Abstract
Giant dielectric ceramics Ba(Nb0.5Fe0.5-x Ti x )O3 (BNFT) have been fabricated by a conventional solid-state reaction. According to X-ray diffraction analysis, the crystal structure of these ceramics can be described by the cubic centrosymmetric with Pm-3m space group. The real part (ε’) of dielectric permittivity and dielectric loss (tanδ) of the BNFT ceramics was measured in a frequency range from 40 Hz to 100 MHz at room temperature. The (ε’) of all these samples displays a high value (~6500) and a small frequency-dependence from 1 kHz to 1 MHz. We have established a link between conductivity activation energy and defect compensation at grain boundaries. The Ti4+-doped Ba(Nb0.5Fe0.5)O3 as a donor makes a great influence on the grain boundary behavior, which restricts the migration of oxygen vacancy and depresses dielectric loss factor for Ba(Nb0.5Fe0.5)O3 ceramics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Oxides with perovskite structure and high dielectric constant are of enormous importance to the electronic industry due to their wide technological applications. They have been used as memory devices, sensors, actuators and capacitor [1, 2]. However, most of the high dielectric constant materials contain lead, which can cause environmental pollution. Therefore, it is necessary to search alternative lead-free compounds for such applications, which should have either comparable or superior dielectric properties [3–6].
It is well known that Ba(Fe0.5Nb0.5)O3 (BFN) ceramics exhibit a giant dielectric response with a dielectric relaxation [3]. Furthermore, BFN is a relaxor-type material which has a partially disordered perovskite structure [7]. At the same time, the mechanisms of giant dielectric constant and relaxation behavior also have been investigated. Raevski et al. [8] explained the giant dielectric behavior associated with the Maxwell–Wagner effect. Wang et al. [4] proved that the dielectric relaxation is induced by oxygen vacancies. In fact, dielectric relaxations contributed by interface polarization and oxygen-vacancy hopping generally coexist at high temperatures [9]. Therefore, the enhancement of ionic disorder at both A and B sites in BFN could modify the transportation of oxygen vacancies and then to detect the mechanism of relaxation behavior of BFN ceramics.
One of the effective methods to improve the dielectric properties, especially decrease dielectric loss factor, is to decrease the concentration of oxygen vacancies. In this work, the substitution of Fe3+ by Ti4+ in BFN was carried out to reveal the relationship between doping concentration and carrier transportation, since Ti+4 has an electronic configuration which has an empty d subshell [10]. Our result suggests that the substitution of Ti4+ in Fe3+ in BFN makes an influence on the concentrations of both single-ionized and doubly ionized oxygen vacancies, improving the dielectric properties.
2 Experimental procedure
Ba(Nb0.5Fe0.5−x Ti x )O3 (x = 0.00, 0.01, 0.03, 0.05) ceramics were prepared by the conventional solid-state processing using starting powders: BaCO3 (99 %), Fe2O3 (99 %), Nb2O5 (99.99 %) and TiO2 (99.99 %) were used as starting materials. Reagents were dried at 393 K for 48 h before weighting. The stoichiometric powders were mixed by ball milling in ethanol for 8 h and then dried and calcined at 1473 K for 3 h. The calcined powders were mixed with 5 wt% polyvinyl alcohol (PVA) solution and then uniaxially pressed into pellets with a diameter of 12 mm under 350 MPa pressure. After burning out PVA, the green disks were, respectively, sintered in air at their own optimized temperatures for 3 h, depending on their x, in the range between 1598 and 1623 K.
Crystal structures of the samples were measured by X-ray diffraction (XRD) using a X-ray diffractometer (X’Pert PRO, PANalytical, Netherlands) with Cu K α (λ = 0.15,418 nm) radiation. The microstructures of the sintered samples were observed under SEM (JSM6380-LV JEOL, Tokyo, Japan) with an appropriate thermal etching. Silver paste electrodes were formed at the two surfaces of the samples and fired at 900 K for 30 min. The dependence of the dielectric permittivity on temperature was measured with precision impedance analyzer (Agilent 4294A, USA) over the frequency range 40 Hz–100 MHz. Temperature was varied in 3 K interval between 300 and 700 K.
3 Results and discussion
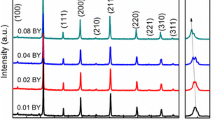
Figure 1 shows X-ray diffraction patterns of the BNFT ceramics with x = 0.00, 0.01, 0.03 and 0.05, respectively. All samples can be described as cubic perovskite structure with space group Pm-3m (ICSD 01-089-3122). No second phase is observed in the patterns. It indicates that Ti4+ diffuses into the perovskite lattice to form a solid solution.
