Abstract
The Ca1−xLaxCu3Ti4O12 (0 ≤ x ≤ 0.3) ceramics have been successfully prepared by the traditional solid-state reaction method at 1090 °C for 10 h. Effects of La3+ doping on the structure and negative temperature coefficient electrical properties of Ca1−xLaxCu3Ti4O12 ceramics are investigated in detail. Scanning electron microscope images demonstrate that the grain size of ceramic samples decreases with the increasing La3+ content. X-ray photoelectron spectroscopy analysis further confirms the coexistence of Cu+/Cu2+ and Ti3+/Ti4+ ions, which is one of the considerable contributors to the electrical conductivity of Ca1−xLaxCu3Ti4O12 ceramics. All the prepared ceramics show a linear relationship between the natural logarithm of the resistivity and the reciprocal of absolute temperature, indicating NTC characteristics. The obtained values of ρ25, B200/400 and Ea for the thermistors are in the range of 2.00 × 105–5.22 × 107 Ω cm−1, 2644–4205 K, 0.228–0.363 eV, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Negative temperature coefficient (NTC) thermistors are thermally sensitive resistors whose resistance exhibits a decrease as temperature increases. A necessary condition for their NTC behavior is the electron jump between neighbour ions of the same type and having different valences [1, 2]. NTC thermistors are widely used for aerospace, temperature compensation and control [1]. Classical NTC materials usually consist of transition metal oxides with spinel structure (AB2O4) [3].
Lately, there has been a rising interest in studying NTC behavior of complex oxide materials for thermistor applications. The perovskite-like material CaCu3Ti4O12 (CCTO) has been widely studied owing to its interesting dielectric properties [4,5,6,7,8,9]. The high dielectric constant of CCTO enable it to be considered as a potential candidate for microelectronic applications such as resonators, filters and cellular phones [7, 9, 10]. Moreover, after comprehensive investigations into its intrinsic mechanism, the hints to the giant dielectric constant of CCTO are focused on the Internal Barrier Layer Capacitance mechanism, suggesting that CCTO ceramic is composed of semiconducting grains and insulating grain boundaries [11], a necessary condition for NTC behavior. What’s more, CCTO contains oxygen deficiencies, which can be compensated by low-valence Ti3+ and Cu+ ions [12]. Bearing in mind that it has the characteristic of semiconductivity, CCTO material can be used for NTC thermistors. However, previous researches on CCTO mostly focus on improving its dielectric and nonlinear electrical properties by element doping [10, 13,14,15]. We have investigated the NTC electrical performances and conduction mechanism of Cu-sited doped CCTO (CaCu3-xMnxTi4O12) ceramics [16]. Based on previous work, considering the ionic radii of Ca2+ (1.34 Å) and La3+ (1.36 Å) [17] with 12-fold coordination number are similar, we propose that by doping La3+ ions on Ca sites, solid solutions can be formed and the NTC electrical properties of CCTO can be adjusted. The main aim in the present work is to research the effect of La3+ doping on the structure and NTC electrical performance of Ca-sited doped CaCu3Ti4O12 ceramics.
1.1 Experiment
The Ca1−xLaxCu3Ti4O12 (x = 0.0, 0.1, 0.2, 0.3) polycrystalline samples were synthetized by the traditional solid-state method. The starting materials were CaCO3 (≥ 99%), La2O3(≥ 99.99%), CuO (≥ 99%) and TiO2 (≥ 99.0%). The raw materials were weighed on the basis of their stoichiometric ratio and ground in mortar for 6 h. Then the powders were calcined at 900 °C in air for 6 h. The calcined powders were then pressed into the cylindrical pellets with about 10 mm in a diameter, then cold isostatic pressing at 300 MPa was used to improve density. All the pellets were sintered at 1090 °C for 10 h.
The crystal structure of as-sintered specimen is studied by X-ray diffraction (XRD; BRUKERD8-ADVANCE, Cu Kα radiation) analysis. The surface microstructure and the compositional distribution of the ceramic samples were analyzed by the Scanning Electron Microscope (SEM; Zeiss SUPRA 55 VP, Germany) in combination with energy dispersive spectroscopy (EDS). X-ray Photoelectron Spectroscopy (XPS; Thermo Scientific, K-Alpha +) was adopted to investigate the chemical states of the ceramic samples. So as to obtain the electrical conductivity, the both parallel sides of the ceramics were coated with platinum paste, and then annealed at 800 °C for 30 min. The relationship between resistances and temperature were measured from 25 up to 400 °C by a digital multimeter.
