Abstract
In this work, the nominal CaCu3−xMgxTi4.2O12 (0.00, 0.05 and 0.10) ceramics were prepared by sintering pellets of their precursor powders obtained by a polymer pyrolysis solution method at 1100 °C for different sintering time of 8 and 12 h. Very low loss tangent (tanδ) < 0.009–0.014 and giant dielectric constant (ε′) ∼ 1.1 × 104–1.8 × 104 with excellent temperature coefficient (Δε′) less than ± 15% in a temperature range of − 60 to 210 °C were achieved. These excellent performances suggested a potent application of the ceramics for high temperature X8R and X9R capacitors. It was found that tanδ values decreased with increasing Mg2+ dopants due to the increase of grain boundary resistance (Rgb) caused by the very high density of grain, resulting from the substitution of small ionic radius Mg2+ dopants in the structure. In addition, CaCu3−xMgxTi4.2O12 ceramics displayed non-linear characteristics with the significant enhancements of a non-linear coefficient (α) and a breakdown field (Eb) due to Mg2+doping. The high values of ε′ (14012), α (13.64) and Eb (5977.02 V/cm) with very low tanδ value (0.009) were obtained in a CaCu2.90Mg0.10Ti4.2O12 ceramic sintered at 1100 °C for 8 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Presently, investigation for high performance capacitors with efficient operation in a wider temperature range, especially in a high temperature region, has been extensively attempted due to the increasing demand of the materials in automobile, aerospace and military mobile communications industries and etc. [1]. In addition to high dielectric constant (ε′) and low loss tangent (tanδ) values, one important factor to be considered for good capacitor is the temperature stability of \({\varepsilon ^\prime }\left( {\Delta {\varepsilon ^\prime }={\text{1}}00 \times (\varepsilon _{T}^{\prime } - \varepsilon _{{{{30}^ \circ }{\text{C}}}}^{\prime })/\varepsilon _{{{{30}^ \circ }{\text{C}}}}^{\prime }} \right)\) that must be less than ± 15% in a given temperature range, which is practically dissimilar for different types of capacitor. For instance, X9R, X8R and X7R capacitors are allowed to operate in a temperature range of − 55 to 200 °C, − 55 to 150 °C and − 55 to 125 °C, respectively. From literatures, a number of articles concerning the temperature stability study of capacitors have been mostly reported for X8R [2,3,4,5] and X7R [6] capacitors, but rarely for X9R capacitor [1, 7, 8]. Recently, in order to achieve the excellent Δε′ that satisfies the categories for X9R capacitor, attempt have been proposed on various investigations by shifting the Curie temperature (Tc) in BaTiO3-based materials and other ferroelectric materials of perovskite type [7,8,9,10,11], as for examples. However, some of these compounds are complex with Pb as a constituent that can be easily decomposed at a high sintering temperature during the preparation process and cause seriously environmental problems due to the toxicity of Pb.
Over the past decade, perovskite CaCu3Ti4O12 (CCTO) [12,13,14,15,16,17] compounds with giant dielectric constant and other novel giant dielectric materials [18,19,20] have been comprehensively studied, focusing on high performance capacitor as well. In general, CCTO [21] exhibits an ultra-high dielectric permittivity (εʹ) without any detectable phase transition over a wide temperature range. In principle, the dielectric properties of CCTO strongly depend on the electrical heterogeneity of grains, consisting of n-type semiconducting grains and insulating grain boundaries (GBs) [15, 17, 22, 23] i.e. the grain and grain boundary resistances are crucial factors that affect the dielectric properties. Recently, many researchers reported that several CCTO-based compounds had demonstrated themselves to accomplish a category for the EIA code X7R capacitor [24, 25]. Moreover, several groups had successfully prepared materials, which could be applied for X8R capacitor by co-doping CCTO [5, 26] and doping Ca2Cu3Ti4O12 (CCTO/CaTiO3) [4] with some metals. To our knowledge CCTO-based ceramics with satisfactional properties for X9R capacitor have not been reported.
Differently, many groups have focused their work on the study of CaCu3Ti4O12/TiO2 ceramics with excess molar concentration of Ti. For instance, Lin et al. [27] reported the successful preparation of TiO2-rich CCTO (CaCu3Ti4+xO12; x = 0.00, 0.5, 1.00 and 1.5) ceramics through a solid state reaction process. In this report, it was found that TiO2 secondary phase trended to increase with increasing Ti content, whereas ε′ and tanδ values decreased to ~ 4233 and ~ 0.03, respectively. Furthermore, nonlinear characteristics could be improved. In the work of Ouyang et al. [28], it was reported that TiO2 phase could modify the microstructural and electrical properties of CCTO ceramics prepared by a sol–gel process. At room temperature (RT) and 0.1 kHz, they found that these ceramic samples exhibited ε′ value of ~ 5.5 × 104 with tanδ value of ~ 0.2. Other than this, Hao et al. [29] had successfully prepared CCTO/xTiO2 (x = 0.0, 0.5, 1.0 and 2.0) ceramics by a sol–gel process. They found that tanδ value at low frequency decreased with increasing Ti content, resulting from the presence of TiO2 secondary phase. Moreover, ε′ and tanδ values were relatively proportional to the amount of TiO2. Although various CCTO/TiO2-based ceramics have been widely studied, it was hard to fulfill all of the requirements for high dielectric performance, especially for the excellent value of Δε′ in a properly given temperature range. However, it is evident that over all dielectric properties and Δε′ of CCTO-based ceramics can be significantly improved by Mg2+ doping [30, 31] and co-doping with others metal [5, 26, 32]. For instance, very high performance dielectric properties with appropriate Δε′ for X8R capacitor could be achieved by substitution of Mg2+ ions on the Cu2+ sites in the crystal structure of Ca2Cu2Ti4O12 ceramics [4]. According to these reports, it is interesting to put forward effort on the study of Mg2+-doped CaCu2Ti4O12/TiO2 ceramics with the presumption that overall dielectric properties and temperature stability of the dielectric constant can be improved and benefit for the X9R capacitor. Therefore, it is the aim of this work to provide a different approach for the improvement of the overall dielectric properties with excellent Δε′ of CCTO-based ceramics by substitution of Mg2+ ions into a binary compound system of CaCu3−xMgxTi4O12/TiO2 ceramics. In order to accomplish this, a well-established polymer pyrolysis solution method (PP method) has been employed for the preparation of high performance CCTO-based ceramics [33, 34]. In this work, CaCu3−xMgxTi4.2O12 (x = 0.00, 0.05 and 0.10) precursor powders were prepared by a PP method. Effect of Mg2+ doping on the dielectric and non-ohmics properties, including the Δε′ were examined and discussed based on the promotion of oxygen vacancy at GB. Surprisingly, very low loss tangent (tanδ < 0.009–0.014) and high dielectric constant (ε′ ∼ 1.1 × 104–1.8 × 104, at 1 kHz and RT) with excellent temperature stability less than ± 15% of Δε′ over a more wider temperature range of − 60 to 210 °C were achieved. In addition, the highest value of α ~ 13.75 and Eb ~ 5977.0 V/cm were obtained. In order to clarify the effect of Mg2+ ions on the dielectric and non-ohmics properties of prepared ceramic samples, impedance measurements were performed.
