Abstract
Er3+ doped Gd2O3 phosphors were prepared by using solution combustion synthesis method. The structural characterization and the luminescence behaviour of the prepared phosphors were determined and compared. Average crystal size for the phosphors yielded was in nanometre range from 6 to 48 nm, which was calculated by using XRD and confirmed by TEM technique. The compositional analysis was done by FTIR. Qualitative information regarding the elemental composition of powder sample was measured using EDX spectroscopy. EDX and FT-IR spectra confirm presence of Er3+ in the Gd2O3:Er3+ phosphors. For thermoluminescence (TL) studies the TL glow curves were recorded under two different excitations i.e. UV and gamma exposures. All the factors like dopant concentration, heating rate, exposure dose etc. were optimized for both the excitations. Various Kinetic parameters were calculated for TL glow curve recorded for all the optimized conditions. Computerized glow curve deconvolution analysis was carried out for optimized TL glow curve. Different kinetic parameters were also calculated for all the deconvoluted peaks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Thermoluminescence is a well known phenomenon used for dosimetric purposes in the field of personnel, environmental and clinical dosimetry [1]. Thermoluminescence behaviour is based on the emission of light by heating a previously exposed sample. This phenomenon is observed due to development of localised energy level inside the forbidden energy band gap by the impurities. So that thermoluminescence dosimetry can be used for detecting the presence of defect levels in a sample [2]. TL is the phenomenon of releasing electrons trapped by defects within a crystal lattice by some ionizing radiations. The TL signal intensity and the irradiation dose provides the sample the potential application in dosimetry. The information regarding the trapping levels can be obtained by thermoluminescence measurements, which includes transfer of charge carriers to their respective traps by different irradiation sources [3–5].
Oxide phosphors are very much suitable for field emission display (FED), vacuum fluorescecnt display, plasma panel display and electroluminescence (EL) devices [6]. Rare earth oxide with trivalent impurities showed great scientific and technological interest for many years due to their attractive physical and chemical properties. Further, RE2O3 (RE = Dy, Eu, Er and Gd) compounds were widely used. Many investigators have focused their attention on the preparation and optical properties of this oxide material [7–10]. All of them, the Gd2O3 with Er3+ is used as high performance luminescent devices and other functional materials based on the electronic, optical and chemical characteristics arising from their 4f electrons, but its thermoluminescence properties is quite untouched by the authors knowledge [11].
In our previous work we have reported the thermoluminescence behaviour of various undoped and doped oxide phosphors [12–16]. Much work has been done in thermoluminescence study of rare earth oxide phosphors. We have reported thermoluminescence behaviour of Er3+ doped Gd2O3 phosphor prepared by solid state reaction method under UV exposure and gamma radiation. The TL glow curve was found at 128 °C for UV exposure and at 180 °C for gamma exposure. For gamma exposure the activation energy was found greater than UV exposure which was due to the formation of deeper traps under gamma radiation [17]. Afterwards effect of Yb3+ codoping on the thermoluminescence behaviour of Er3+ doped Gd2O3 phosphor prepared by solid state reaction method under UV exposure and gamma radiation has been studied. The study reveals that the phosphors gives dual peak in TL glow curve due to the formation of two different trap levels which were generated by two different impurities present in the phosphor. These samples also show similar behaviour with UV and gamma radiations. The activation energy for gamma radiation was more than UV exposure due to formation of deeper traps [18].
In the preset study, the thermoluminescence behaviour of Gd2O3:Er3+ phosphor prepared by combustion synthesis method has been carried out. The TL studies were performed on Gd2O3:Er3+ phosphor by irradiating with UV and gamma irradiations. The material is heated at different heating rate for its optimization and the light output was measured as a function of temperature of the material and a TL glow curve was plotted. By using of this optimized heating rate, other factors were optimized such as UV exposure time, effect of Er3+ concentration and gamma dose. The relationship between the TL intensity and the irradiation dose provides the sample for potential application in different field. The peak temperature is the measure of energy depth of the trapped electrons in the material, while the area under the TL peak often indicates the number of electrons transferred into these traps.
