Abstract
The Yb3+ doped Gd2O3 phosphors have been prepared by combustion route method. The structural, morphological, compositional and vibrational analysis of the Yb3+ doped Gd2O3 phosphor have been studied by X-ray diffraction, transmission electron microscopy, energy dispersive X-ray and Fourier transform infrared spectroscopy. The results indicated that Yb3+ doped Gd2O3 phosphor synthesized by combustion had particle size ranging from 15 to 38 nm, for the different concentration of the Yb3+. The optical and luminescence properties of prepared phosphors had been studied by using 980 nm laser excitation. The effects of Yb3+ doping and annealing temperature on luminescence properties were also studied. Photoluminescence spectra showed that nanocrystals exhibited emission at near infrared region corresponding to different Stark labels of Yb3+ ions. The theoretical mechanism of luminescence was explored. The results indicate that this material may be suitable for infrared laser devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years much attention has been paid for the development of efficient luminescent materials in phosphor industry for different applications. Luminescence properties of these materials depend upon so many factors such as synthesis method, size, shape, phase and doping concentration. The Yb3+ ion attract us for its simple electronic structure, having only two level 2F7/2lower and upper level 2F5/2 which are separated by about 10,000 cm−1 shows high quantum efficiency, low quantum defects, large excited-state absorption and energy-transfer upconversion [1–5].
Oxide host phosphors constitutes as one of the important luminescent materials which can have high stability, low synthesis temperature, high luminescence efficiency etc. [6–8]. The rare earth and transition metal ions activated Gd2O3 phosphor show superior properties for many applications such as displays, catalysts, sensors, fuel cells etc. Till date various approaches namely sol-gel, hydrothermal, co-precipitation, solid state reaction etc. were used for the preparation of Gd2O3 phosphors [9–12].
In the present paper we focus to develop high efficiency and rich performing laser application materials. Among them, we choose combustion synthesis method to synthesize Gd2O3:Yb3+ phosphor. The preparation method offers a great advantage such as low cost, flexibility on choosing host materials, high yield, large surface area etc. [13, 14]. On realizing the importance of the host matrix and rare earth ion activators, the present investigation was carried out. Yb3+ activated Gd2O3 phosphor were prepared by low temperature combustion reaction in air atmosphere using Urea as a fuel.
Experimental
Nanophosphor of Gd2O3 doped with 5 mol% - 45 % Yb3+ ions were prepared via a combustion synthesis procedure. For preparation of phosphor aqueous solution containing Urea, Gd(NO3)3 and Yb(NO3)3 were prepared. A constant percentage of urea and Gd2O3 in ratio of 2:1 was mixed to prepare the precursor solution. The solution was concentrated by heating until excess free water evaporated and spontaneous ignition occurred. In the beginning, solution undergoes dehydration with the liberation of large amount of gaseous products. At the point of spontaneous combustion, the solution begins burning and releases lot of heat; the solution vaporizes instantly and becomes a burning solid with liberation of gaseous by- products such as oxide of carbons and nitrogen until the combustion was finished. When it was cooled down to room temperature, the resultant particles were crushed by mortar and pestle and then annealed at different temperatures in the range 600 °C to 1400 °C for 2 h [15–18].
The crystallinity as well as the particle size of the phosphor was monitored by X-ray diffraction measurement. The X-ray powder diffraction data was collected by using PAN-analytical 3 kW X’pert Powder (Multifunctional), X-ray diffractometer using Cu LFF High Resolution X-ray tube. The X-rays were produced using a sealed tube and the wavelength of X-ray was 0.154 nm. Molecular structure was determined by FTIR analysis done by Nicolet Instruments Corporation USA MAGNA-550. Energy dispersive X-ray analysis (EDX) was used for elemental analysis of the phosphor. Particle diameter and surface morphology of prepared phosphor determined by Transmission Electron Microscopy (TEM) using Philips CM-200. The PL emission spectra were recorded with a spectrofluorophotometer (SHIMADZU, RF-5301 PC), which was also used to record the excitation spectra. The 980 nm excitation was given by 980 nm laser source.