2 Experimental details
CaCu3−xMgxTi4.2O12 (x = 0.00, 0.05 and 0.10) precursor powders were prepared by a PP method. Cu(NO3)2·3H2O (99.5% Carlo Erba), Ca(NO3)2·4H2O (99.99% Kanto), TiC16H28O6 (75 wt% in isopropanol), Mg(NO3)2·6H2O (99.9% Kanto), (NH4)2S2O4 (99.95% Carlo Erba) and acrylic acid were used as starting materials. The complete details for the preparation of these powders were given elsewhere [34]. By the end of the process, the viscous blue gels were dried at 350 °C for 2 h, then ground to fine powders and calcined at 900 °C for 10 h. These calcined powders were pressed using the uniaxial compression at a pressure of 150 MPa into pellets of approximately4.75 mm in radius and ∼ 1.2 mm in thickness. To obtain CaCu3−xMgxTi4.2O12 (x = 0.00, 0.05 and 0.10) ceramics, these pellets were separated for sintering at the same temperature of 1100 °C for 8 h and the others for 12 h. Effect of sintering time on the dielectric and non-ohmics properties, including the temperature stability of εʹ were studied. The obtained ceramic samples CaCu3−xMgxTi4.2O12 sintered at 1100 °C for 8 h with different Mg concentrations are denoted as Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 for x = 0.00, 0.05 and 0.10, respectively. Similarly, for those samples sintered at 1100 °C for 12 h, Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 symbols are denoted for them with x = 0.00, 0.05 and 0.10, respectively.
Structural and phase composition of ceramic samples were investigated by X-ray diffraction (PW3040 Philips; Cu-Kα radiation; λ = 0.15406 nm). Microstructure and the dispersion of Mg, Ca, Cu, Ti and O elements in ceramic samples were studied by field emission scanning electron microscope (FESEM) coupled with energy dispersive X-ray spectroscopy (EDXS) (LEO SEM VP1450, UK). Prior to the measurements of dielectric constant (ε′) and dielectric loss tangent (tanδ) of ceramic samples, both surfaces of each sample were polished, subsequently cleaned and coated with Au using a Polaron SC500 sputtering unit. The measurements of dielectric constant (ε′) and tanδ of ceramic samples were performed in wide temperature (− 50 to 210 °C) and frequency (100 Hz–1 MHz) ranges using an impedance gain phase analyzer (Hewlett Packard Model 4294A). Nonlinear characteristics of ceramic samples were studied at RT using a high voltage measurement unit (Keithley Model 247). Nonlinear coefficients (α) were calculated by Eq. (1) and breakdown electric field (Eb) were determined at a current density (J) of 1 mA/cm2.
where E1 and E2 are the electric fields, corresponding to J1 = 1 mA/cm2 and J2 = 10 mA/cm2, respectively.