2 Experimental
2.1 Synthesis
Gd2O3:Er3+ phosphors were synthesized by the combustion method. Urea was used as fuel. The precursor materials Gd(NO3)3·6H2O (99.99%), Er(NO3)3·5H2O (99.95%) and urea (NH2CONH2) were dissolved in distilled water. A urea to metal nitrate molar ratio of 2:1 was employed to prepare well crystallized phosphor [19]. The solution was concentrated by heating until excess free water evaporated and spontaneous ignition occurred (Scheme 1). In the beginning, solution undergoes dehydration with the liberation of large amount of gaseous products. At the point of spontaneous combustion, the solution begins burning and releases lot of heat; the solution vaporized instantly and becomes a burning solid with liberation of gaseous by products such as oxide of carbons and nitrogen the combustion was finished. When it cooled down to room temperature, the resultant particles were crushed by mortar and pestle and then annealed at different temperatures 600, 700, 800 and 900 °C for 2 h [20].
To determine the influence of dopant Er3+ concentration on the luminescence properties, the Gd2O3:Er3+ phosphors with different Er3+ concentration (from 0.5 to 7%) have been prepared by using above mentioned procedure.
2.2 Instrument details
The crystallinity as well as the particle size of the phosphor was monitored by X-ray diffraction measurement. The X-ray powder diffraction data was collected by using PAN-analytical 3 kW X’pert Powder (Multifunctional), X-ray diffractometer using Cu LFF High Resolution X-ray tube. The X-rays were produced using a sealed tube and the wave length of X-ray was 0.154 nm. Molecular structure was determined by FTIR analysis done by Nicolet Instruments Corporation USA MAGNA-550. Energy dispersive X-ray analysis (EDX) was used for elemental analysis of the phosphor. Particle diameter and surface morphology of prepared phosphor determined by Transmission electron microscopy (TEM) using Philips CM-200. TL glow curve was recorded at room temperature by using TLD reader I1009 (Nucleonix Sys. Pvt. Ltd. Hyderabad).The obtained phosphor under the TL examination is given UV radiation using 254 nm UV source, gamma irradiation using Co60 source. All of the measurements were performed at room temperature.
3 Result and discussion
3.1 X-ray diffraction analysis
The structure and phase purity for the Er3+ doped Gd2O3 phosphors were confirmed by XRD analysis. In order to investigate the effect of temperature on structural properties of nanoparticles, the prepared phosphors were heated at 600 and 900 °C for 2 h, respectively (Fig. 1). The effect of Er3+ concentration on structural properties had also been studied with different Er3+ (0.5–1.5%) concentration annealed at 900 °C for 2 h (Fig. 2).
The crystal size was calculated from the XRD pattern using the Scherrer’s equation [21].
Here, D is the crystalline size of the (hkl) plane, λ is the wavelength of the incident X-ray radiation [(0.154056 nm)], β is the full width at half maximum (FWHM) in radians, and θ is the diffraction angle of the (hkl) plane. For monoclinic Gd2O3:Er3+, crystal, size was calculated with variable annealing temperature as well as for variable Er3+ concentration. For variable annealing temperature the crystal sizes were found in the range of 6–24 nm (Table 1). For variable Er3+ concentration the crystal size were found 24–48 nm at 900 °C annealing temperature (Table 2). It was observed that change in concentration of Er3+ ion does not have any appreciable effect on phase and the crystal size increases with increasing Er3+ concentration. It may be due to the small grain growth of Er3+ doped Gd2O3 phosphor as comprised with pure Gd2O3 phosphor [22]. All diffraction patterns are in good agreement with JCPDS no. 43-1015 confirmed monoclinic structure of prepared sample.
3.2 Transmission electron microscopic (TEM) analysis
The TEM images of the Gd2O3:Er3+ phosphor are presented in Fig. 3. It was used to characterize the morphology, structure and particle size for the sample. The images show that the size distribution was broad and the average diameter was 8–13 nm for freshly prepared phosphor. The average crystallite size for Gd2O3:Er3+ phosphor annealed at 900 °C is around 31 nm. These values are in good agreement with the one estimated from Scherer’s equation. [22].
