Abstract
In this paper, the effects of Li2O–B2O3–Bi2O3–SiO2 (LBBS) glass on the phase formation, sintering characteristic, the microstructure and microwave dielectric properties of temperature-stable (Mg0.95Co0.05)2TiO4–Li2TiO3 ceramics were investigated. (Mg0.95Co0.05)2TiO4–Li2TiO3 powders were obtained by using the traditional solid-state process. A small amount of LBBS doping can effectively reduce sintering temperature and promote the densification of the ceramics. X-ray diffraction analysis revealed not only the primary phase (Mg·Co)2TiO4 associated with Li2TiO3 minor phase but also a third phase (Mg·Co)TiO3. The dielectric constant and Qf values vary with the doping amount of LBBS and sintering temperatures. With the compensation of the positive temperature coefficient (τ f ) of Li2TiO3 and the negative τ f of (Mg0.95Co0.05)2TiO4, the τ f of the specimens fluctuates around zero. The (Mg0.95Co0.05)2TiO4 ceramic with 2.5 wt% LBBS addition and sintering at 900 °C for 4 h exhibited excellent microwave dielectric properties: ɛ r = 19.076, Qf = 126100 GHz, and τ f = 0.98 ppm/°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of microwave communication, the demand for microwave ceramic components has intensified. To realize the miniaturization and integration of microwave devices, low temperature co-fired ceramic (LTCC) technology has been studied extensively. Given its high conductivity and low cost, silver (Ag) has been widely used as internal electrodes in LTCC devices [1,2,3,4,5]. The melting point of silver is in the vicinity of 960 °C. Thus, the sintering temperature of LTCC materials must be lower than 960 °C [6,7,8]. In recent years, three main methods have been used to reduce the sintering temperature of these dielectric ceramics; these methods are low melting glass addition, chemical processing, and small particle size of starting materials [9,10,11,12]. A small τ f of LTCC materials is also important to obtain stable LTCC microwave components [13,14,15,16]. Mg2TiO4 ceramics are frequently used dielectric ceramics because of their excellent microwave dielectric properties, namely, ɛ r value of ~14, a high Qf value of ~150,000, and τ f of ~−50 ppm/°C when sintered at 1350 °C. Mg2+ ions can be substituted by Co2+ ions to form (Mg·Co)2TiO4 compositions. (Mg0.95Co0.05)2TiO4 ceramics with a spinel-type structure have excellent dielectric properties with a ɛ r value ∼15.7, a Qf value of ~286,000 GHz and a τ f value of ~−52.5 ppm/°C [17,18,19]. However, the τ f value of (Mg0.95Co0.05)2TiO4 ceramics is significantly negative. Thus, the practicality of this material is limited. Li2TiO3 not only has a relatively low sintering temperature but also has a positive τ f (ɛ r = 12, Qf = 15,000, τ f = 35.05 ppm/°C) [20]. To achieve a near-zero τ f value, the (Mg0.95Co0.05)2TiO4 and Li2TiO3 are compounded at proper contents. However, the sintering temperature of (Mg0.95Co0.05)2TiO4–Li2TiO3 is still higher than 950 °C. Thus, an appropriate sintering aid must be used to reduce the sintering temperature of (Mg0.95Co0.05)2TiO4–Li2TiO3.
In this work, Li2O–B2O3–Bi2O3–SiO2 (LBBS) glass was used as a sintering aid to lower the sintering temperature of (Mg0.95Co0.05)2TiO4–Li2TiO3 compound ceramics. The effects of this glass on the sintering behavior, microstructure, and microwave dielectric properties of the ceramics were systematically investigated.
2 Experimental procedure
High-purity MgO (99.5%), Co2O3 (99.5%), Li2CO3 (99.5%) and TiO2 (99.5%), were used as starting materials. The powders were prepared separately according to the desired stoichiometry Li2TiO3 and (Mg0.95Co0.05)2TiO4 and then milled with ZrO2 balls in distilled water for 12 h. The prepared (Mg0.95Co0.05)2TiO4 powders were dried and calcined at 1150 °C for 4 h in air. The prepared Li2TiO3 powders were dried and calcined at 850 °C for 4 h in air. LBBS glass was prepared using a quenching method. The oxide raw materials were mixed and melted at 1150 °C for 2 h using an alumina crucible at a Li2CO3:B2O3:BiO3:SiO2 molar ratio of 2:2:1:1. The solution was then quickly removed from the furnace and poured with cold water to obtain the glass. Then, 0.5–3.5 wt% LBBS was added to 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 (0.44:0.56 was the weight ratio of the (Mg0.95Co0.05)2TiO4 and Li2TiO3 powders, which was calculated by their respective τ f to obtain near-zero τ f in the compound materials), ground in distilled water for 12 h in a ball mill with ZrO2 balls, dried and mixed with 20 wt% acryloid as binder, and then granulated. The granulated powders were uniaxially pressed into pellets, which were sintered at 850–950 °C for 4 h.