3 Results and discussion
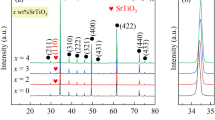
Rietveld refinement profile fits for the XRD patterns of Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics are shown in Fig. 1a–c and those of Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics are shown in Fig. 1d–f, respectively. As seen in Fig. 1a–f the profile fits for the main peaks of all ceramics correspond to those of the standard CaCu3Ti4O12 (ICSD card No. 95714) with the detection of a minor diffraction peak of TiO2 secondary phase (ICSD card No. 26715) in all ceramics. From Rietveld refinement fitting profiles the CaCu3Ti4O12:TiO2 ratios were determined from the main diffraction peaks of each CaCu3−xMgxTi4.2O12 ceramic and found to have the same value of approximately 94:6. In addition, these main diffraction peaks in each of the XRD pattern can be perfectly indexed based on the bcc structure of a space group Im3 (204). Complete details for the analysis of various R (Rexp, Rwp, and Rp) values and goodness of fit (GOF) were given elsewhere [35]. From Rietveld refinement results, GOF values are found to be in a narrow range of approximately 1.0–1.4 for all ceramics. The percentages of R (Rexp, Rwp, and Rp) factors for all ceramics are in the appropriate range of approximately 5–10%. Lattice parameters (a) of Ti02/8, Ti02–Mg05/8, Ti02–Mg10/8, Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics were determined and found to be 7.387 (2), 7.388 (2), 7.386 (3), 7.389 (8), 7.388 (1) and 7.390 (8) Å, respectively. It is obvious that the lattice parameters (a) display no significant change with increasing Mg2+ concentration. This confirms the substitution of Mg2+ ion on the Cu site rather than the Ca site (having Ca2+ ion of large ionic radius 1.00 Å) due to the nearly equal ionic radius of Cu2+ (0.73 Å) and Mg2+ (0.72 Å). Moreover, the obtained a values of all ceramics are comparable with those of CCTO ceramics (a = 7.391 Å) reported in literatures [21]. According to the Rietveld refinement fitting profile results, the theoretical densities (Dxrd) of Ti02/8, Ti02–Mg05/8, Ti02–Mg10/8, Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics were determined and found to be 5.0591, 5.0574, 5.0608, 5.0586, 5.0555 and 5.0534 g cm−3, respectively. It is remarkable that Dxrd values of all CaCu3−xMgxTi4.2O12 (Ti02–Mg) ceramics are less than that of CaCu3Ti4.2O12 (Ti02) ceramic due to the lighter atomic mass of Mg atom (24.304 amu) than that of Cu atom (63.546 amu).
Since ε′ and tanδ values with nonlinear J–E properties of CCTO ceramics are strongly dependent on their microstructure as generally reported in literatures [36]. Thus, morphology and microstructure of all ceramic samples were examined. As shown in Fig. 2a–f, are back scattered FESEM images of polished surface of CaCu3−xMgxTi4.2O12 ceramics sintered at 1100 °C for 8 h and 12 h, respectively. These FESEM images reveal the microstructural evolution of grains in all ceramic samples as a result of sintering process. The mean grain sizes of Ti02/8, Ti02–Mg05/8, Ti02–Mg10/8, Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics were found to be 8.433 ± 1.321, 5.343 ± 1.069, 7.016 ± 2.111, 8.446 ± 1.756, 6.626 ± 1.677 and 7.348 ± 1.571 µm, respectively. It is obvious that the mean grain sizes of Mg-doped ceramic samples sintered with different sintering time of 8 and 12 h slightly decrease, comparing with their undoped counterparts. This might result from the solute drag mechanism due to the substitution of Mg2+ ion of lighter atomic mass and smaller atomic radius, compare with those of Cu2+ ion, into the nominal composition of CaCu3−xMgxTi4.2O12 ceramics. Slight change of the mean grain sizes is suggested to originate from the less difference in ionic radii of Mg2+ (0.72 Å) dopant and Cu2+ (0.73 Å) host ions. Furthermore, effect of sintering time can result in a slight increase of the mean grain sizes of all ceramics.
To observe the segregation of Mg2+ ions in the microstructure of all CaCu3−xMgxTi4.2O12 (x = 0.00, 0.05 and 0.10) ceramics sintered at 1100 °C for 8 and 12 h, the EDXS spectra obtained at grain and GB with back scattered FESEM images of each sample were examined. For illustration, back scattered FESEM image of Ti02–Mg10/8 ceramic is shown in Fig. 3a with a marked point (1) for grain and point (2) for GB. However, EDXS spectra of all ceramic samples are not shown. The EDXS results indicate that Mg2+ ions can segregate at grains and GBs with preference to be at GB. The Ca:Cu:Ti:Mg:O ratios at grain (1) and GB (2) of Ti02–Mg10/8 ceramic were determined and found to be 6.30:32.76:0.45:32.72:27.78 At.% and 6.80:30.56:0.00:33.28:29.36 At.%, respectively. The determined Ca:Cu:Ti:Mg:O ratios at grain and GB for other ceramic samples are listed in Table 1. In addition, sintering time significantly affect the distribution of Mg2+ in grains and GBs as can be seen in Table 1 that Mg2+ ion in Ti02–Mg10/12 ceramic can be detected at both of grain and GB regions, whereas that of Ti02–Mg10/8 ceramic can be only detected at grain regions. This result is similar to that reported in literature [4]. The dispersion of each Ca, Cu, Ti, Mg and O element in all CaCu3−xMgxTi4.2O12 ceramics were determined and observed by elements mapping using FESEM. As shown in Fig. 3b–f are the back scattered FESEM mapping images of Ca, Cu, Ti, Mg and O elements in Ti02–Mg10/8 ceramic, corresponding to its back scattered FESEM image shown in Fig. 3a. These results confirm the existence of all major elements of Ca, Cu, Ti, Mg and O with homogeneous dispersion of them in grains and GBs. It is obviously seen in Fig. 3e that segregation of Mg2+ dopant in any specific region of the ceramic is not observed.