3.3 Energy dispersive X-ray (EDX) spectroscopy
Prepared samples were analyzed by energy dispersive X-ray (EDX) analysis to obtain the chemical composition of the prepared phosphors. In the spectrum intense peak of Gd, Er and O are present which confirms the formation of Gd2O3:Er3+ phosphor (Fig. 4).
3.4 Thermoluminescence behaviour of Er3+ doped Gd2O3 phosphors
The TL response of the Gd2O3:Er3+ phosphor was recorded for two different radiations i.e. 254 nm UV exposure and 60Co gamma exposure. The TL glow curve of phosphor recorded under 254 nm UV exposure for 5 min exposure time at 5 °C s−1 heating rate has TL glow peak at 98 °C (Fig. 5). The TL glow curve of the phosphor recorded for gamma exposure of 1 kGy gamma dose at the heating rate of 5 °C s−1 has the TL glow peak at 188 °C. In TL glow curve of gamma dose irradiated sample peak position was found at higher temperature than UV irradiated sample because in gamma irradiation deeper traps were found, whereas UV irradiation forms shallow traps.
3.4.1 Effect of annealing on TL glow curve of Er3+ doped Gd2O3 phosphor
Annealing refers to a heat treatment given to a material so as to modify some of the material properties. It is a process in which the material is heated above its recrystallization temperature, for certain duration, maintaining a suitable temperature, and then finally cooled. Annealing is used to relieve internal stresses in the material there by refining the structure by making it more homogeneous. Considering the above facts the prepared phosphors were annealed at different temperatures ranging from 600 to 900 °C (Fig. 6). No change in peak position was observed with increasing annealing temperature for both UV and gamma exposures. The TL intensity increases with increasing annealing temperature. The increase in TL intensity with annealing temperature is due to the fact that the higher annealing temperature minimizes the residual TL signal by emptying the high temperature traps and restores the original sensitivity of glow curve [23].
3.4.2 Effect of heating rate on TL glow curve of Er3+ doped Gd2O3 phosphor
In TL measurements thermal quenching is a major problem that significantly depends on the heating rate used and, therefore, the heating rate of the material has been optimized. Therefore the TL glow curves of the Er3+ doped Gd2O3 phosphor were recorded as a function of heating rate for both UV and gamma exposures. To study the effect of heating rate for UV exposure the TL glow curve was recorded under variable heating rates from 4 to 8 °C s−1 for fixed 5 min UV exposure time (Fig. 7a–d). It has also been investigated for the phosphor exposed to gamma radiations for a dose of 1 kGy (Fig. 8a–d). It was observed that, with increases in the heating rate the peak intensities as well as the total area of the peaks increases, maximizes and then decreases. As the heating rate was increased from 4 to 7 °C s−1, a slight increase in the peak intensity was observed with peak temperature shifting to the higher temperature side. However with further increase in heating rate to 8 °C s−1, the TL intensity again decreased and peak shifts towards higher temperature. This behaviour can be explained by the fact that with lower heating rates the charge carries travelling towards there combination centres for producing the desired luminescence have enough time to get retrapped and do not involve in producing actual luminescence. Whereas, when the heating rate is high, the thermal quenching affects the TL intensity therefore TL intensity decreases at higher heating rates. This requires an optimum heating rate at which maximum recombination may occur. Using the above results the heating rate was optimized to 7 °C s−1 [24].