Results and discussion
XRD analysis
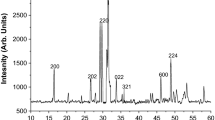
The structure and phase purity of combustion derived Yb3+ doped Gd2O3 phosphor is confirmed by XRD. It was observed that the phosphor prepared by combustion method has a monoclinic structure. The XRD patterns of Yb3+doped Gd2O3 phosphor annealed at different temperatures is shown in Fig. 1. In order to investigate the effect of temperature on structural properties of nanoparticles, different portions of phosphor containing 5 % Yb3+ were heated at 600, to 1400 °C for 2 h, respectively. The same type of observation has also been reported on Gd2O3: Eu nanocrystal by Jacobsohn et al. [19].
The monoclinic structure with the crystalline planes of (111), (200), (220), (311), (222), (400) and (331) are matched with that of pure Gd2O3 (JCPDS, File No. 43–1015) [20]. The average crystallite size was estimated using Scherrer’s equation [21].
Where λ is wavelength (0.154 nm) of X-rays, β; full width at half maximum θ; diffraction angle, k; shape factor (0.9) and Dhkl represents the size along (h k l) direction. The particle size was found to be in the range 6–22 nm for different annealing temperature. Particle was found in the range of 15–38 nm with variable Yb3+ concetrations.
The diffraction pattern indicates that the samples are crystallized in monoclinic crystal structure along with some additional peaks indicated the existence of Yb3+impurities. There is no additional signature in Yb3+doped Gd2O3phosphor which imply that the dopants were incorporated into the structure of Gd2O3 which indicates the formation of single phased phosphors. This XRD analysis indicates that the dopant do not participate in overall crystal formation except replacing/sitting between few atoms. The small variation in particles size was observed (Table 1). The concentration of oxygen vacancy is increased with increasing dopant concentration [22]. On increasing annealing temperature no significant change had been observed up to 900 °C. After 900 °C temperature, variation in the phase of the prepared phosphor observed and at 1400 °C the phase changes from monoclinic to cubic (Fig. 1). Relation between annealing temperature and particle size had been represented (Fig. 2).
The effect of Yb3+ ion concentration on structural properties had also been studied with different Yb3+ (5 % to 45 %) concentration annealed at 900 °C for 2 h (Fig. 3). Change in concentration of Yb3+ ion does not have any appreciable effect on phase and small variation in particle size of the prepared phosphor has been observed (Fig. 4) (Table 2).
Fourier transformation inferred spectroscopy (FITR)
To understand the vibrational modes of Gd2O3, FTIR spectra have been recorded for Yb3+ doped Gd2O3phosphor for different annealing temperature (Fig. 5). FTIR spectra revealed that the peak at 1382 cm−1 is may be due to surface-adsorbed NO3 − group, stretching vibration of NO [23]. The peak at 1500–1600 cm−1 correspond to the stretching mode of C = O in a carbonyl functional group on the crystal surface. The broad peak centred around 3450 cm-1 corresponds to the O-H symmetric stretching from the surface hydroxyl group. These surface groups enhance the depopulation of excited states of the RE3+ ion so that they can be act as a “sink”. Hydroxyl groups provide an effective pathway for the radiationless energy transfer of OH vibration and quench the emission intensity. The bands around 542 and 440 cm−1 are assigned to the Gd-O vibration of Gd2O3 [24].
FT-IR spectra illustrate changes for the broad band in the range 2500–3700 cm−1 where the hydroxyl group stretching vibration appear. It is clear from the spectra that the bands belonging to OH stretching decreases and gets almost invisible above 800 °C. Change in dopant concentration does not have any effect on FTIR spectra of Gd2O3:Yb3+ phosphor.