The frequency dependence of ε′ and tanδ for Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics are shown in Fig. 4a and its inset. The determined ε′ values (at 30 °C and 1 kHz) of Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics were found to be 46,434, 12,902 and 14,012, respectively. These ε′ values are summarized in Table 2. Notably, ε′ value of Ti02–Mg05/8 ceramic decreases by a factor of approximately four times compare with that of undoped Ti02/8 ceramic. However, for further increasing Mg-doping content, ε′ of Ti02–Mg10/8 ceramic slightly increase to a value, which is higher than that of Ti02–Mg05/8 ceramic. It is obvious that the ε′ value of Mg2+-doped Ti02 ceramic is lower than that of undoped Ti02 ceramic due to the lower concentration of oxygen vacancy at grain boundary (GB), which is consistent with those reported in literature [37,38,39,40,41]. In addition, Han et al. reported that free charge carrier at GB could play an important role on the improvement of dielectric properties of Y-doped CCTO ceramics [39]. Moreover, the ε′ values of Mg-doped Ti02/8 ceramics are almost stable over a wide frequency range from 100 Hz to 1 MHz, while that of undoped Ti02/8 ceramic greatly decrease with increasing frequency. It is also remarkable that ε′ values of Ti02–Mg05/8 and Ti02–Mg10/8 ceramics are approximately 3.5 and 3.3 times lower than that of undoped Ti02/8 ceramic. However, these ε′ values are still large enough (higher than 104 at 30 °C and 1 kHz) and sufficient for capacitor application. In addition to high ε′ value, tanδ value of high performance capacitor should be less than 0.05. As shown in the inset of Fig. 4a, the red dashed line signifies tanδ value equal to 0.05. At 30 °C and 1 kHz, tanδ values of Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics are found to be 0.685, 0.011 and 0.009, respectively. These tanδ values are summarized in Table 2. It is obvious that tanδ values of Ti02–Mg05/8 and Ti02–Mg10/8 ceramics are smaller than 0.05 and both values are approximately 30 times less than that of Ti02/8 ceramic in a wide frequency range of 100 Hz–0.1 MHz. Figure 4b and its inset show the frequency dependence of ε′ and tanδ of Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics. At 30 °C and 1 kHz, ε′ and tanδ values are found to be 199,083, 18,000 and 11,086; and 0.823, 0.014 and 0.009 for Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics, respectively. All of these ε′ and tanδ values are summarized in Table 2. It is interesting that tanδ values of the Ti02–Mg05/12 and Ti02–Mg10/12 ceramics are about 58 and 91 times less than that of undoped Ti02/12 ceramic, while the ε′ values of them are approximately 11 and 18 times less than that of undoped Ti02/12 ceramic. It worth noting that increasing sintering time from 8 to 12 h, tanδ value of undoped Ti02 ceramic greatly increase, whereas those of Ti02–Mg05 and Ti02–Mg10 ceramics slightly increase to a stable value. Moreover, ε′ values of Ti02 and Ti02–Mg05 ceramics are greatly increase with increasing sintering time from 8 to 12 h, whereas that of Ti02–Mg10 ceramic slightly decrease. It is obvious that the increase of sintering time at the sintering temperature of 1100 °C can greatly promote oxygen vacancy and Ti3+, which play an important role on the improvement of electrical properties of these samples in consistent with those reported in literature [16, 42, 43]. Notably, the very low tanδ (∼ 0.009 to 0.014) and giant ε′ (∼ 11,086 to 18,000) values of the Mg-doped Ti02 ceramics are comparable with those reported in literatures [44,45,46]. Furthermore, the lowest tanδ value of 0.009 (at RT and 1 kHz) is achieved in both Ti02–Mg10/8 and Ti02–Mg10/12 ceramics. This tanδ value is approximately 1.4, 1.6, 4.0 and 4.3 times less than those obtained in (Y + Mg) co-doped CCTO [5], Ca2Cu3−xMgxTi4O12 [4], Na1/2Y1/2Cu3−xMgxTi4O12 [47] and (Sm3+and Mg2+) co-doped CCTO [32] ceramics, respectively. It is notable that, all Mg-doped Ti02 ceramics sintered for 8 and 12 h demonstrate giant ε′ and very low tanδ values. Thus, it can be concluded that all Mg-doped Ti02 ceramics demonstrate very low tanδ and high ε′ over a wide frequency range, which is appropriate for capacitor application.
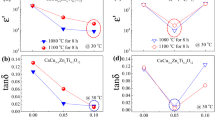
The temperature dependence of ε′ at 1 kHz for all ceramic samples sintered at 1100 °C for 8 and 12 h are shown in Fig. 5a, b, respectively. As seen in Fig. 5a, b, ε′ values of undoped Ti02/8 and Ti02/12 ceramics greatly increase with increasing temperature, whereas those of Mg-doped Ti02 ceramics are almost stable in a temperature range of − 60 to 210 °C. The greatly temperature dependence of ε′ in undoped Ti02 ceramics is similar to that observed in CCTO ceramics [53, 54]. Figure 5c, d display the temperature dependence of tanδ at 1 kHz for all ceramic samples sintered at 1100 °C for 8 and 12 h, respectively. It is obvious in Fig. 5c, d that when the temperature is higher than 100 °C, tanδ of all Mg-doped Ti02 ceramics is higher than 0.05. In addition, tanδ values of both undoped Ti02 ceramics are higher than 0.05 through the whole temperature range from − 50 to 210 °C. The large increase in tan δ at high temperatures of both undoped Ti02 ceramics is consistent with their great increase in ε′. It is likely that the large value of tanδ in a high temperature region is the most serious problem one that limit the potent application of CCTO-based compounds at high temperature. Therefore, it is evident that Mg doping in Ti02 ceramics can result not only in the temperature stability of ε′, but also simultaneously improve temperature stability of tanδ.