TL glow curve of Gd2O3:Er3+ (1%) phosphors for different heating rate at 5 min UV exposure time a for freshly prepared phosphor, b heating rate versus peak temperature for combustion synthesis fresh phosphor, c for phosphor annealed at 900 °C, d heating rate versus peak temperature for phosphor annealed at 900 °C
TL glow curve of Gd2O3:Er3+ (1%) phosphors for different heating rate at 1 kGy gamma ray a for freshly prepared phosphor, b heating rate versus peak temperature for combustion synthesis fresh phosphor, c for phosphor annealed at 900 °C, d heating rate versus peak temperature for phosphor annealed at 900 °C
3.4.3 Effect of radiation dose on TL glow curve
3.4.3.1 Effect of UV exposure time
Effect of UV exposure time on TL glow curve of the Gd2O3:Er3+ phosphor was studied. The TL glow curve for the phosphors were recorded using 5–20 min UV exposure time by heating the sample at a heating rate of 7 °C s−1 (Fig. 9a–d). It was observed that the TL peak is broad and less intense for lower UV exposure time. The intensity increases with increasing UV exposure time up to 15 min. The TL intensity decreased with further increase in UV exposure time. The UV exposure time do not affect the peak positions it only affects the intensity and broadening of the TL peak. Under UV radiation the TL response is generated from the traps present at the surface because UV radiation can’t have enough energy to penetrate deeper inside so that it will not induce lattice defects. As the UV exposure time increases the density of surface defects increases which leads to increase in peak intensity. After a certain UV exposure dose further increase in UV exposure the intensity starts to decrease this fall in TL intensity is usually a consequences of competition between radiative and non radiative centre or between different kinds of trapping centres [25, 26].
3.4.3.2 Effect of gamma exposure
Effect of gamma exposure was also studied for the Gd2O3:Er3+ phosphors have been studied. The TL glow curve for the phosphors was recorded using gamma irradiation in the dose range from 0.5 to 2 kGy (Fig. 10a–d). All the analysis was carried out at room temperature by warming the samples at a heating rate of 7 °C s−1. It was observed that the TL peak was broader in lower gamma doses and become well defined and more intense at higher gamma doses. The gamma dose forms the deeper trap levels. Due to the deeper traps no competition was observed between different trapping centres so that with increase in gamma dose continuous increase in intensity of glow curve is observed.
3.4.4 Effect of Er3+ concentration on TL glow curve for UV exposure
Effect of different concentration of Er3+ from 0.5 to 2 mol% on TL response of Gd2O3:Er3+ nanophosphor was studied (Fig. 11a–d). For this the TL glow curve with the variable concentration of Er3+ (0.5–2 mol%) were recorded for constant UV exposure of 15 min and at constant heating rate of 7 °C s−1. It was noticed that the integrated area of the curve for UV exposure increases with increasing Er3+ concentration up to 1 mol%. Further the glow peak structure remains same for all the concentration of Er3+; however the TL intensity decreases with Er3+ concentration. The TL glow curve shows that the dopant concentration (Er3+) shows the linearly up to 1 mol% of Er3+ than the concentration quenching occurs and the trap levels destroy. The TL intensity decreases further with increase in Er3+ concentration.
Effect of Er3+ concentration on TL glow curve of Gd2O3:Er3+ phosphor under UV exposure a for combustion synthesis fresh phosphor, b Er3+ concentration versus intensity for combustion synthesis fresh phosphor, c for phosphor annealed at 900 °C, d Er3+ concentration versus intensity for phosphor annealed at 900 °C
3.4.5 Analysis of trapping parameters for optimized TL glow curves
The trapping parameters order of kinetics, activation energy shape factor and other trapping parameters were calculated by applying Chen’s formula for the TL glow curve at all the optimized conditions [27]. The activation energy for TL glow curve for combustion synthesized fresh phosphor has value 0.66 eV for UV exposure and 0.814 eV for gamma exposure, for 900 °C annealed phosphor has value 0.66 eV for UV exposure and 0.806 eV for gamma exposure. Value of activation energy for gamma exposure is greater for all the phosphors which confirm the formation of deeper traps in the crystal lattice. The value of shape factor for UV exposure was in 0.482 and for gamma exposure the value is in between 0.517 the values are near to the 0.52 which shows that the prepared phosphor shows second order kinetics (Table 1).
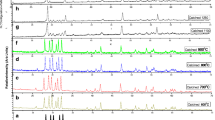
3.5 Analysis of glow curves by GCCD curves fitting and trapping parameters
The computerised deconvoluted curves and the theoretical curve fitted with the experimental curve for optimized conditions is recorded for nanocrystalline powder samples exposed to UV radiation as well as for gamma radiation (Fig. 12a–f). Trapping parameters of all the above peaks are also calculated and summarized in Table 2. From the data it is clear that all the peaks follow second order of kinetics and hence there is no retrapping takes place. The energy levels (activation energy) of various traps (corresponding to various peaks) are very much different. Therefore, it is clear that there are some deep and shallow traps. The competition among them might be giving various releasing and retrapping probabilities, which might have resulted in different frequency factors. The traps could be either electron traps or hole traps or of both kinds.