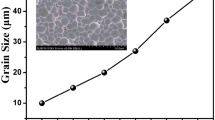
Figure 2 shows the SEM micrographs of the thermally etched surfaces of the samples. No second phase is observed on the SEM micrographs, which is agreement with XRD results. Using a linear intercept method on the photographs, the average grain size was determined to be 1.92 μm for the sample x = 0.00, 3.57 μm for x = 0.01, 2.62 μm for x = 0.03 and 2.1 μm for x = 0.05. It seems that the small-concentration addition of Ti promotes the grain growth of the ceramics.
Temperature dependence of dielectric constant (ε’) under different frequencies of the BNFT ceramics is shown in Fig. 3. A giant dielectric constant (up to the order of 105) with frequency dispersion is observed in the system. Two dielectric relaxation processes are clear in the doped samples. The low-temperature one was marked by Peak I, while the high-temperature one was marked by Peak II in Fig. 3. It is noted that the 100-Hz peak does not occur completely due to high conductivity at high temperatures. All dielectric relaxation peaks move toward higher temperature with the increase of the measuring frequencies (marked by arrow), which indicates a thermally activated process. The relaxation peaks are associated with interface polarization or oxygen-vacancy hopping, which is similar to Refs. [4, 11]. The origin of the relaxations could be revealed by impedance spectroscopy.
Temperature dependence of the dielectric constant (ε’) and loss factor of the BNFT ceramics at 10 kHz are shown in Fig. 4. All samples present a good temperature stability of dielectric constant in a broad temperature range from 450 to 700 K. Moreover, the dielectric loss is reduced significantly between 350 and 700 K for x = 0.01, presumably as the conductivity decreases. The dielectric loss factor of the sample is lower than 0.2 near 380 K.
Figure 5 shows the frequency dependence of the real part (ε’) of dielectric constant and dielectric loss factor (tanδ) of BNFT ceramics. A high (ε’) value is observed at low frequencies, which is related to the interface polarization between ceramics and electrode [12]. The ε’ of all these samples displays a high value (~6500) and a small frequency-dependence from 1 kHz to 1 MHz. As shown in Fig. 5, the dielectric loss measured from 40 Hz to 10 kHz decreases firstly with increasing the donor Ti4+ and then increases. It suggests that the resistance of grain boundaries has the same change. Generally, impedance spectroscopy analyses could reveal the mechanism responsible for the dielectric behavior of BNFT system, particularly to obtain information on non-intrinsic dielectric response. Figure 6 shows Cole–Cole plots (a plot drawn between imaginary and real parts of the impedance) at various temperatures. For all samples, two semicircular arcs are observed in the complex plane, which can be fitted using the simplified equivalent circuit consisting of two parallel RC elements connected in series with one RC element R g C g, representing the grain interiors, R gb C gb, representing the grain boundary responses. The simplified equivalent circuit is shown in the inset of Fig. 6a. The high-frequency semicircular arc is attributed to grain response (inset in each picture in Fig. 6), while the big arc corresponds to grain boundary response. Although not all the plots are depressed semicircles, it also can be seen the center of the circles do not fall on the real axis but fall on a straight line below the real axis. It suggests the relaxations are out of pure Debye type. For two parallel RC elements connected in series, Z(ω), Z’(ω) and Z”(ω) can be expressed as
so
and
where R and C are the resistance and capacitance, respectively. The subscripts g and gb refer to the grains and grain boundaries, respectively. ω is the angular frequency. Therefore, the giant dielectric constant in BNFT system can be explained by the IBLC model. In general, the higher the resistance of grain boundaries, the lower the dielectric loss in the low-frequency region. Ceramics whose resistance decreases with increasing temperature present a negative temperature coefficient (NTC) behavior [9]. This is due to the appearance of space charges and thermally activated charge carriers at high temperatures, as Pastor [13] and Liu et al. [14] have explained.
In order to make sure the physical nature of dielectric relaxations of the BNFT ceramics, normalized imaginary part of impedance of BNFT at different temperatures is plotted in Fig. 7. Each curve has a peak corresponding to a frequency at a given temperature. For a thermal activation behavior, the relationship between frequency and temperature follows an Arrhenius law [14], which is given by
where ω is angle frequency, ω 0 is pre-exponential factor, k B is the Boltzmann constant 8.617 × 10−5 eV/K, T is Kelvin temperature, E rel is the activation energy of thermally activated carriers. Activation energy can be obtained: E a = 1.03 eV for x = 0.00, E a = 0.59 eV for x = 0.01, E a = 0.52 eV for x = 0.03 and E a = 0.48 eV for x = 0.05, according to the slope of the straight line obtained from lnω–1000/T plot in Fig. 8. The decrease of E a with increasing Ti4+ content may be associated with the ionic disorder because the transportation of charge carriers could be enhanced in a more disorder system or lattice distortion structure [9].