2 Results and discussion
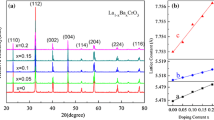
Figure 1 shows the XRD patterns of Ca1−xLaxCu3Ti4O12 ceramics sintered at 1090 °C for 10 h. All the diffraction peaks in XRD patterns for all samples were completely indexed to be the CCTO phase having a body-centered cubic structure with space group Im-3. The structure was refined by using TOPAS. Lattice constant of the Ca1−xLaxCu3Ti4O12 ceramics is listed in Table 1. From the Table 1, it can be seen that the lattice constant increased with increasing La content. This result can be understood based on the fact that the substitution of larger La3+ for Ca2+ causes a lattice expansion and thereby resulting in an increase in the lattice constant. But a small amount of secondary phase (CaTiO3) was also detected in the La3+-doped CCTO ceramics. The appearance of CaTiO3 may be relevant to the liquid phase sintering mechanism of CCTO and the doping of La3+. The sintering mechanism of CCTO suggests that the rate of grain growth of CCTO ceramics is inhibited by CaTiO3 solid particles, and the second phase CaTiO3 can be incorporated into CCTO lattices to form a solid solution in the last stage of sintering processes [18], which is in accordance with following SEM results. The doping of La3+ may reduce the solid solubility limit of CaTiO3 in CCTO ceramics, and result in the appearance of second-phase CaTiO3.
The SEM morphology of the polished and thermally etched surfaces of ceramic specimens is shown in Fig. 2. The specific process of thermal etching for the ceramic samples is as follows: the polished ceramic samples were thermally etched at 950 °C for 40 min in air atmosphere. It can be seen that the substitution of La3+ for Ca2+ has an obvious effect on the microstructure of CCTO ceramics. Discontinuous grain growth occurred in all the ceramic samples, and the terrace-ledge and bump area morphologies observed on the grain surfaces for the ceramics may result from a spiral growth of crystals by screw dislocation [5]. This morphology is present under thermal etching, and is easy to develop at lower etching temperatures or shorter etching times [5]. It is also noted that the grain size of ceramic samples decreased with the increasing La3+ content. This is mainly attributed to the appearance of second-phase CaTiO3 produced by the doping of La3+, which impedes the movement of grain boundaries, thus reducing the growth of grains. This result is agreement with the discussion based on the XRD results. From Fig. 2, it can be seen that there is a pore in the Ca0.7La0.3Cu3Ti4O12 (x = 0.3) ceramic samples. This result can be explained as follows [19]: with the assist of liquid phase, the rate of grain boundary movement is significantly larger than that of pore migration, which may result in the generation of residual pores.
The EDS analysis was used to confirm the compositional distributions of the Ca1−xLaxCu3Ti4O12 ceramics. The atomic percentages of elements are listed in Table 2 where the molar ratio is also presented. From the Table 2, it can be seen that the ratio of Ca/La is close to the stoichiometric ratio, which is consistent with our experimental design.
Figure 3 shows the fitted XPS results of Cu 2p3/2. The peaks with highest and intermediate energy correspond to Cu2+, and the highest binding energy is attributed to the coordination of Cu2+ with six oxygen atoms [20]. The lowest binding energy states of three components of Cu 2p3/2 peak are attributed to Cu+ [21]. These results identify the existence of Cu+ and Cu2+ in ceramic specimens. From Fig. 3, we can obtain the Cu 2p3/2 curve-fitted line which is a superposition of lines of the calibration compounds with Cu+ and Cu2+, as shown in Fig. 4. It can be seen that peak positions of Cu 2p3/2 exhibited a chemical shift to lower binding energy with increasing La3+ contents, suggesting an increase in the concentration of Cu+ ions.
Ti 2p XPS spectra are shown in Fig. 5. The peaks of 2p3/2 and 2p1/2 peaks can be divided into two peaks, suggesting the contribution from Ti3+ and Ti4+. These results are in accordance with previous studies [22]. From Fig. 5, we can obtain the Ti 2p curve-fitted line which is a superposition of lines of the calibration compounds with Ti3+ and Ti4+, as shown in Fig. 6. It can be seen that peak positions of Ti 2p exhibited a chemical shift to lower binding energy with increasing La3+ contents, suggesting an increase in the concentration of Ti3+ ions.