Computerized glow curve deconvolution of Gd2O3:Er3+ phosphors a for combustion synthesized fresh phosphor under UV exposure, b combustion synthesized fresh phosphor under gamma radiation, c combustion synthesized phosphor annealed at 900 °C under UV exposure, d combustion synthesized phosphor annealed at 900 °C under gamma radiation
Dosimetric characteristics of TL materials are mainly depending on kinetic parameters. Kinetic parameters quantitatively describe the trapping-emitting centres responsible for the TL emission. Therefore, determination of the kinetic parameters is an active area of research for better understanding of TL process. There are various methods for evaluating the trapping parameters. When the single glow peak is present, the methods such as peak shape, various heating rates and initial rise methods are suitable methods to determine trapping parameters. The kinetic parameter for deconvoluted glow peak have been calculated by using Chen’s peak shape method [27]. The activation energy lies in between 0.742–1.245 and 0.83–0.85 eV for UV exposed freshly prepared phosphor by combustion synthesis and annealed at 900 °C respectively. For gamma exposed samples activation energy lies in between 0.79–0.93 and 0.8–0.903 eV respectively. The value of shape factor lies in between 0.48 and 0.51 which is near to 0.52 so the results confirms the presence of second order kinetics for all the TL glow peaks (Table 2).
4 Conclusions
Er3+ doped Gd2O3 phosphor was prepared by using combustion synthesis method. The structural characterization confirms the formation of monoclinic phosphor by combustion synthesis method. The XRD results shows the phosphor have crystal size around 6–24 nm. The TL studies were carried out under UV and gamma exposure. Various parameters related to TL like heating rate, exposure time and annealing temperature were optimized for both the irradiations. Optimized heating rate for all the TL analysis is 7 °C s−1. The optimized UV exposure time for all the analysis was found at 15 min. The optimized Er3+ concentration for TL analysis was 1 mol%. The trapping parameters were calculated for the TL glow curve at all the optimized conditions. It was observed that the value of activation energy for gamma exposure is greater for all the phosphors which confirm the formation of deeper traps in the crystal lattice. The CGCD was applied on the TL glow curve recorded at optimized condition for both UV and Gamma exposure. The value of shape factor confirms the presence of second order kinetics for all the TL glow peaks.
References
F. Daniels, C.A. Boyd, D.F. Saunders, Science 117, 343 (1953)
P.D. Townsend, J.C. Kelly, Colour Centres and Imperfections in Insulators and Semiconductors (Sussex University Press, London, 1993), p. 70
R. Chen, S.W.S. Mckeever, Theory of Thermoluminescence and Related Phenomenon (World Scientific Publisher, Beijing, 1997)
P. Aliberti, S.K. Shrestha, R. Li, M.A. Green, G.J. Conibeer, J. Cryst. Growth 327, 84–88 (2011)
R. Chen, Y. Kirsh, Analysis of Thermally Stimulated Process (Pergamon, Oxford, 1981)
S. Erdei, R. Roy, G. Harshe, H. Juwhari, D. Agarwal, F.W. Ainger, W.B. White, Mater. Res. Bull. 30, 745–753 (1995)
Y.L. Song, Q.W. Tian, R.J. Zou, Z.G. Chen, J.M. Yang, J.H. Hua, J. Alloys Compd. 509, 6539–6544 (2011)
K. Bernot, L. Bogani, A. Caneschi, D. Gatteschi, R. Sessoli, J. Am. Chem. Soc. 128, 7947–7956 (2006)