Arrhenius plots of the relaxation frequency of BNFT ceramics. The squares are the experimental points, and the solid line is the least-squares straight-line fit according to Eq. (1)
Figure 9 shows the frequency dependence of the ac conductivity σ ac at various temperatures. The σ ac remains near constant value at low frequency but increases with increasing frequency due to Anderson-localized charge carriers contribution to the conductivity by hopping process [15, 16]. The increase of σ ac with increasing temperature could be attributed to thermally activated defect carries, such as localized charge carries or oxygen vacancies [9]. Obviously, the conductivity can be expressed by the so-called universal dielectric response law [17].
Frequency dependence of the AC conductivity at different temperatures for a x = 0.00, b x = 0.01, c x = 0.03, d x = 0.05. The symbols are the experimental results. The solid lines are the best-fitting result according to Eq. (2)
where σ dc is the dc bulk conductivity, σ 0 and s (0 < s ≤ 1) are two temperature-dependent adjusting constants. Equation (5) describes typical thermally assisted tunneling between localized states. The law describes one phenomenon that is associated with many-body interactions between charges and dipoles [18, 19]. The temperature dependence of σ dc follows the Arrhenius law given by:
where σ 0 is pre-exponential factor, k B is the Boltzmann constant, E con is the dc activation energy of conduction. The conductivity activation energy (E con) of both grain and grain boundary are calculated and listed in Table 1, from the slope of the straight line obtained from lnσ dc–1000/T plot in Fig. 10. The conductivity activation energy of grain boundary decreases with the increasing Ti concentration, which suggests the increase of oxygen-vacancy concentration. It is known donor doping will restrict the concentration of oxygen vacancies; however, the substitution of Ti4+ (donor) for Fe3+ leads to an increase of oxygen vacancies. This could be associated with the concentration fraction of single-ionized and doubly ionized oxygen vacancies, as shown in Eqs. (7)–(10). In stoichiometric compounds, oxygen vacancies could be easily created by loss of oxygen from the crystal lattice during sintering at high temperatures or at low oxygen-partial-pressure sintering [20].
Arrhenius plots of the DC conductivity of BNFT ceramics. The squares are the experimental points, and the solid line is the least-squares straight-line fit according to Eq. (3)
where \( V_{O} \) is the loss of lattice oxygen, \( V_{O}^{ \cdot } \) or \( V_{O}^{ \cdot \cdot } \) is the presence of single-ionized and doubly ionized oxygen vacancy, respectively, and ε’ is the released or captured electron [14].
The electron ionized from oxygen vacancy can be bonded to Fe3+ in the form of
With the increasing Ti concentration, Fe2+ could be easily created by the substitution of Fe3+ by Ti4+, which suggests the increase of single-ionized oxygen vacancies.
The conductivity activation energy of grain for x = 0.00, 0.01, 0.03 and 0.05 is similar to the potential barrier of the hopping electron between the Fe3+ and Fe2+ ions (0.29 eV) [21]. Considering the charge balance, Fe3+ ion is reduced to compensate the existence of oxygen vacancies. It has been reported [14] that the oxygen vacancies exist in the single-ionized state with activation values in the range 0.3–0.5 eV and 0.6–1.2 eV for doubly ionized oxygen vacancies, which can act as an acceptor to introduce the p-type carries [22]. Consequently, the dielectric loss factor is depressed for small donor doping (x = 0.01) in the BNT ceramics at low frequencies; however, it increases for the high-concentration doping. Meanwhile, no obvious change of dielectric loss factor can be found at high frequencies, as shown in Fig. 4. It suggests the substitution of Fe3+ by Ti4+ makes an influence at grain boundaries rather than grains. The migration of oxygen vacancy is restricted for the small-concentration doping, as shown in Fig. 4, which also leads to the decrease of dielectric loss factor. However, the loss factor increases for the high-concentration doping (Fig. 4) due to both Fe3+ and Ti4+ ionizing. For pure BFN, the concentration of doubly ionized oxygen vacancy is dominant according to the values of activation energy (Table 1). However, the activation energy reduces significantly for the Ti4+ doped into the BFN, which indicates that single-ionized oxygen vacancy occurs according to the value of the activation energies at grain boundaries. Single-ionized oxygen vacancy increases while doubly ionized oxygen vacancy decreases for further increasing the donor Ti4+; meanwhile, the concentration of single-ionized oxygen vacancy also increases due to the electron captured by Fe3+. Therefore, the activation energy of oxygen vacancy decreases monotonously.