As shown in Fig. 5e, f are the Δε′ values of ceramic samples sintered at 1100 °C for 8 and 12 h, respectively. All of these values are evaluated at the frequency of 1 kHz from the following equation,
where \(\varepsilon _{T}^{\prime }\) and \(\varepsilon _{{30}}^{\prime }\) are ε′ at a temperature of T and 30 °C, respectively. In addition to high ε′ and low tanδ, a good capacitor should have Δε′ < ±15% over a wide temperature range. As seen in Fig. 5e, f, Δε′ of both undoped Ti02 ceramics abruptly change in a very narrow temperature range of 10 °C. In contrast to this, Δε′ < ± 15% of both Mg-doped Ti02 ceramics sintered for 8 h, as shown in Fig. 5e, are found to slowly vary in a more wider temperature range of − 60 to 170 °C and − 60 to 210 °C for Ti02–Mg05/8 and Ti02–Mg10/8, respectively. Similar behavior of Δε′ < ±15% is observed in Ti02–Mg05/12 and Ti02–Mg10/12 ceramics, as shown in Fig. 5f with temperature stability in a range of − 60 to 180 °C and − 60 to 210 °C, respectively. It worth noting that dielectric properties of both Ti02–Mg05/8 and Ti02–Mg05/12 ceramics satisfy the EIA X5R, X7R and X8R standard capacitor specification. Interestingly, Ti02–Mg10/8 and Ti02–Mg10/12 ceramics satisfy the EIA X5R, X7R, X8R and X9R standard capacitors. In addition to the application for X–R capacitors, all Mg-doped Ti02 ceramics have a potent use for other EIA code X–P capacitors, which is defined with the temperature stability condition that Δε′ < ± 10% should be in a temperature range of − 55 to 125 °C and − 55 to 150 °C for the EIA code X7P and X8P capacitors, respectively. In Fig. 5e, f, the blue dashed lines are drawn to signify Δε′ < ±10%. As illustrated in Fig. 5e, it is clear that Δε′ < ± 10% for Ti02–Mg05/8 and Ti02–Mg10/8 are in temperature ranges of − 60 to 150 °C and − 60 to 170 °C, respectively. Similarly, these temperature ranges of Δε′ < ± 10% are found to be − 60 to 160 °C and − 60 to 170 °C for Ti02–Mg05/12 and Ti02–Mg10/12 ceramics, respectively. Therefore, it can be concluded that, Ti02–Mg05 and Ti02–Mg10 ceramics sintered for 8 and 12 h not only satisfy for the EIA code X–R capacitors, but also beneficial for the EIA code X–P capacitors. Furthermore, the temperature stability of Ti02–Mg05 ceramics also slightly increases with the increasing sintering time from 8 to 12 h. Notably, the achievement of excellent dielectric properties and a wide range temperature stability of ε′ in Mg-doped TiO2 ceramics are difficult to attain in other CCTO-based giant dielectric materials.
In this work, although the Δε′ < ± 15% of Mg-doped Ti02 ceramics is stable in a temperature range comparable to those of other co-doped CCTO ceramics [5, 32], its performance of temperature stability is better than those reported in a binary system of Ca2Cu2−xMgxTi4O12 ceramics [4] and other Mg-doped CaCuTi4O12 ceramics [44,45,46]. Thus, it is verified that Mg-doped CaCu3Ti4.2O12 ceramics prepared by the PP method can be successfully modified and categorized for the EIA code X9R capacitors. In this work, it is evident and can be concluded that dielectric properties, high ε′ and low tanδ with the excellent temperature coefficient Δε′, of Ti02 ceramics can be significantly improved by Mg2+ doping, follow with a proper sintering process treatment.
The nonlinear J–E properties of all ceramic samples were examined at RT. As shown in Fig. 6a, b are nonlinear J–E characteristic curves of Mg-doped CaCu3Ti4.2O12 ceramic samples sintered at 1100 °C for 8 and 12 h, respectively. Nonlinear coefficient (α) and breakdown field (Eb) of all prepared ceramics can be determined from these J–E curves. The obtained α and Eb values of Ti02, Ti02–Mg05 and Ti02–Mg10 ceramics are summarized in Table 2. It worth noting that α and Eb values of Mg-doped Ti02 ceramics significantly increase with increasing Mg2+ doping content. In addition, both α and Eb values are reduced by increasing sintering time. It is suggested that the enhancement of α and Eb values might arise from the immense increase of Rgb [14, 55]. It is remarkable in Table 2 that the maximum Eb values observed in Ti02–Mg10/8 and Ti02–Mg10/12 ceramics are closely correlated with the highest values of Rgb and the lowest tanδ values with very high thermal stability of ε′. The values of α and Eb are comparable with those reported in literatures [13, 22, 24, 56]. Furthermore, the α values obtained in this work are higher than those of 5.1–7.9 reported for CaCu3Ti4+xO12+2x (0.00 < x < 1.5) ceramics sintered at 1100 °C for 3 h [27]. In order to understand the electrical respond at GBs of these ceramic samples, the potential barriers are discussed based on Schottky barrier [57]. Based on this type of barrier, the electrical current density (J) and the applied electrical field (E) are related by the relationship, \(\ln \left( {{J \mathord{\left/ {\vphantom {J {A{T^2}}}} \right. \kern-0pt} {A{T^2}}}} \right)=\frac{1}{{{k_B}T}}\left( {\beta {E^{1/2}} - {\phi _B}} \right)\) [58, 59], where A is the Richardson constant (∼ 1202 × 102 mA cm−2 K−2), kB is the Boltzmann constant (1.3806 × 10−23 m2 kg s−2 K−1), β is a constant related to the potential barrier width, and φB is the barrier high. As seen in Fig. 6c, d a good linear relationship of ln (J/AT2) versus E1/2 is obtained for ceramic samples sintered at 1100 °C for 8 and 12 h. From the fitting results, the φB and β values of all ceramic samples are obtained and summarized in Table 2. It is also found that the φB value of Ti02 ceramic is greatly increased with Mg2+ doping. It is suggested that the formation of the electrostatic potential barriers at GBs is due to the present of Mg2+ ions. Moreover, the α and Eb values are directly promotional to the φB and 1/β values, respectively. In addition, it is suggested that reducing of the β value can increase the Eb value, whereas the α is improved by the increasing of φB values. Notably, φB values (0.957–0.970 eV) of these Mg2+-doped Ti02 ceramics are comparable with the φB values of the Na1/2Sm1/2Cu3Ti4O12 ceramics (∼ 0.964 eV) [60].