The crystalline phases of the sintered ceramics were analyzed by using X-ray diffraction (XRD: DX-2700) using Cu Kα radiation, and microstructure observations were performed by using scanning electron microscopy (SEM: JOEL JSM6490LV). The relative densities were obtained by the ratios of the bulk and theoretical densities. The microwave dielectric characteristics of the sintered samples were measured using the Hakki–Coleman method with Agilent N5230A network analyzer in a resonant cavity. The temperature coefficient of resonant frequency (τ f ) can be calculated by the following equation: τ f = (f 80 − f 20)/[f 20×(80 − 20)], where f 80 and f 20 represent the resonant frequency at 80 and 20 °C, respectively [21,22,23].
3 Results and discussion
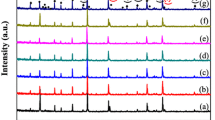
Figure 1 shows the XRD patterns of 0.44(Mg0.95CO0.05)2TiO4–0.56Li2TiO3 ceramics with x wt% LBBS (x = 0.5, 1, 1.5, 2, 2.5, 3, 3.5) and sintered at 900 °C. The XRD patterns shows that all samples have three phases, namely, the primary phase (Mg·Co)2TiO4 associated with Li2TiO3 and the third phase (MgvCo)TiO3. The formation of the second phase is attributed to the decomposition of (Mg0.95Co0.05)2TiO4 into (Mg·Co)2TiO4 and (Mg·Co)TiO3, which was confirmed in a previous report [17]. LBBS addition was not found in the XRD patterns, thereby suggesting that LBBS existed in an amorphous phase [24].
Figure 2 shows the SEM micrographs of 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 ceramics sintered at 900 °C for 3 h in air; these ceramics were added with different amounts of LBBS glass. LBBS have a strong effect on the average grain size and compactness of the compound ceramics. The sample with 0.5 wt% LBBS glass has a porous microstructure with a small average grain size. With the increase in the glass content, the average grain size enlarged obviously, and intergranular pores reduced significantly. This phenomenon is due to the low melting point of LBBS glass, which formed a liquid-phase layer and facilitated the liquid-phase sintering process. When the LBBS content reached 2.5 wt%, the microstructure became denser, and few intergranular pores were found. However, after incorporating large contents of LBBS glass at more than 3.5 wt%, the SEM micrographs showed a molten microstructure without a clear grain.
The effect of LBBS content on the apparent densities is shown in Fig. 3. The relative density of the samples increased slightly with the increase in the LBBS content, peaked with 2.5 wt% LBBS, and then decreased with a further increase in LBBS content. This phenomenon was also consistent with the change in microstructure. With the increase in the amount of LBBS glass, the densification of the samples increased and the number of intergranular pores reduced until the densification peaked with 2.5 wt% LBBS. The densification and the number of intergranular pores decreased with a further increase in LBBS content.
Figure 4 shows the ɛ r values of 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 samples with different doping amounts of LBBS and sintered at various temperatures. As expected, the tendency of ɛ r values is consistent with the tendency of relative density. With the addition of LBBS glass, ɛ r values increased gradually until they peaked with 2.5 wt% LBBS. Then, ɛ r slightly decreased with further LBBS addition. The same tendency of ɛ r values and relative density shows that the permittivity was significantly affected by the relative density.
Figure 5 shows the Qf values of LBBS-doped 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 ceramics that were sintered at a temperature range from 850 to 950 °C. The Qf values of the sample sintered at 900 and 950 °C initially increased and peaked with 2.5 wt% LBBS and then decreased with the increase in the LBBS content. The Qf value changed minimally when the sample was sintered at 850 °C. Microwave dielectric loss may be divided into intrinsic and extrinsic losses. Intrinsic losses are primarily dependent on the crystal structure and are mainly caused by lattice vibration modes. Extrinsic losses are related to many factors, such as second phases, oxygen vacancies, grain sizes, and densification or porosity [3, 13, 19]. Therefore, the initial increase in the Qf value may be due to increased densification and average grain size. The subsequent decrease in the Qf values may due to the increasing number of intergranular pores and the excessively high loss of the liquid glass phase.
