Abstract
The present paper reports thermoluminescence (TL) glow curves of Eu3+-doped Y2O3 phosphor with different ultraviolet (UV) exposure times. The glow peak shows second-order kinetics of Eu3+-doped Y2O3, and corresponding kinetic parameters were evaluated using the peak shape method. Calculations of trap depth were carried out using different methods. The kinetics order, activation energy, and frequency factor were calculated. The recorded glow curve shifts towards higher intensity with longer UV exposure. The heating rate used for recording TL was 3.0 °C s−1. Particle size and structure were verified by X-ray diffraction (XRD) pattern and morphology by scanning electron microscopy (SEM) imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphors are important constituents of field-emission displays (FEDs) and cathode-ray tubes (CRTs). Europium-doped Y2O3 is a common red phosphor in lighting and display applications, and nanoscale Y2O3:Eu3+ is a candidate for new applications in FEDs and high-definition televisions (HDTVs) [1]. Y2O3 has a large band gap (5.8 eV) and high dielectric constant of 14–18, and is also optically isotropic with a refractive index of 1.91, as reported elsewhere [2]. Yttrium sesquioxide (Y2O3) ceramics have been intensively investigated for different technological purposes. For decades, yttrium oxide has been an important material in the ceramic industry, from being a constituent of ceramic superconductors [3] to well-known yttria-stabilized zirconia (YSZ) ceramics [4]. Y2O3 is used in electronic applications as part of metal–oxide–semiconductor (MOS) heterostructures in MOS transistors [5]. It also plays an important role in the preparation of novel light-emitting materials [6–9]. Apart from these properties, other characteristics of lanthanide-doped oxides also play an important role in the use of these materials in technology. Thermoluminescence (TL) is the emission of light from a sample when it is heated due to previous absorption of energy from irradiation. UV-induced TL in rare-earth-doped oxide phosphors and its possible use in UV dosimetry have been investigated, showing that Eu-doped Y2O3 is sensitive enough to measure background UV radiation such as from sunlight, bulb light, etc. [1, 11].
Experimental
The method used for preparation of Y2O3:Eu3+ was the solid-state reaction method. The appropriate oxides and fluorides (Y2O3, Eu2O3, and CaF2) were thoroughly ground in a mortar for 45 min then fired at 1,300 °C for about 1 h. Thermally stimulated luminescence glow curves were recorded at room temperature by using a TLD reader (I1009 supplied by Nucleonix Sys. Pvt. Ltd., Hyderabad) [13, 14]. The TL of the obtained phosphor was examined with UV exposure at 365 nm at a heating rate for the TL measurement of 3 °C s−1. The sample was characterized at Inter University Consortium (IUC), Indore using X-ray diffraction. XRD data were collected over the range 20–70° at room temperature. XRD measurements were carried out using a Bruker D8 Advance X-ray diffractometer. The X-rays were produced using a sealed tube at wavelength of 0.154 nm (Cu Kα). The X-rays were detected using a fast counting detector based on silicon strip technology (LynxEye detector; Bruker). Particle size was calculated using the Debye–Scherrer formula. Particle size and morphological investigations of the Y2O3:Eu3+ phosphor prepared by this process were carried out by scanning electron microscopy (SEM, LEO 440 system).
Results and discussion
Structural characterization
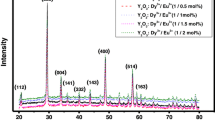
The XRD patterns of Eu3+-doped Y2O3 phosphor are shown in Fig. 1. Four different peaks were obtained, at 2θ values of 29.12°, 33.78°, 48.46°, and 57.56°, corresponding to diffraction at (222), (400), (440), and (622) planes, respectively. The sharp peaks indicate the microcrystalline behavior of the sample. All diffraction patterns are in good agreement with JCPDS reference no. 86-1107, proving all powders to be pure cubic Y2O3 phase having lattice symmetry. The size of the particles was computed from the width of the first peak using the Debye–Scherrer formula [10]
where λ is the X-ray wavelength, θ is the diffraction pattern angle, and β is the corrected full-width at half-maximum (FWHM) of the XRD peaks (corresponding to 2θ).