In order to further study the effect of Mg2+ doping on the resistance of grain (Rg) and grain boundary (Rgb), the Z* plots at 120 °C of all ceramic samples were performed as shown in Fig. 7a. Generally, the semicircular arcs at low and high frequency ranges in Z* plots correspond to Rgb and Rg, respectively [12, 61]. As seen in Fig. 7a for illustration, only the low frequency semicircular arcs are observed in Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramic samples. This behavior is similar to those of ceramic samples sintered for 12 h. In Fig. 7a and the inset (1), Rgb values of Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics were estimated and found to be 3310, 1,030,000 and 1,750,000 Ω cm, respectively. These values are summarized in Table 2. As seen in the inset (2) of Fig. 7a, Rg values of Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics are estimated from a nonzero intercept on the Z′ axis at high frequency region and found to be 25, 59 and 45 Ω cm, respectively. Similarly, Rgb and Rg values at 120 °C of Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics were also estimated and summarized in Table 2. It is notable in Table 2 that Rgb values of Ti02/8 and Ti02/12 ceramics increase with increasing Mg2+ dopants, which is similar to those observed in literatures [5, 44, 47]. Furthermore, increasing sintering time from 8 h to 12 h can also increase Rgb values for Mg-doped Ti02 ceramic samples with the opposite decreasing of these values as observed in undoped Ti02 ceramic samples. Thus, it is obvious that sintering time can significantly influence on the total resistance of the insulating parts i.e. Rgb of ceramic samples, as well. This might primarily originate from the GBs and/or outer surface layers. In addition, Rgb is observed to relate with the very high temperature coefficient of ε′ i.e. undoped Ti02 ceramic with low value of Rgb exhibits a poor temperature stability of ε′, whereas Mg-doped Ti02 ceramic samples with high Rgb value demonstrate the excellent temperature stability of ε′. Therefore, Mg-doped Ti02 ceramic samples of high performance dielectric properties with the excellent temperature stability of ε′ can be well described by their large Rgb values.
a Impedance complex plane plots (Z*) at 120 °C of Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics with inset (1) displays a magnification of the overlapped region and (2) displays an expanded view of high frequency data close to the origin. b Frequency dependence of − Z″ in the temperature range of 130–210 °C for Ti02–Mg10/8 ceramic
As shown in Fig. 8 and its inset are the temperature dependence of grain boundary conductivity (σgb) for ceramic samples sintered at 1100 °C for 8 and 12 h, respectively. As seen in Fig. 8, the plots follow the Arrhenius law,
where the values of σgb is defined by σgb = 1/Rgb, σ0 is the pre-exponential term, Egb is the activation energy for conduction at grain boundaries, kB is the Boltzmann constant, and T is the absolute temperature. Egb values are generally calculated from the slopes of the plots of Ln σgb versus 1000/T and found to be 0.419, 0.611 and 0.623 eV for Ti02/8, Ti02–Mg05/8 and Ti02–Mg10/8 ceramics, respectively. As for Ti02/12, Ti02–Mg05/12 and Ti02–Mg10/12 ceramics, the Egb values are found to be 0.413, 0.620 and 0.625 eV, respectively. It worth noting that, Egb values of Ti02–Mg/8 and Ti02–Mg/12 ceramics increase with increasing Mg2+ doping concentration, which is consistent with the increase of their Rgb values, indicating the decrease of oxygen vacancy concentration at GB. All calculated Egb values in this work are comparable with the conduction activation energy at GB of other CCTO ceramics (Egb ∼ 0.47–0.74 eV) [16, 42, 62] and Ln3+-doped CaCu3Ti4O12 ceramics (Egb ∼ 0.55–0.76 eV) [63]. Remarkably, the highest Egb values are observed in both Ti02–Mg10/8 and Ti02–Mg10/12 ceramics, corresponding with the lowest tanδ values and the highest Eb values as revealed in Fig. 6a, b and the results summarized in Table 2. Consequently, it can be concluded that Mg2+ doping of Ti02 ceramics can result in a large increase of Rgb, Eb and Egb values with the excellent temperature stability of ε′ and very high performance of dielectric properties (giant ε′ and very low tanδ).
4 Conclusion
Very high temperature stability of ε′ < ± 15% in a range of − 60–210 °C with giant ε′ of 1.1 × 104–1.4 × 104 and very low tanδ of 0.009 are achieved in the CaCu2.9Mg0.1Ti4.2O12 ceramics sintered at 1100 °C for 8 and 12 h. Sintering time can immensely improve the ε′ of CaCu3Ti4.2O12 ceramic with increasing tanδ value, as well. Mg2+ doping of CaCu3Ti4.2O12 ceramic results in the decrease of ε′ value with very low tanδ value, consistent with the immense increase of GB resistance (Rgb). Longer sintering time can reduce the Rgb of CaCu3Ti4.2O12 ceramic, but increase those of Mg-doped CaCu3Ti4.2O12 ceramics. In addition, the nonlinear J–E properties of Mg-doped CaCu3Ti4.2O12 ceramics (α and Eb values) significantly increase with increasing Mg2+ doping content, especially for the Eb value. Consequently, it can be concluded that excellent dielectric properties i.e. very high ε′ and low tanδ with excellent temperature coefficient of CaCu3Ti4.2O12 ceramics prepared in this work by the PP method can be significantly improved by Mg2+ doping follow with a proper sintering process treatment. This improvement can be explained by the enhancement of electrical responses at GBs resulting from the existence of Mg2+ ions. Moreover, it is suggested that CaCu2.9Mg0.1Ti4.2O12 ceramics sintered at 1100 °C for 8 and 12 h can be applicable for high temperature semiconductor devices such as X8R and X9R capacitors.