Figure 6 shows the τ f values of 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 samples sintered at 900 °C for 4 h in air. τ f can be tuned by the formation of a solid solution or mixtures of dielectrics with opposite τ f values [13]. (Mg0.95Co0.05)2TiO4 has τ f values of −50 ppm/°C, and Li2TiO3 has τ f values of 35.05 ppm/°C. Therefore, through recombination, Li2TiO3 with positive τ f values and (Mg0.95Co0.05)2TiO4 with negative τ f values can obtain a composite with near-zero τ f values.
4 Conclusion
In this work, the effect of LBBS glass doping on sintering temperature, microstructure, and microwave dielectric properties of 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 ceramics were investigated. Combining Li2TiO3 with positive τ f and (Mg0.95Co0.05)2TiO4 with negative τ f could obtain 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 samples with near-zero τ f values. With moderate LBBS addition, high Qf values could be obtained. When the amount of LBBS doping was 2.5% and with sintering at 900 °C, the 0.44(Mg0.95Co0.05)2TiO4–0.56Li2TiO3 ceramics presented excellent microwave dielectric properties: ɛ r = 19.076, Qf = 126,100 GHz and τ f = 0.98 ppm/°C, which is suitable for LTCC applications.
References
M.T. Sebastian, H. Jantunen, Int. Mater. Rev. 53, 57 (2008)
C.S. Hsi, Y.R. Chen, H.I. Hsiang, J. Mater. Sci 46, 4695 (2011)
M. Guo, S. Gong, G. Dou, D. Zhou, J. Alloys Compd. 509, 5988 (2011)
Z.B.O. Frit 954, 2008 (2009)
G. hua Chen, L. jiang Tang, J. Cheng, M. hong Jiang, J. Alloys Compd. 478, 858 (2009)
A. Feteira, D.C. Sinclair, J. Am. Ceram. Soc. 91, 1338 (2008)
G. Yao, P. Liu, H. Zhang, J. Am. Ceram. Soc. 96, 1691 (2013)
H. Chen, H. Su, H. Zhang, T. Zhou, B. Zhang, J. Zhang, X. Tang, Ceram. Int. 1088, 1 (2014)
Z. Zhang, H. Su, X. Tang, H. Zhang, T. Zhou, and Y. Jing, Ceram. Int. 40, 1613 (2014)
C.-L. Huang, J.-Y. Chen, J. Am. Ceram. Soc. 92, 379 (2009)
N.X. Wu, J.J. Bian, Int. J. Appl. Ceram. Technol. 8, 1494 (2011)
J. Tong, B. Zhang, W. Huang, H. Yang, Mater. Lett. 95, 168 (2013)
S. Zhang, H. Su, H. Zhang, Y. Jing, X. Tang, Ceram. Int. 42, 15242 (2016)
C.F. Tseng, P.H. Chen, P.A. Lin, J. Alloys Compd. 632, 810 (2015)
G. Yao, P. Liu, H. Zhang, J. Am. Ceram. Soc. 96, 3119, (2013)
W. Lei, W.Z. Lu, X.C. Wang, Ceram. Int. 38, 99 (2012)
C.-L. Huang, J.-Y. Chen, B.-J. Li, Mater. Chem. Phys. 120, 217 (2010)
C.L. Huang, J.Y. Chen, J. Alloys Compd. 485, 706 (2009)
M. Guo, G. Dou, S. Gong, D. Zhou, J. Eur. Ceram. Soc. 32, 883 (2012)
A. Sayyadi-Shahraki, E. Taheri-Nassaj, S.A. Hassanzadeh-Tabrizi, H. Barzegar-Bafrooei, J. Alloys Compd. 597, 161 (2014)
J.S. Chen, G.H. Chen, X.L. Kang, T. Yang, Z.C. Li, Y. Yang, C.L. Yuan, C.R. Zhou, J. Mater. Sci. Mater. Electron. 28, 317 (2017)
H. Zhou, N. Wang, X. Tan, J. Huang, X. Chen, J. Mater. Sci. Mater. Electron. 27, 11850 (2016)
F. Gu, G. Chen, C. Yuan, C. Zhou, T. Yang, Y. Yang, Mater. Res. Bull. 61, 245 (2015)
R.-L. Jia, H. Su, X.-L. Tang, Y.-L. Jing, Chinese Phys. B 23, 47801 (2014)
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos. 61471096 and 51372031, Special Support Program of Guangdong Province under Grant No. 2014TX01C042 and Science and Technology Department of Sichuan Province 2016GZ0258, 2016JQ0016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Su, H., Lai, Y. et al. Microwave dielectric properties of temperature-stable (Mg0.95Co0.05)2TiO4–Li2TiO3 composite ceramics for LTCC applications. J Mater Sci: Mater Electron 28, 14190–14194 (2017). https://doi.org/10.1007/s10854-017-7275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7275-x