)
H. Zhou, N. Wang, X. Tan, J. Huang, X. Chen, Glass-free Li2ZnTi3O8 low temperature cofired ceramics by pretreating raw materials. J. Mater. Sci.: Mater. Electron. 27, 11850–11855 (2016)
L.-X. Pang, D. Zhou, Microwave dielectric properties of low-firing Li2MO3 (M = Ti, Zr, Sn) ceramics with B2O3-CuO addition. J. Am. Ceram. Soc. 93, 3614–3617 (2010)
Z. Ding, H. Su, X. Tang, H. Zhang, B. Liu, Low-temperature-sintering characteristic and microwave dielectric properties of (Zn0.7Mg0.3)TiO3 ceramics with LBSCA glass. Ceram. Int. 41, 10133–10136 (2015)
S. Zhang, H. Su, H.W. Zhang, Y.L. Jing, X.L. Tang, Microwave dielectric properties of CaWO4-Li2TiO3 ceramics added with LBSCA glass for LTCC applications. Ceram. Int. 42, 15242–15246 (2016)
M. Bari, E. Taheri-Nassaj, H. Taghipour-Armaki, Phase evolution, microstructure, and microwave dielectric properties of reaction-sintered Li2ZnTi3O8 ceramic obtained using nanosized TiO2 reagent. J. Electron. Mater. 44, 3670–3676 (2015)
Y. Wu, D. Zhou, J. Guo, L.-X. Pang, H. Wang, X. Yao, Temperature stable microwave dielectric ceramic 0.3Li2TiO3–0.7Li(Zn0.5Ti1.5)O4 with ultra-low dielectric loss. Mater. Lett. 65, 2680–2682 (2011)
H. Zuo, X. Tang, H. Zhang, Y. Lai, Y. Jing, H. Su, Low-dielectric-constant LiAlO2 ceramics combined with LBSCA glass for LTCC applications. Ceram. Int. 43, 8951–8955 (2017)
H. Chen, H. Su, H. Zhang, Y. Gui, H. Zuo, L. Yang, X. Tang, Low temperature sintering and microwave dielectric properties of the LBSCA-doped (Zn0.95Co0.05)2SiO4 ceramics. J. Mater. Sci.: Mater. Electron. 26, 2820–2823 (2015)
J. Bi, Y. Niu, H. Wu, Li4Mg3Ti2O9: a novel low-loss microwave dielectric ceramic for LTCC applications. Ceram. Int. 43, 7522–7530 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos. 61471096 and 61771104 and Sichuan science and technology program. Special Projects on Science and Technology of Guizhou Province [2016]3011. And Dongguan entrepreneurial talent program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
lei, T., Chen, J., Xu, Z. et al. Microwave dielectric properties of the low-temperature-fired Li2ZnTi3O8–Li2TiO3 ceramics for LTCC applications. J Mater Sci: Mater Electron 29, 14705–14709 (2018). https://doi.org/10.1007/s10854-018-9607-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9607-x