Transmission electron microscopy
Transmission electron microscopic analysis was carried out to identify the effect of annealing temperature (Fig. 6) and the effect of Yb3+ concentration (Fig. 7) on particle size of the prepared phosphor. Transmission electron microscopy study indicates that the average particle size of prepared phosphor was in 20–35 nm range. Moreover, the agglomeration of powder particles was also observed. The transmission electron microscope results are in good correlation with the XRD results.
Energy dispersive X-ray (EDX) analysis
The elemental analysis was carried out by using energy dispersive X-ray (EDX) analysis. It is clear from the analysis that the prepared phosphor has Gd, O and Yb as constituent element, which indicates the formation of Gd2O3:Yb3+ phosphor (Fig. 8).
Optical properties
The emission spectra of Yb3+ doped Gd2O3 phosphor were recorded and the effect of annealing temperature and Yb3+ ion concentration on emission spectra were studied. Single Yb3+ ion have only two Stark splitting energy manifolds, the ground state 2F7/2 and the excited state 2F5/2 around 10,000 cm−1. Due to the thermal vibration of crystal lattice at room temperature, the manifolds 2F5/2 and 2F7/2 of Yb3+ ions are expected to split. So a series of NIR emissions with different peak values originate from the same transition of 2F5/2 → 2F7/2 are observed. For Yb3+, the ground 2F7/2 state consists of four Stark splitting levels labelled from 1 to 4 toward higher energy, and the excited 2F5/2 state consists of three Stark splitting levels labelled from 5 to 7 toward higher energy [4, 5].
The near infrared (NIR) emission spectra of Gd2O3:Yb3+ were recorded with the excitation of 980 nm laser diode at room temperature. The samples show intense emissions within the region of 960–1100 nm (Fig. 9). A strong peak at 1027 nm and weak shoulders at 972 nm, 1006 nm and 1071 nm are present, which are assigned to the f–f transitions of Yb3+ from 2F5/2 to 2F7/2 [25]. The excitation of 980 nm irradiation causes the 1 → 6 transition. The PL peak at 1027 nm is assigned to the 5 → 3 transition. Besides the main peak at 1027 nm, PL peaks are observed at 972 and 1071 nm which are assigned to 5 → 1 and 5 → 4 transitions, respectively. Another PL peak at 1006 nm might be overlaid peaks corresponding to 6 → 2, 6 → 3, and 6 → 4 transitions (Fig. 10). Furthermore, strong interaction of Yb3+ ions with lattice vibrations causes strong vibronic sidebands or supplementary Stark splitting levels due to resonances between Stark levels and phonon, resulting in a formation of a broad background peak [26–28].
Effect of annealing temperature on emission spectra
To observe the effect of heat on the photoluminescence behaviour of the Gd2O3:Yb3+ phosphor the emission spectra were recorded after annealing the prepared phosphor under 600 °C to 1400 °C for 2 h. The PL intensity becomes higher with increasing annealing temperature. The PL intensities of the samples heated at 600 °C, 900 °C and 1400 °C are 2.5 and 5.6 and 12.8 time, respectively, higher than that of as-prepared sample (Fig. 11). The increasing annealing temperature decreases OH− group’s contamination in the prepared Gd2O3:Yb3+ phosphors above 800 °C. However luminescence quenching due to hydroxide group was not observed. Therefore it can be concluded that there is no OH− influence for quenching of luminescence in Gd2O3:Yb3+ annealed at 900 °C.
Heating at 1400 °C eliminates lattice distortion and promotes crystallite growth, which is responsible for the enhancement of near infrared emission. However, near infrared emission intensity increases after heating below 900 °C, although this heating does not have large influence on crystallite size and lattice distortion. Another important factor is multiphonon relaxation by adsorbed species on particle surface which quenches near infrared emission of Yb3+ through their vibrational excitation. Energy of 2F5/2 → 2F7/2 transition of Yb3+ ≈ 10.000 cm−1, which is about three times larger than energy of O─H stretching vibration, 3000–3500 cm−1, and about six times larger than energy of C─O stretching vibration, ≈1500 cm−1. Probability of multiphonon relaxation for O─H stretching vibration therefore, higher than that of the latter. In fact, heating at 1400 °C markedly causes weakening of the absorption due to O─H stretching vibration as well as crystallite growth, and hence enhances near infrared emission, although the absorption due to C─O stretching vibration is still high [27–29].