4 Conclusions
Lead-free Ba(Nb0.5Fe0.5−x Ti x )O3 (x = 0.00, 0.01, 0.03, 0.05) ceramics have been fabricated by the conventional solid-state reaction. The dielectric properties and conductivity mechanism were investigated by impedance spectroscopy and universal dielectric response law. Dielectric loss factor is depressed for the small-concentration doping but enhanced for high-concentration substitution. The migration of oxygen vacancy is restricted by the small-concentration Ti4+ doping. The activity energies of dielectric relaxation for x = 0.00, 0.01, 0.03 and 0.05 are 1.03 eV, 0.59 eV, 0.52 eV and 0.48 eV, respectively. The decrease of the activation energies associates with the defect compensation and the concentration fraction between single-ionized oxygen vacancy and doubly ionized oxygen vacancy.
References
C.C. Homes, T. Vogt, S.M. Shapiro, S. Wakimoto, A.P. Ramirez, Science 293, 673 (2001)
S.Y. Chung, I.D. Kim, S.J. Kang, Nat. Mater. 3, 774 (2004)
S. Saha, T.P. Sinha, J. Phys.: Condens. Matter 14, 249 (2002)
Z. Wang, X.M. Chen, L. Ni, X.Q. Liu, Appl. Phys. Lett. 90, 022904 (2007)
F. Zhao, Z. Yue, J. Pei, D. Yang, Z. Gui, L. Li, Appl. Phys. Lett. 91, 052903 (2007)
S. Saha, T.P. Sinha, Phys. Rev. B 65, 134103 (2002)
P.K. Patel, K.L. Yadav, H. Singh, A.K. Yadav, J. Alloys Compd. 591, 224 (2014)
I.P. Raevski, S.A. Prosandeev, A.S. Bogatin, M.A. Malitskaya, L. Jastrabik, J. Appl. Phys. 93, 4130 (2003)
Y.M. Huang, D.P. Shi, L.J. Liu, G.Z. Li, S.Y. Zheng, L. Fang, Appl. Phys. A 114, 891 (2014)
G.D. Dwivedi, A.G. Joshi, H. Kevin, P. Shahi, A. Kumar, A.K. Ghosh, H.D. Yang, S. Chatterjee, Solid State Commun. 152, 360 (2012)
M. Gangulya, S. Paridaa, E. Sinhaa, S.K. Routa, A.K. Simanshub, A. Hussainc, I.W. Kimc, Mater. Chem. Phys. 131, 535 (2011)
L.J. Liu, H.Q. Fan, L. Wang, X.L. Chen, P.Y. Fang, Philos. Mag. 88, 537 (2008)
M. Pastor, J. Alloys Compd. 463, 323 (2008)
L.J. Liu, Y.M. Huang, C.X. Su, L. Fang, M.X. Wu, C.Z. Hu, Appl. Phys. A 104, 1047 (2011)
S.R. Elliott, Adv. Phys. 36, 135 (1987)
A.R. Long, Adv. Phys. 31, 553 (1982)
A.K. Jonscher, Dielectric relaxation in solids (London: Chelsea). J. Phys. D Appl. Phys. 32, 57 (1999)
A.K. Jonscher, Nature 267, 673 (1977)
A.K. Jonscher, J. Mater. Sci. 16, 2037 (1981)
G.Z. Li, Z. Chen, X.J. Sun, L.J. Liu, L. Fang, B. Elouadi, Mater. Res. Bull. 65, 260 (2015)
K. Kalantari, I. Sterianou, S. Karimi, M.C. Ferrarelli, S. Miao, D.C. Sinclair, L.M. Reaney, Adv. Funct. Mater. 21, 3737 (2011)
P.F. Liang, Y.Y. Li, Y.Q. Zhao, L.L. Wei, Z.P. Yang, J. Appl. Phys. 113, 224102 (2013)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 11264010, 11564010, 51402196), the Natural Science Foundation of Guangxi (GA139008) and the China Postdoctoral Science Foundation (Grants 2014M552229 and 2015T80915).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, X., Deng, J., Liu, S. et al. Grain boundary defect compensation in Ti-doped BaFe0.5Nb0.5O3 ceramics. Appl. Phys. A 122, 864 (2016). https://doi.org/10.1007/s00339-016-0407-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-016-0407-y