References
S. Gao, S. Wu, Y. Zhang, H. Yang, X. Wang, Mater. Sci. Eng. B 176, 68–71 (2011)
C. Sun, X. Wang, C. Ma, L. Li, J. Am. Ceram. Soc. 92, 1613–1616 (2009)
Z. Hu, B. Cui, M. Li, L. Li, J. Mater. Sci.: Mater. Electron. 24, 3850–3855 (2013)
J. Jumpatam, B. Putasaeng, T. Yamwong, P. Thongbai, S. Maensiri, J. Eur. Ceram. Soc. 34, 2941–2950 (2014)
J. Boonlakhorn, B. Putasaeng, P. Kidkhunthod, P. Thongbai, Mater. Des. 92, 494–498 (2016)
S.M. Galeb, H. Maher, U.S. Patent US6727200 B2 (2004)
B. Tang, S.-R. Zhang, X.-H. Zhou, Y. Yuan, L.-B. Yang, J. Electroceram. 25, 93–97 (2010)
Z. Shen, X. Wang, L. Li, J. Mater. Sci.: Mater. Electron. 28, 3768–3773 (2017)
X. Huang, W. Zhang, J. Xie, Q. Xu, L. Zhang, H. Hao, H. Liu, M. Cao, J. Mater. Sci.: Mater. Electron. 28, 4204–4210 (2017)
C. Su, H. Hao, Q. Xu, Y. Lu, M. Appiah, C. Diao, M. Cao, Z. Yao, H. Liu, J. Mater. Sci.: Mater. Electron. 27, 6140–6149 (2016)
Z. Liu, H. Fan, S. Lei, X. Ren, C. Long, J. Eur. Ceram. Soc. 37, 115–122 (2017)
T.B. Adams, D.C. Sinclair, A.R. West, Phys. Rev. B 73, 094124 (2006)
J. Boonlakhorn, P. Kidkhunthod, B. Putasaeng, T. Yamwong, P. Thongbai, S. Maensiri, J. Mater. Sci.: Mater. Electron. 26, 2329–2337 (2015)
S.-Y. Chung, J.-H. Choi, J.-K. Choi, Appl. Phys. Lett. 91, 091912 (2007)
J. Jumpatam, B. Putasaeng, T. Yamwong, P. Thongbai, S. Maensiri, Ceram. Int. 39, 1057–1064 (2013)
Y. Huang, D. Shi, Y. Li, G. Li, Q. Wang, L. Liu, L. Fang, J. Mater. Sci.: Mater. Electron. 24, 1994–1999 (2013)
Z. Kafi, A. Kompany, H. Arabi, A. Khorsand Zak, J. Alloys Compd. 727, 168–176 (2017)
C. Long, T. Li, H. Fan, Y. Wu, L. Zhou, Y. Li, L. Xiao, Y. Li, J. Alloys Compd. 658, 839–847 (2016)
G. Liu, H. Fan, J. Xu, Z. Liu, Y. Zhao, RSC Adv. 6, 48708–48714 (2016)
X. Liu, H. Fan, J. Shi, Q. Li, Sci. Rep. 5, 12699 (2015)
M.A. Subramanian, D. Li, N. Duan, B.A. Reisner, A.W. Sleight, J. Solid State Chem. 151, 323–325 (2000)
P. Leret, J.F. Fernandez, J. de Frutos, D. Fernández-Hevia, J. Eur. Ceram. Soc. 27, 3901–3905 (2007)
Z. Yang, L. Zhang, X. Chao, L. Xiong, J. Liu, J. Alloys Compd. 509, 8716–8719 (2011)
J. Boonlakhorn, P. Kidkhunthod, B. Putasaeng, P. Thongbai, Ceram. Int. 43, 2705–2711 (2017)
S. Vangchangyia, T. Yamwong, E. Swatsitang, P. Thongbai, S. Maensiri, Ceram. Int. 39, 8133–8139 (2013)
J. Boonlakhorn, P. Kidkhunthod, P. Thongbai, S. Maensiri, Ceram. Int. 42, 8467–8472 (2016)
Y.-H. Lin, J. Cai, M. Li, C.-W. Nan, J. He, Appl. Phys. Lett. 88, 172902 (2006)
X. Ouyang, M. Habib, P. Cao, S. Wei, Z. Huang, W. Zhang, W. Gao, Ceram. Int. 41, 13447–13454 (2015)
W. Hao, P. Xu, M. Wang, S. Yang, W. Yupeng, H. Wu, L. Sun, E. Cao, Y. Zhang, J. Alloys Compd. 740, 1159–1164 (2018)
M. Li, G. Cai, D.F. Zhang, W.Y. Wang, W.J. Wang, X.L. Chen, J. Appl. Phys. 104, 074107 (2008)
L. Ni, X.M. Chen, Solid State Commun. 149, 379–383 (2009)
J. Boonlakhorn, P. Kidkhunthod, P. Thongbai, J. Eur. Ceram. Soc. 35, 3521–3528 (2015)
P. Thongbai, B. Putasaeng, T. Yamwong, S. Maensiri, J. Alloys Compd. 509, 7416–7420 (2011)
E. Swatsitang, A. Niyompan, T. Putjuso, J. Mater. Sci.: Mater. Electron. 24, 3514–3520 (2013)
E. Jansen, W. Schäfer, G. Will, J. Appl. Crystallogr. 27, 492–496 (1994)
J.-W. Lee, J.-H. Koh, Ceram. Int. 41, 10442–10447 (2015)
S. Liu, X. Sun, B. Peng, H. Su, Z. Mei, Y. Huang, J. Deng, C. Su, L. Fang, L. Liu, J. Electroceram. 37, 137–144 (2016)
J. Deng, L. Liu, X. Sun, S. Liu, T. Yan, L. Fang, B. Elouadi, Mater. Res. Bull. 88, 320–329 (2017)