Effect of Yb3+ ion concentration on PL emission spectra
To study the effect of Yb3+ ion concentration on luminescence behaviour of Gd2O3:Yb3+ phosphor the emission spectra were recorded for the Gd2O3:Yb3+ containing different Yb3+ concentration (Fig. 12). All the samples were annealed under 900 °C before recording the emission spectra. The phosphor was annealed at 900 °C as after this temperature structure changes from monoclinic to cubic is observed. It is clear from the spectra that the Yb3+ ion concentration does not show any change in peak positions of emission spectra. The intensity of emission peaks increases with increasing Yb3+ ion concentration (Fig. 13). No quenching in NIR emission for higher Yb3+ was observed.
Conclusion
Gd2O3:Yb3+ phosphors were prepared by combustion route method using urea as fuel. Structural characterization reveals that the prepared phosphor has monoclinic structure. The particle size of the prepared phosphor was found around 6–28 nm for different annealing temperature and around 15–38 nm for variable Yb3+ ion concentration. In optical characterization near infrared emission (NIR) was observed at 1027 nm corresponding to the 2F5/2 → 2F7/2 transition of Yb3+ under 980 nm excitation. Subsequent heat treatment causes crystalline growth and dehydration, resulting in an enhancement of NIR emission. No quenching in NIR emission with variable Yb3+ concentration was observed.
References
C. Feldmann, T. Justel, C.R. Ronda, P.J. Schmidt, Inorganic luminescent materials: 100 years of research and application. Adv. Funct. Mater. 13, 511–516 (2003)
H. Guo, N. Dong, M. Yin, W. Zhang, L. Lou, S. Xia, Visible upconversion in are earth ion-doped Gd2O3nanocrystals. J. of Phy. Chem. B 108, 19205–19209 (2004)
T.Y. Fan, IEEE J. Quant. Elec. 29, 1457 (1993)
L. Laversenne, Y. Guyot, C. Goutaudier, M.T. Cohen-Adad, G. Boulon, Optimization of spectroscopic properties of Yb3+-doped refractory sesquioxides: cubic Y2O3, Lu2O3 and monoclinic Gd2O3. Opt. Mat. 16, 475–483 (2001)
W.F. Krupke, IEEE J. Sel. Top. Quantum Electron. 6, 1287 (2000)
U. Griebner, S. Rivier, V. Petrov, M. Zorn, G. Erbert, M. Weyers, X. Mateos, M. Aguiló, J. Massons, F. Dıaz, Opt. Express 13, 3456 (2005)
C. Manjunatha, B.M. Nagabhushana, D.V. Sunitha, H. Nagabhushana, S.C. Sharma, G.B. Venkatesh, R.P.S. Chakradhar, J. Lumin. 134, 432–440 (2013)
A.B.B. Umesh, B. Eraiah, H. Nagabhushana, G. Nagaraju, J. Alloys Compd 509, 1146–1151 (2011)
Y. Bai, K. Yang, Y. Wang, X. Zhang, Y. Song, Opt. Commun. 281, 2930 (2008)
A. Brenier, G. Boulon, J. Lumin. 82, 285 (1999)
R.K. Tamrakar, D.P. Bisen, N. Brahme, K. Upadhyay, “Structural and Luminescence Behavior of Gd2O3: Er3+ Phosphor Synthesized by Solid State Reaction Method, Optik – Int. J. Light. Electron Opt. (2015), http://dx.doi.org/10.1016/j.ijleo.2015.06.045
R.K. Tamrakar, D.P. Bisen, thermoluminescence studies of ultraviolet and gamma irradiated erbium(III)- and ytterbium(III)-doped gadolinium oxide phosphors. Mater. Sci. Semicond. Process. 33, 169–188 (2015)