V.P. Menushenkov, J. Magn. Magn. Mater. 290, 1274–1277 (2005)
N. Dhananjaya, H. Nagabhushana, B.M. Nagabhushana, B. Rudraswamy, C. Shivakumara, R.P.S. Chakradhar, J. Alloys Compd. 509, 2368–2374 (2011)
X. Zhang, J. Wang, K. Guo, H. Chen, X. Yang, J. Zhao, J. Alloys Compd. 517, 149–156 (2012)
R.K. Tamrakar, K. Upadhyay, D.P. Bisen, Gamma ray induced thermoluminescence studies of yttrium(III) oxide nanopowders doped with gadolinium. J. Radiat. Res. Appl. Sci. 7(4), 526–531 (2014)
R.K. Tamrakar, D.P. Bisen, K. Upadhyay, S. Tiwari, Synthesis and thermoluminescence behavior of ZrO2:Eu3+ with variable concentration of Eu3+ doped phosphor. J. Radiat. Res. Appl. Sci. 7(4), 486–490 (2014)
R.K. Tamrakar, V. Dubey, Synthesis, structural characterization and thermoluminescence glow curve study of gadolinium-doped Y2O3 nanophosphor. J. Taibah Univ. Sci. 3(10), 317–323 (2014)
R.K. Tamrakar, N. Tiwari, R.K. Kuraria, D.P. Bisen, V. Dubey, K. Upadhyay, Effect of annealing temperature on thermoluminescence glow curve for UV and gamma ray induced ZrO2:Ti phosphor. J. Radiat. Res. Appl. Sci. 8(1), 1–10 (2015)
R. Tamrakar, V. Dubey, N.K. Swamy, R. Tiwari, S.V.N. Pammi, P.V. Ramakrishna, Thermoluminescence studies of UV-irradiated Y2O3:Eu3+ doped phosphor. Res. Chem. Intermed. 39(8), 3919–3923 (2013)
R.K. Tamrakar, D.P. Bisen, I.P. Sahu, N. Brahme, UV and gamma ray induced thermoluminescence properties of cubic Gd2O3:Er3+ phosphor. J. Radiat. Res. Appl. Sci. 7(4), 417–429 (2014)
R.K. Tamrakar, D.P. Bisen, Thermoluminescence studies of ultraviolet and gamma irradiated erbium(III)-and ytterbium(III)-doped gadolinium oxide phosphors. Mater. Sci. Semicond. Process. 33, 169–188 (2015)
S.K. Singh, K. Kumar, S.B. Rai, Multifunctional Er3+–Yb3+ codoped Gd2O3 nanocrystalline phosphor synthesized through optimized combustion route. Appl. Phys. B 94(1), 165–173 (2009)
R.K. Tamrakar, D.P. Bisen, K. Upadhyay, Effect of annealing on down-conversion properties of monoclinic Gd2O3:Er3+ nanophosphors. Luminescence (2014). doi:10.1002/bio.2824
A. Guinier, X-Ray Diffraction (Freeman, San Francisco, 1963)
V.D. Mote, J.S. Dargad, B.N. Dole, Effect of Mn doping concentration on structural, morphological and optical studies of ZNO nano particles. Nanosci. Nanoeng. 1(2), 116–122 (2013)
Y.S. Horowitz, TL Dose Response, in Thermoluminescence and Thermoluminescence Dosimetry (CRC Press, Boston, 1984), p. 89
S. Bahl, A. Pandey, S.P. Lochab, V.E. Aleynikov, A.G. Molokanov, P. Kumar, Synthesis and thermoluminescence characteristics of gamma and proton irradiated nanocrystalline MgB4O7:Dy, Na. J. Lumin. 134, 691–698 (2013)
V. Pagonis, R. Chen, J.L. Lawless, A quantitative kinetic model for Al2O3:C:TL response to ionizing radiation. Radiat. Meas. 42(2), 198–204 (2007)
J.L. Lawless, R. Chen, D. Lo, V. Pagonis, A model for non-monotonic dose dependence of thermoluminescence (TL). J. Phys. Condens. Matter 17(4), 737 (2005)
R. Chen, Y. Kirsh, The Analysis of Thermally Stimulated Processes (Pergamon Press, Oxford, 1981)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamrakar, R.K., Upadhyay, K. Thermoluminescence behaviour of Gd2O3:Er3+ phosphor prepared by combustion synthesis method. J Mater Sci: Mater Electron 28, 4267–4278 (2017). https://doi.org/10.1007/s10854-016-6050-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6050-8