SEM results
Figure 2 shows SEM micrographs of Eu3+-doped Y2O3 phosphor synthesized by the solid-state method. The phosphor shows a compact distribution over the surface and good connectivity between grains. It shows flake-type formation with particle size distribution around 500 nm.
TL glow curves of Eu3+-doped Y2O3 phosphor
With the help of TL glow curve we calculated trapping parameters such as the trap depth (E), escape frequency factor (s), and kinetics order for glow peaks obtained under ultraviolet excitation. The TL glow curve of Y2O3:Eu3+ phosphor showed second-order kinetics. Y2O3:Eu3+ phosphor was found to be a very good red phosphor. The phosphor was subjected to UV irradiation at 365 nm from a UV source at a heating rate of 3 °C s−1. Each time, 2 mg of irradiated phosphor was taken for TL measurements.
The TL glow curves for phosphor for different UV exposure times are shown and compared in Fig. 3. In all the glow curves, only a single peak was observed, at around 138, 143, and 144 °C, respectively. It was observed that the peak temperature increased with increasing UV exposure time. The kinetic parameters of UV-irradiated Y2O3:Eu3+ phosphor are presented in Table 1. In Table 2, the trap depth for the prominent glow peaks of the Y2O3:Eu3+ phosphor is given.
This phosphor could be used in fluorescent lamps, projection television tubes, as well as plasma display panels. It can be sensitized for excitation at 365 nm using Bi and, reduced efficiency at 254 nm [12]. The presented study results show satisfactory agreement for the 365-nm UV source. The intensity of the TL glow curve increases with increasing UV exposure time.
Conclusions
From the above study results it can be concluded that Y2O3:Eu3+ phosphor can be prepared by solid-state synthesis. The synthesized sample follows second-order kinetics. The E a (activation energy) and frequency factor lie in the range of 0.516–0.568 eV and 2 × 107–1 × 108 s−1, respectively. The trap depth decreases with increasing UV exposure time.
References
R. Chen, Y. Krish, Analysis of Thermally Stimulated Processes (Pergamon, New York, 1981)
S. Shinoya, W.M. Yen, Phosphor Handbook 2, vol. 1 (CRC, Boca Raton, 1999)
P. Regnier, M. Sapin, C. Thomas de Montpreville, Supercond. Sci. Technol. 2, 173 (1989)
M. Boaro, J.M. Vohs, R.J. Gorte, J. Am. Ceram. Soc. 86, 395 (2003)
J.J. Chambers, G.N. Pearson, J. Appl. Phys. 90, 918 (2001)
F. Vetrone, J.-C. Boyer, J.A. Capobianco, A. Speghini, M. Bettinelli, Chem. Mater. 15, 2737 (2003)
H. Wang, C.K. Lin, X.M. Liu, J. Lin, M. Yu, Appl. Phys. Lett. 87, 181907 (2005)
Y. Zhou, J. Lin, S.B. Wang, J. Solid State Chem. 171, 391 (2003)
A. Konrad, U. Herr, R. Tidecks, F. Kummer, K. Samwer, J. Appl. Phys. 90, 3516 (2001)
A. Guinier, X-ray Diffraction (Freeman, San Francisco, 1963)
C. Furetta, Handbook of Thermoluminescence (World Scientific, Singapore, 2003)
W.M. Yen, M.J. Weber, Inorganic Phosphors, Compositions, Preparation and Optical Properties (CRC, London, 2004)
V. Dubey, N.S. Suryanarayana, J. Kaur, J. Miner. Mater. Character. Eng. 9(12), 1101 (2010)
R.K. Tamrakar, D.P. Bisen, Res. Chem. Intermed. (2012). doi:10.1007/s11164-012-0816-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamrakar, R., Dubey, V., Swamy, N.K. et al. Thermoluminescence studies of UV-irradiated Y2O3:Eu3+ doped phosphor. Res Chem Intermed 39, 3919–3923 (2013). https://doi.org/10.1007/s11164-012-0908-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0908-z