K.C. Patil, M.S. Hegde, T. Rattan, S.T. Aruna, Chemistry of Nanocrystalline : Combustion Synthesis, Properties and Applications (World Scientific Publishing Co. Pvt. Ltd., UK, 2008)
Y. Li, Hong G. J. Lumin. 124, 297 (2007)
R.K. Tamrakar, D.P. Bisen, K. Upadhyay, N. Bramhe, Down-conversion luminescence property of Er3+ and Yb3+ co-doped Gd2O3 crystals prepared bycombustion synthesis and solid state reaction method. Superlattice. Microst. 81, 34–48 (2015)
Y. Li, G. Hong, Synthesis and luminescence properties of nanocrystalline Gd2O3:Eu3+ by combustion process. J. of Lumin. 124, 297–301 (2007)
R.K. Tamrakar, D.P. Bisen, C.S. Robinson, I.P. Sahu, N. Brahme, Ind. J. Mat. Sci. 396147, 7 (2014)
R.K. Tamrakar, D.P. Bisen, Combustion synthesis and optical properties of ceria doped gadolinium oxide Nano powder. AIP Conf. Proc. 273, 1536 (2013). doi:10.1063/1.4810206
L.G. Jacobsohn, B.L. Bennett, R.E. Muenchausen, S.C. Tornga, J.D. Thompson, O. Ugurlu, D.W. Cooke, A.L. Lima Sharma, J. Appl. Phys. 103, 104303 (2008)
D. Grier, G. McGarthy, Fargo, ND (USA, ICCD, Grantin Aid, 1991)
A. Guinier, X-ray diffraction (Freeman, San Francisco, Calif, USA, 1963)
A.M. Pires, M.F. Santos, M.R. Davolos, E.B. Stucchi, The effect of Eu ion doping concentration in Gd2O3 fine spherical particles. J. of All. And Comp. 344, 276–279 (2002)
F. Vetrone, J.C. Boyer, J.A. Capobianco, Effect of Yb3+codoping on the upconversion emission in nanocrystalline Y2O3: Er3+. J. Phys. Chem. 107, 1107–1112 (2003)
N. Zhang, R. Yi, L. Zhou, G.V. Gao, R. Shi, G.V. Qiu, xiaohe Liu, lanthanide hydroxide nanorods and their thermal decomposition to lanthanide, oxide nanorods. Mater. Chem. Phys. 114, 160–167 (2009)
G. Boulon, Opt. Mater. 34, 499–512 (2012)
H. Cañibano, G. Boulon, L. Palatella, Y. Guyot, A. Brenier, M. Voda, R. Balda, J. Fernandez. J. Lumin. 318 (2003) 102–103.
Y. Hasegawa, Y. Wada, S. Yanagida, J. Photochem. Photobiol. C 5, 183 (2004)
R.K. Tamrakar, D.P. Bisen, N. Brahme, Effect of Yb3+ concentration on photoluminescence properties of cubic Gd2O3 phosphor. Infrared Phys. Technol. 68, 92–97 (2015)
R.K.Tamrakar, D.P. Bisen, K. Upadhyay, I.P. Sahu, Comparative study and role of Er3+ and Yb3+ concentrations on upconversion process of Gd2O3: Er3+ Yb3+ phosphors prepared by solid-state reaction and combustion method. J. Phys. Chem. C 119(36), 21072--21086 (2015)
Acknowledgments
We are very grateful to NIT Raipur for XRD characterization and also thankful to Dr.Mukul Gupta for his encouragement. I am very thankful to SAIF, IIT Bombay for other characterization such as SEM, TEM, FTIR and EDX. Very thankful to Dr. KVR Murthy M.S. University, Baroda (Chairman Luminescence Society of India) for PL study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamrakar, R.K., Bisen, D.P., Upadhyay, K. et al. Effect of synthesis annealing temperature & Yb3+ concentration on photoluminescence properties of monoclinic Gd2O3 phosphor. J Opt 44, 337–345 (2015). https://doi.org/10.1007/s12596-015-0307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-015-0307-0