The plots of natural logarithm of the resistivity (lnρ) dependence on the reciprocal absolute temperature (1000/T) for the NTC thermistors are shown in Fig. 7. As can be seen the resistivity decreased with increasing temperature, indicating the NTC behavior. It is also pointed out that the relationship between the lnρ and 1000/T is approximate in linear over the measurement region of temperature. The linearity is based on the small polaron hopping transport model [23], which is able to be expressed by the following equation: ρ = ρ0exp(Ea/kT), where ρ0 is the resistivity as the temperature tends to infinity, Ea is the activation energy, k is the Boltzmann constant, and T is the absolute temperature. According to previous study [24], the electronic transport for CCTO is described by small-polaron hopping which is able to be explained as follows: (1) Cu2+ becomes non-stable and reduces to Cu+ during the heating process, which can be compensated by the substitution of Ti4+ on the Cu site to maintain the charge balance. This procedure can be described by the reaction \({\text{Ti}}_{Ti}^{ \times } + 2{\text{Cu}}^{2 + } \to 2{\text{Cu}}^{ + } + {\text{Ti}}_{Cu}^{ \cdot \cdot }\); (2) The Cu+ will re-oxidized to Cu2+ to accompanied by the released electrons entering the Ti 3d conduction band when cooling down, which results in the reduction of Ti4+–Ti3+. The expression of this process can use the reaction Cu+ + Ti4+ → Cu2+ + Ti3+. This explanation indicates that the major carrier is electron and CCTO is a n-type semiconductor [25,26,27]. Thus, small-polaron hopping transport mechanism for the CCTO is attributed to the electron hopping between the Cu2+ and Cu+ cations, and between Ti4+ and Ti3+ cations. Besides, we can detect that the resistivity reduced with rising La3+ content. When La3+ substitutes for Ca2+, there is a formation of electron, which results in a higher electron concentration, thereby reducing the energy barrier for polaron hopping, and reducing the resistivity. That is to say, the substitution of La3+ for Ca2+ promotes the conversion of Cu2+–Cu+ and Ti4+–Ti3+. The defect formation mechanism can be formulated by the reactions: \({\text{La}}_{2} {\text{O}}_{3} \to 2{\text{La}}_{\text{Ca}}^{ \cdot } + 2e' + 3{\text{O}}_{\text{O}}^{ \times }\), \({\text{Cu}}^{2 + } + e' \to {\text{Cu}}^{ + }\), \({\text{Ti}}^{4 + } + e' \to {\text{Ti}}^{3 + }\). The results presented herein are consistent with previous XPS analysis. The increasing of Cu+ and Ti3+ concentrations will decrease the resistivity due to the n-type conductivity of the CCTO. According to our previous XRD results, a small amount of secondary phase CaTiO3 is present in the La3+-doped CCTO ceramics. The appearance of second-phase CaTiO3 is in favor of the hopping conductivity due to the charge transport produced from doubly charged oxygen vacancies and electrons [28], and thus decreasing the resistivity.
The resistivity at 25 °C, B200/400 and Ea for NTC materials are shown in Table 1. The sensitivity of thermistor material can be described by constant B which can be calculated as follows:
where R1 is the resistance at temperature T1, and R2 is the resistance at temperature T2. As can be obtained from Table 1, the values of ρ25,B200/400 and Ea are in the range of 2.00 × 105–5.22 × 107 Ω cm−1, 2644–4205 K, 0.228–0.363 eV, respectively. In addition, it can be seen that the Ea values decreased with the increasing La3+ content. When the La3+ ion is doped into the CCTO ceramics, the Cu+ and Ti3+ concentrations will increase based on the following defect formation mechanism: \({\text{La}}_{2} {\text{O}}_{3} \to 2{\text{La}}_{\text{Ca}}^{ \cdot } + 2e^{\prime} + 3{\text{O}}_{\text{O}}^{ \times }\), \({\text{Cu}}^{2 + } + e^{\prime} \to {\text{Cu}}^{ + }\), \({\text{Ti}}^{4 + } + e^{\prime} \to {\text{Ti}}^{3 + }\). This results in a higher electron concentration, thereby reducing the energy barrier for polaron hopping, and thus reducing the Ea values.
Figure 8 shows the ρ25 and B200/400 constant as a function of x. As shown in Fig. 8, it can be seen that the values of ρ25 and B200/400 decreased with the increasing La3+ content. This phenomenon is due to the substitution of La3+ for Ca2+ that lowers the energy barrier for polaron hopping, thus decreasing the resistivity and B values. All the results above demonstrate that the adjustment of electrical properties of the Ca1−xLaxCu3Ti4O12 NTC materials can be accomplished through altering the La3+ content.
3 Conclusion
The crystal structure, microstructure and NTC electrical properties of Ca1−xLaxCu3Ti4O12 ceramics have been investigated in the present study. The major phase of as-sintered ceramics is the CaCu3Ti4O12 phase. The grain size of the Ca1−xLaxCu3Ti4O12 ceramics decreases with increasing La3+ content. Through the XPS analysis, the co-existing of Cu+/Cu2+ and Ti3+/Ti4+ is confirmed, which are proposed to be the intrinsic conduction mechanism for its NTC properties. It is also found that the resistivity decreases with increasing La3+ content. In addition, the adjustment of electrical performance can be accomplished through altering the La3+ content. It is further concluded that these compounds can be applied as promising materials for NTC thermistors.