F. Han, S. Ren, J. Deng, T. Yan, X. Ma, B. Peng, L. Liu, J. Mater. Sci.: Mater. Electron. 28, 17378–17387 (2017)
G. Li, Z. Chen, X. Sun, L. Liu, L. Fang, B. Elouadi, Mater. Res. Bull. 65, 260–265 (2015)
X. Sun, J. Deng, S. Liu, T. Yan, B. Peng, W. Jia, Z. Mei, H. Su, L. Fang, L. Liu, Appl. Phys. A 122, 864 (2016)
Y. Huang, L. Liu, D. Shi, S. Wu, S. Zheng, L. Fang, C. Hu, B. Elouadi, Ceram. Int. 39, 6063–6068 (2013)
S. Zheng, D. Shi, L. Liu, G. Li, Q. Wang, L. Fang, B. Elouadi, J. Mater. Sci.: Mater. Electron. 25, 4058–4065 (2014)
L. Sun, R. Zhang, Z. Wang, E. Cao, Y. Zhang, L. Ju, J. Alloys Compd. 663, 345–350 (2016)
L. Singh, U.S. Rai, K.D. Mandal, A.K. Rai, Appl. Phys. A 112, 891–900 (2013)
L. Singh, U.S. Rai, K.D. Mandal, J. Alloys Compd. 555, 176–183 (2013)
J. Jumpatam, A. Mooltang, B. Putasaeng, P. Kidkhunthod, N. Chanlek, P. Thongbai, S. Maensiri, Ceram. Int. 42, 16287–16295 (2016)
J. Deng, X. Sun, S. Liu, L. Liu, T. Yan, L. Fang, B. Elouadi, J. Adv. Dielectr. 06, 1650009 (2016)
F. Han, J. Deng, X. Liu, T. Yan, S. Ren, X. Ma, S. Liu, B. Peng, L. Liu, Ceram. Int. 43, 5564–5573 (2017)
X. liu, L. Liu, F. Han, S. Liu, H. Xiang, L. Fang, J. Mater. Sci.: Mater. Electron. 27, 12128–12133 (2016)
L. Liu, H. Fan, P. Fang, X. Chen, Mater. Res. Bull. 43, 1800–1807 (2008)
L. Liu, H. Fan, X. Chen, P. Fang, J. Alloys Compd. 469, 529–534 (2009)
X. Huang, H. Zhang, M. Wei, Y. Lai, J. Li, J. Alloys Compd. 708, 1026–1032 (2017)
S. Jesurani, S. Kanagesan, M. Hashim, I. Ismail, J. Alloys Compd. 551, 456–462 (2013)
S.-Y. Chung, I.-D. Kim, S.-J.L. Kang, Nat. Mater. 3, 774–778 (2004)
P. Thongbai, J. Boonlakhorn, B. Putasaeng, T. Yamwong, S. Maensiri, J. Am. Ceram. Soc. 96, 379–381 (2013)
Y. Jiancong, L. Yuan-Hua, L. Huafei, C. Bo, N. Ce-Wen, J. Am. Ceram. Soc. 94, 1966–1969 (2011)
L.J. Liu, L. Fang, Y.M. Huang, Y.H. Li, D.P. Shi, S.Y. Zheng, S.S. Wu, C.Z. Hu, J. Appl. Phys. 110, 6 (2011)
L. Liu, Y. Huang, Y. Li, D. Shi, S. Zheng, S. Wu, L. Fang, C. Hu, J. Mater. Sci. 47, 2294–2299 (2012)
W. Somphan, P. Thongbai, T. Yamwong, S. Maensiri, Mater. Res. Bull. 48, 4087–4092 (2013)
D.C. Sinclair, T.B. Adams, F.D. Morrison, A.R. West, Appl. Phys. Lett. 80, 2153–2155 (2002)
Q. Zheng, H. Fan, C. Long, J. Alloys Compd. 511, 90–94 (2012)
J. Boonlakhorn, P. Thongbai, B. Putasaeng, T. Yamwong, S. Maensiri, J. Alloys Compd. 612, 103–109 (2014)
Acknowledgements
This work was financially supported by the National Research Council of Thailand (NRCT) under Rajamangala University of Technology Rattanakosin (Grant No. 2562). It was also supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (PHD/0207/2558). The Nanotec-KKU Center of Excellence on Advanced Nanomaterials for Energy Production and Storage, Khon Kaen, Thailand and Integrated Nanotechnology Research Center (INRC), Department of Physics, Faculty of Science, Khon Kaen University, Khon Kaen, 40002 Thailand are also grateful for their co-financial support. The authors express their appreciation to the National Metal and Materials Technology Center (MTEC), Thailand Science Park, Pathumthani, Thailand for dielectric measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swatsitang, E., Prompa, K. & Putjuso, T. Very high thermal stability with excellent dielectric, and non-ohmics properties of Mg-doped CaCu3Ti4.2O12 ceramics. J Mater Sci: Mater Electron 29, 12639–12651 (2018). https://doi.org/10.1007/s10854-018-9381-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9381-9