References
A. Feteira, J. Am. Ceram. Soc. 92, 967 (2009)
R.N. Jadhav, S.N. Mathad, V. Puri, Ceram. Int. 38, 5181 (2012)
K. Park, S. Yun, J. Mater. Sci.: Mater. Electron. 15, 359 (2004)
M.A. Subramanian, D. Li, N. Duan, B.A. Reisner, A.W. Sleight, J. Solid State Chem. 151, 323 (2000)
T. Fang, H. Shiau, J. Am. Ceram. Soc. 87, 2072 (2005)
B.S. Prakash, K.B.R. Varma, Phys. B 382, 312 (2006)
S.F. Shao, J.L. Zhang, P. Zheng, C.L. Wang, J.C. Li, M.L. Zhao, Appl. Phys. Lett. 91, 042905 (2007)
Y. Zhu, J.C. Zheng, L. Wu, J. Hanson, P. Northrup, W. Ku, A.I. Frenkel, Phys. Rev. Lett. 99, 037602 (2007)
B.P. Zhu, Z.Y. Wang, Y. Zhang, Z.S. Yu, J. Shi, R. Xiong, Mater. Chem. Phys. 113, 746 (2009)
J. Deng, X. Sun, S. Liu, L. Liu, T. Yan, L. Fang, B. Elouadi, J. Adv. Dielectr. 06, 1650009 (2016)
T.B. Adams, D.C. Sinclair, A.R. West, J. Am. Ceram. Soc. 89, 3129 (2006)
L. Ni, X.M. Chen, Appl. Phys. Lett. 91, 323 (2007)
N. Lei, M.C. Xiang, J. Am. Ceram. Soc. 93, 184 (2010)
J. Boonlakhorn, P. Kidkhunthod, N. Chanlek, P. Thongbai, J. Eur. Ceram. Soc. 38, 137 (2018)
J. Wang, Z. Lu, T. Deng, C. Zhong, Z. Chen, J. Eur. Ceram. Soc. 38, 3505 (2018)
B. Zhang, Q. Zhao, A. Chang, H. Ye, S. Chen, Y. Wu, Ceram. Int. 40, 11221 (2014)
M.J. Forbess, S. Seraji, Y. Wu, C.P. Nguyen, G.Z. Cao, Appl. Phys. Lett. 76, 2934 (2000)
S. Vangchangyia, E. Swatsitang, P. Thongbai, S. Pinitsoontorn, T. Yamwong, S. Maensiri, V. Amornkitbamrung, P. Chindaprasirt, J. Am. Ceram. Soc. 95, 1497 (2012)
D. Han, J. Zhang, P. Liu, G. Li, S. Wang, J. Eur. Ceram. Soc. 38, 3261 (2018)
P.R. Bueno, R. Tararan, R. Parra, E. Joanni, M.A. Ramírez, W.C. Ribeiro, E. Longo, J.A. Varela, J. Phys. D-Appl. Phys. 42, 55404 (2009)
J. Ghijsen, L.H. Tjeng, J.V. Elp, H. Eskes, J. Westerink, G.A. Sawatzky, M.T. Czyzyk, Phys. Rev. B 38, 11322 (1988)
L. Ni, X.M. Chen, Appl. Phys. Lett. 91, 122905 (2007)
A.N. Kamlo, J. Bernard, C. Lelievre, D. Houivet, J. Eur. Ceram. Soc. 31, 1457 (2011)
J. Li, M.A. Subramanian, H.D. Rosenfeld, C.Y. Jones, B.H. Toby, A.W. Sleight, Chem Mater 16, 5223 (2004)
F.D. Morrison, D.C. Sinclair, A.R. West, J. Am. Ceram. Soc. 84, 474 (2001)
M.A. Ponce, M.A. Ramirez, F. Schipani, E. Joanni, J.P. Tomba, M.S. Castro, J. Eur. Ceram. Soc. 35, 153 (2015)
M.H. Whangbo, M.A. Subramanian, Chem. Mater. 18, 3257 (2006)
W.L. George, R.E. Grace, J. Phys. Chem. Solids 30, 881 (1969)
Acknowledgments
We would like to acknowledge financial support from the National Natural Science Foundation of China (Grant No. 61871377) and the West Light Foundation of the Chinese Academy of Sciences (Grant No. 2015-XBQN-B-13).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, X., Zhang, B. & Chang, A. New negative temperature coefficient ceramics in La-doped CaCu3Ti4O12 system. J Mater Sci: Mater Electron 30, 10217–10223 (2019). https://doi.org/10.1007/s10854-019-01358-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01358-1